Abstract
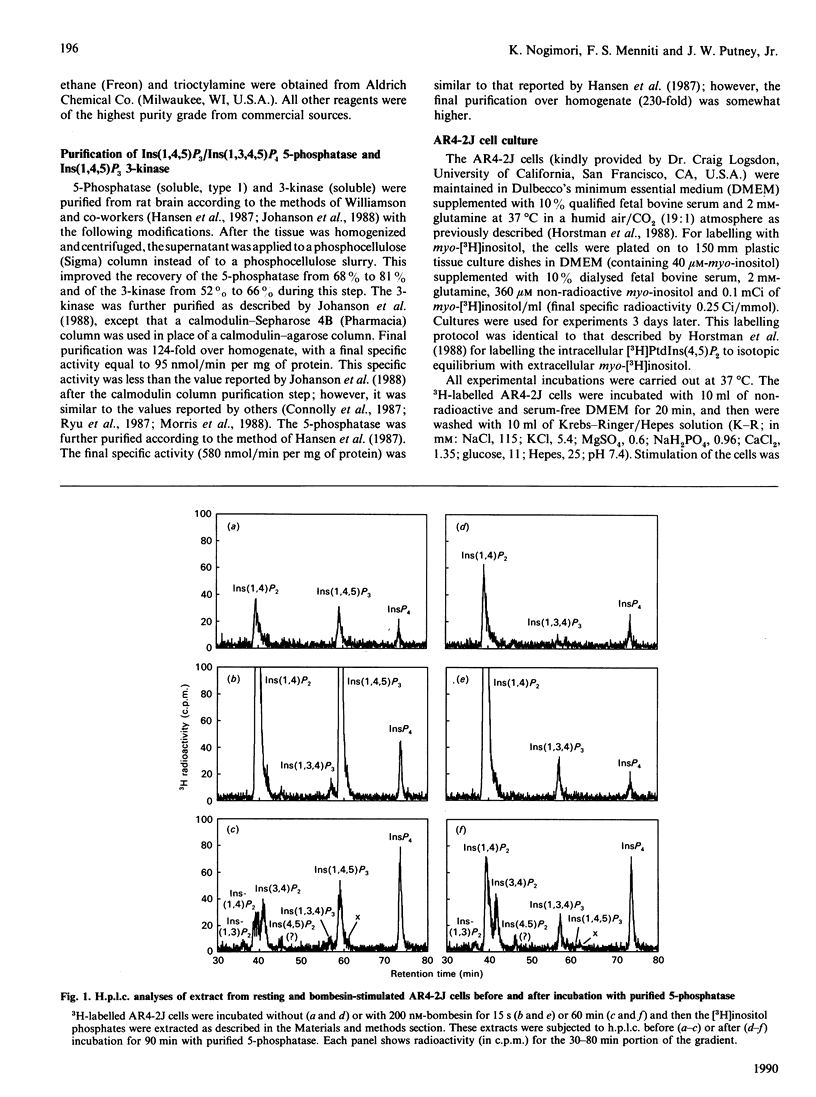
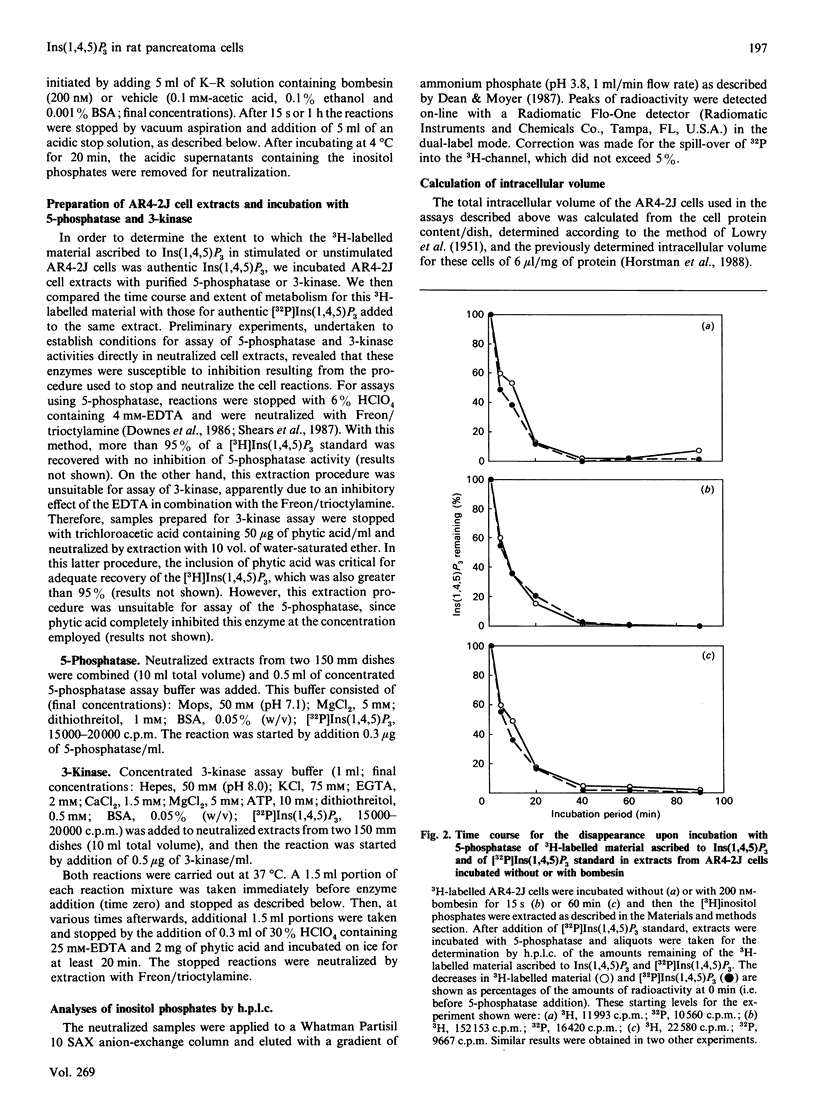
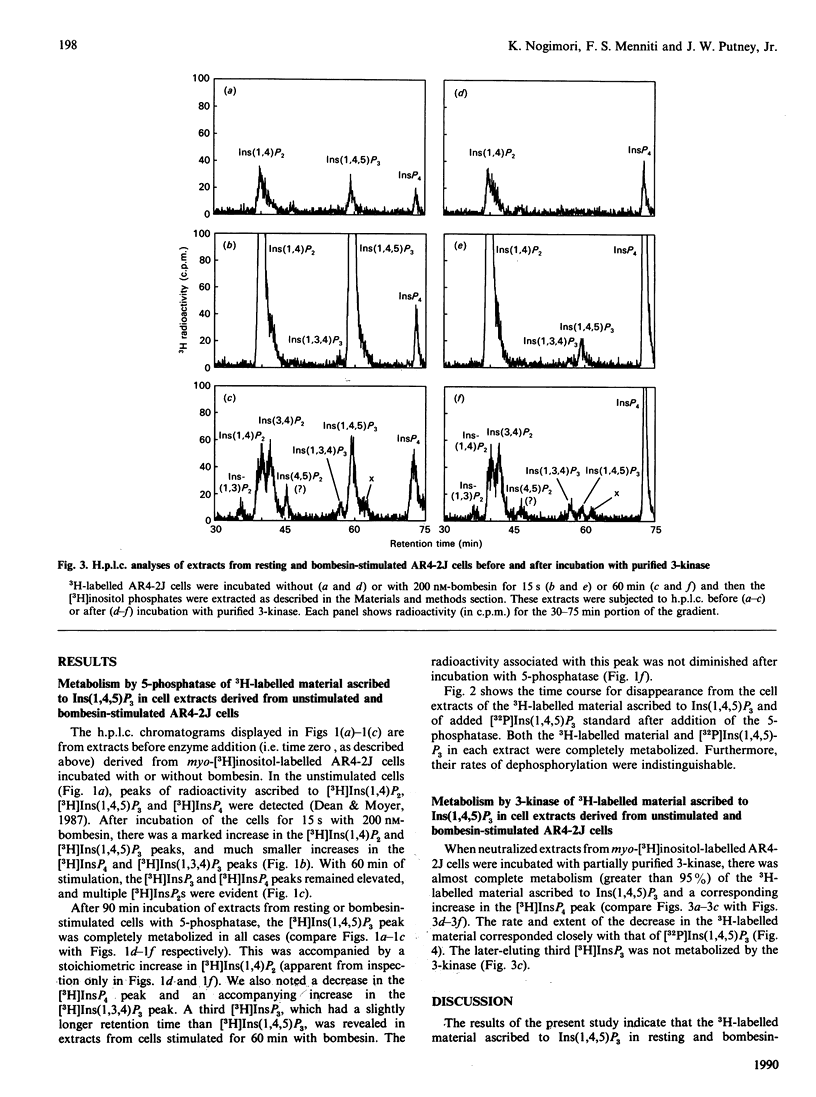
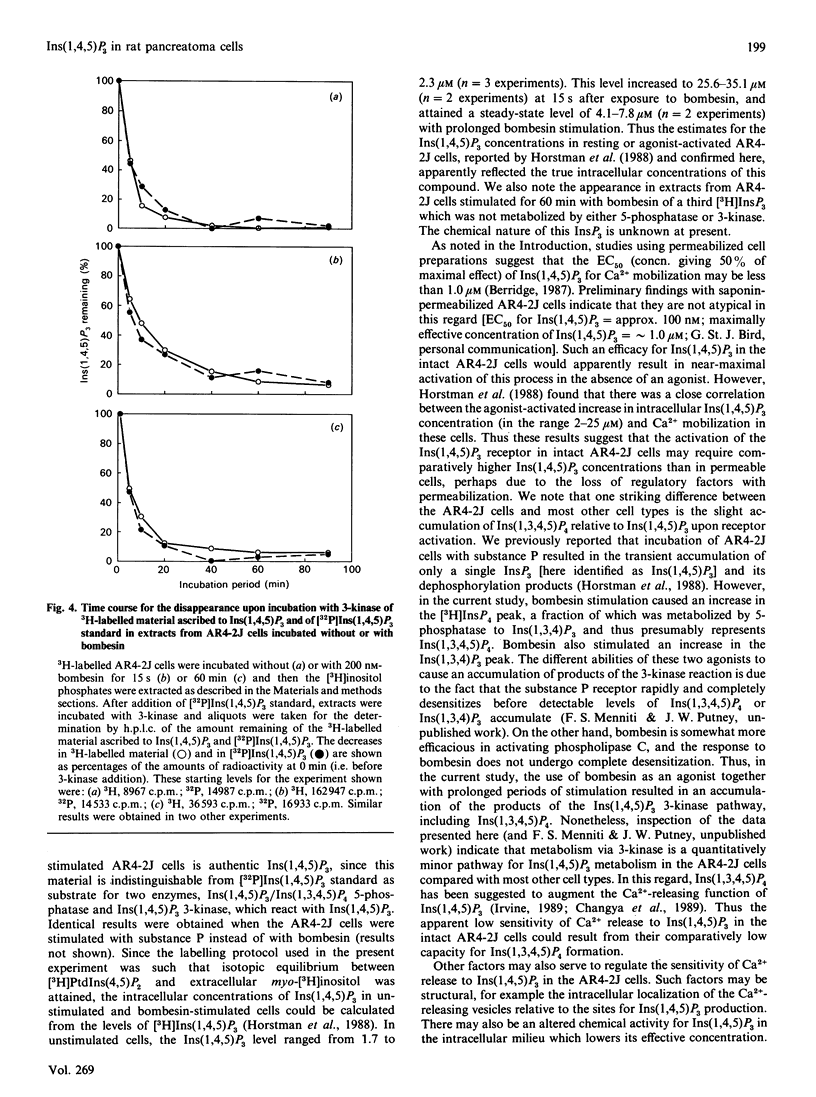
The identity of 3H-labelled material ascribed to Ins(1,4,5)P3 in resting or bombesin-stimulated myo-[3H]inositol-labelled AR4-2J cells was investigated by determining its ability to serve as substrate for partially purified Ins(1,4,5)P3/Ins(1,3,4,5)-P4 5-phosphatase and Ins(1,4,5)P3 3-kinase. This 3H-labelled material was metabolized by these two enzymes at rates which were indistinguishable from those for an internal [32P]Ins(1,4,5)P3 standard, establishing its identity as authentic Ins(1,4,5)P3. In addition, and in contrast with findings in earlier studies utilizing substance P as an agonist, prolonged stimulation with bombesin resulted in an increase in an InsP4 which was degraded by Ins(1,4,5)P3/Ins(1,3,4,5)P4 5-phosphatase. These findings serve to confirm the previous estimate of Horstman, Takemura & Putney [(1988) J. Biol. Chem. 263, 15297-15303] for the intracellular concentrations of Ins(1,4,5)P3 in resting (2 microM) and