Abstract
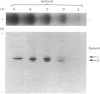
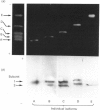
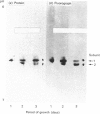
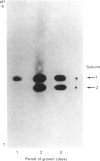
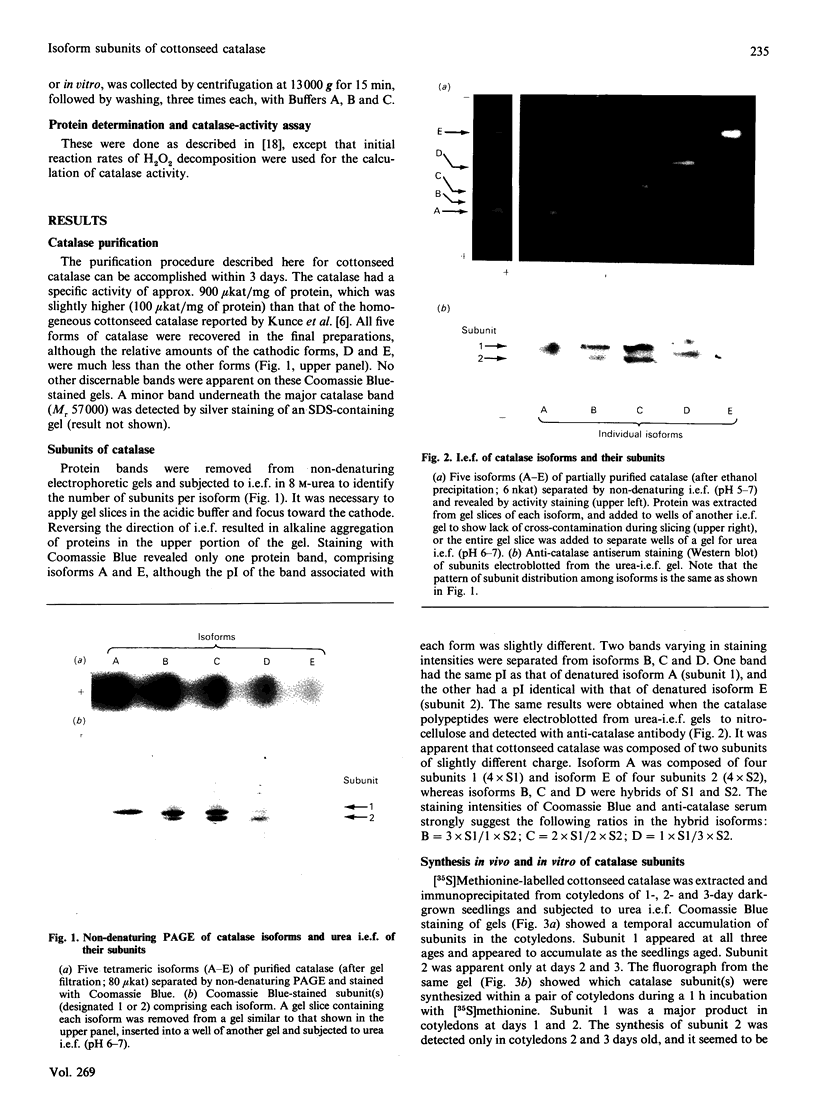
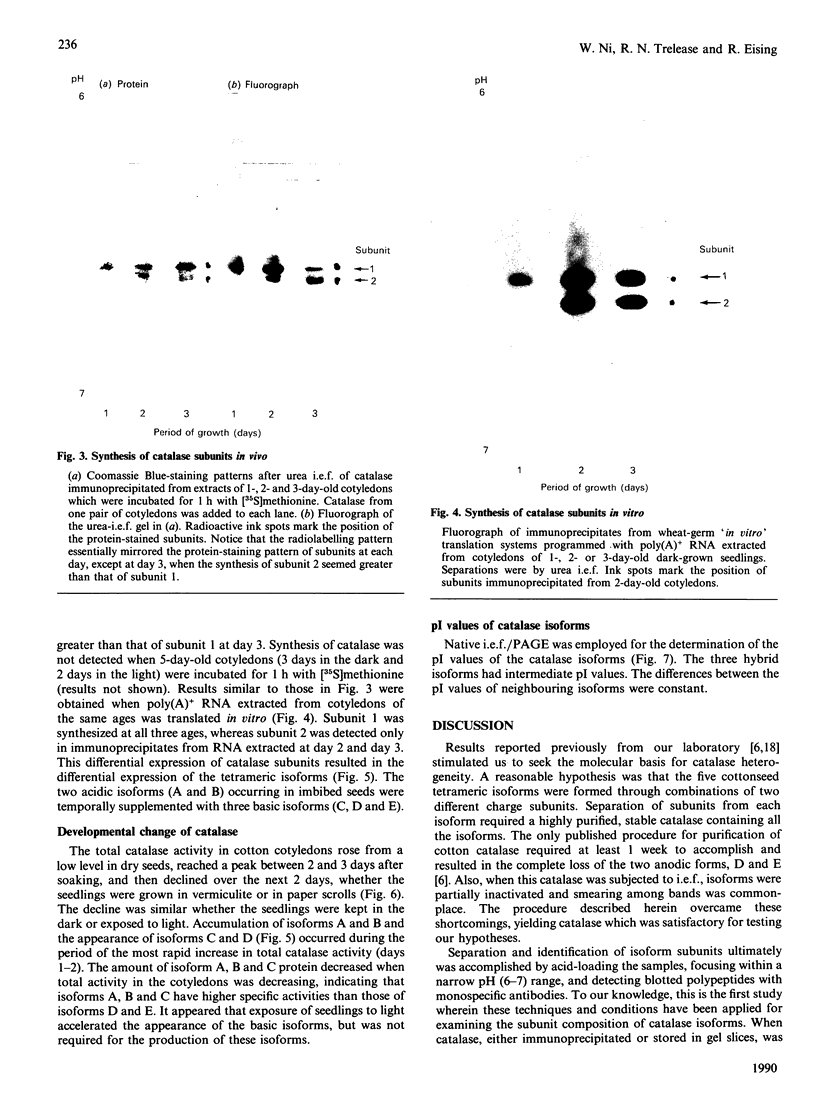
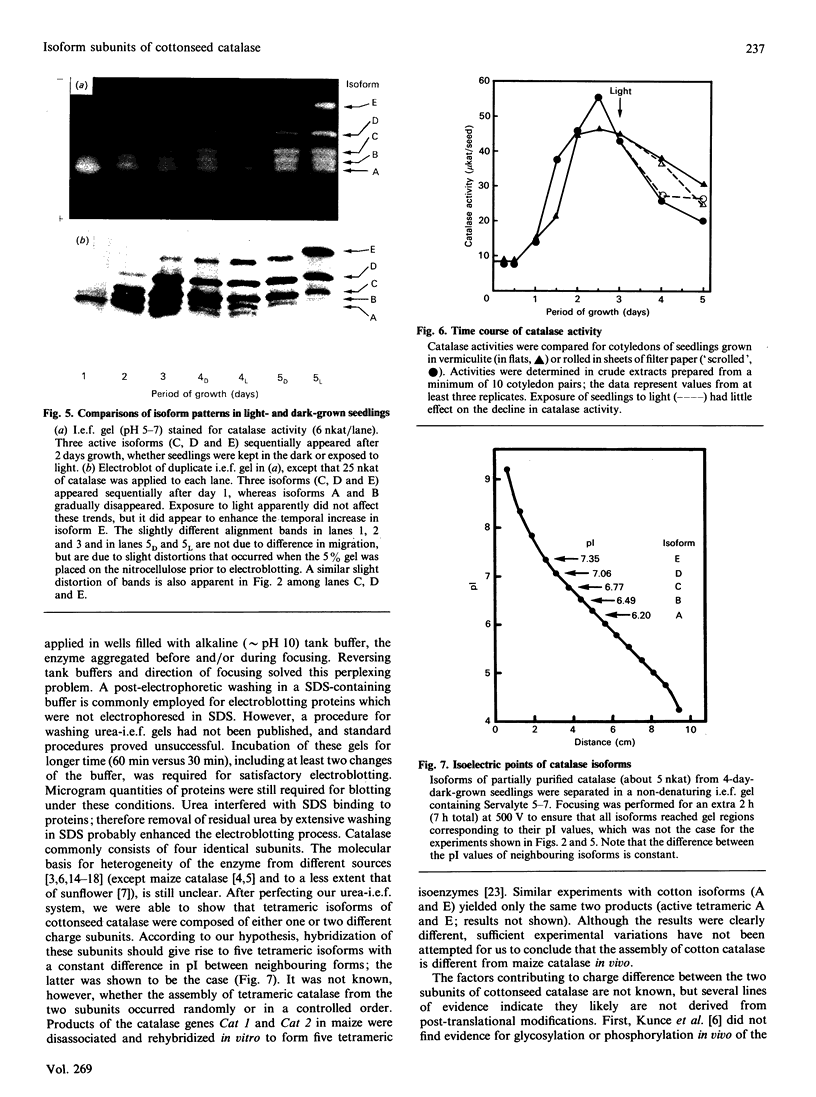
Five charge isoforms of tetrameric catalase were isolated from cotyledons of germinated cotton (Gossypium hirsutum L.) seedlings. Denaturing isoelectric focusing of the individual isoforms in polyacrylamide gels indicated that isoforms A (most anodic) and E (most cathodic) consisted of one subunit of different charge, whereas isoforms B, C and D each consisted of a mixture of these two subunits. Thus the five isoforms apparently were formed through combinations of two subunits in different ratios. Labelling cotyledons in vivo with [35S]methionine at three daily intervals in the dark, and translation in vivo of polyadenylated RNA isolated from cotyledons at the same ages, revealed synthesis of two different subunits. One of the subunits was synthesized in cotyledons at all ages studied (days 1-3), whereas the other subunit was detected only at days 2 and 3. This differential expression of two catalase subunits helped explain previous results from this laboratory showing that the two anodic forms (A and B) found in maturing seeds were supplemented with three cathodic forms (C-E) after the seeds germinated. These subunit data also helped clarify our new findings that proteins of isoforms A, B and C (most active isoforms) accumulated in cotyledons of plants kept in the dark for 3 days, then gradually disappeared during the next several days, whereas isoforms D and E (least active isoforms) remained in the cells. This shift in isoform pattern occurred whether seedlings were kept in the dark or exposed to continuous light after day 3, although exposure to light enhanced this process. These sequential molecular events were responsible for the characteristic developmental changes (rise and fall) in total catalase activity. We believe that the isoform changeover is physiologically related to the changeover in glyoxysome to leaf-type-peroxisome metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eising R., Gerhardt B. Catalase Synthesis and Turnover during Peroxisome Transition in the Cotyledons of Helianthus annuus L. Plant Physiol. 1989 Mar;89(3):1000–1005. doi: 10.1104/pp.89.3.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising R., Trelease R. N., Ni W. T. Biogenesis of catalase in glyoxysomes and leaf-type peroxisomes of sunflower cotyledons. Arch Biochem Biophys. 1990 Apr;278(1):258–264. doi: 10.1016/0003-9861(90)90256-x. [DOI] [PubMed] [Google Scholar]
- Fita I., Rossmann M. G. The active center of catalase. J Mol Biol. 1985 Sep 5;185(1):21–37. doi: 10.1016/0022-2836(85)90180-9. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987 Jun;84(2):450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. K., Yuen K. C., Denell R. E., Consigli R. A. Efficient transfer of proteins from acetic acid-urea and isoelectric-focusing gels to nitrocellulose membrane filters with retention of protein antigenicity. Anal Biochem. 1983 Aug;133(1):126–131. doi: 10.1016/0003-2697(83)90232-4. [DOI] [PubMed] [Google Scholar]