agonist-stimulated (25 microM) AR4-2J cells. The implications of these findings for the physiological regulation of intracellular Ca2+ through this intracellular messenger are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Quantitative changes in inositol 1,4,5-trisphosphate in chemoattractant-stimulated neutrophils. J Biol Chem. 1986 Nov 25;261(33):15644–15647. [PubMed] [Google Scholar]
- Brass L. F., Joseph S. K. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J Biol Chem. 1985 Dec 5;260(28):15172–15179. [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bansal V. S., Bross T. E., Irvine R. F., Majerus P. W. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987 Feb 15;262(5):2146–2149. [PubMed] [Google Scholar]
- Daniel J. L., Dangelmaier C. A., Smith J. B. Formation and metabolism of inositol 1,4,5-trisphosphate in human platelets. Biochem J. 1987 Aug 15;246(1):109–114. doi: 10.1042/bj2460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. M., Moyer J. D. Separation of multiple isomers of inositol phosphates formed in GH3 cells. Biochem J. 1987 Mar 1;242(2):361–366. doi: 10.1042/bj2420361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C. A., Johanson R. A., Williamson M. T., Williamson J. R. Purification and characterization of two types of soluble inositol phosphate 5-phosphomonoesterases from rat brain. J Biol Chem. 1987 Dec 25;262(36):17319–17326. [PubMed] [Google Scholar]
- Horstman D. A., Takemura H., Putney J. W., Jr Formation and metabolism of [3H]inositol phosphates in AR42J pancreatoma cells. Substance P-induced Ca2+ mobilization in the apparent absence of inositol 1,4,5-trisphosphate 3-kinase activity. J Biol Chem. 1988 Oct 25;263(30):15297–15303. [PubMed] [Google Scholar]
- Johanson R. A., Hansen C. A., Williamson J. R. Purification of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. J Biol Chem. 1988 Jun 5;263(16):7465–7471. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Murray K. J., England P. J., Downes C. P., Michell R. H. Partial purification and some properties of rat brain inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1988 Apr 1;251(1):157–163. doi: 10.1042/bj2510157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenberg T., Scholz P., Bergmann G., Mayr G. W. The quantitative spectrum of inositol phosphate metabolites in avian erythrocytes, analysed by proton n.m.r. and h.p.l.c. with direct isomer detection. Biochem J. 1989 Dec 1;264(2):323–333. doi: 10.1042/bj2640323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. H., Lee S. Y., Lee K. Y., Rhee S. G. Catalytic properties of inositol trisphosphate kinase: activation by Ca2+ and calmodulin. FASEB J. 1987 Nov;1(5):388–393. doi: 10.1096/fasebj.1.5.2824270. [DOI] [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Downes C. P. An analysis of myo-[3H]inositol trisphosphates found in myo-[3H]inositol prelabelled avian erythrocytes. Biochem J. 1989 Sep 15;262(3):727–737. doi: 10.1042/bj2620727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tarver A. P., King W. G., Rittenhouse S. E. Inositol 1,4,5-trisphosphate and inositol 1,2-cyclic 4,5-trisphosphate are minor components of total mass of inositol trisphosphate in thrombin-stimulated platelets. Rapid formation of inositol 1,3,4-trisphosphate. J Biol Chem. 1987 Dec 25;262(36):17268–17271. [PubMed] [Google Scholar]