- Kunce C. M., Trelease R. N. Heterogeneity of catalase in maturing and germinated cotton seeds. Plant Physiol. 1986 Aug;81(4):1134–1139. doi: 10.1104/pp.81.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunce C. M., Trelease R. N., Turley R. B. Purification and biosynthesis of cottonseed (Gossypium hirsutum L.) catalase. Biochem J. 1988 Apr 1;251(1):147–155. doi: 10.1042/bj2510147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Fujiki Y., Mortensen R. M., Lazarow P. B. Acyl-Coa oxidase and hydratase-dehydrogenase, two enzymes of the peroxisomal beta-oxidation system, are synthesized on free polysomes of clofibrate-treated rat liver. J Cell Biol. 1984 Dec;99(6):2241–2246. doi: 10.1083/jcb.99.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. The antioxidant enzyme genes Cat and Sod of maize: regulation, functional significance, and molecular biology. Isozymes Curr Top Biol Med Res. 1987;14:19–44. [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaftaris A. S., Scandalios J. G. The multi-locus catalase gene-enzyme system of maize: a model system for the study of gene regulation and enzyme differentiation and function in higher plants. Isozymes Curr Top Biol Med Res. 1983;7:59–77. [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Maturation of catalase precursor proceeds to a different extent in glyoxysomes and leaf peroxisomes of pumpkin cotyledons. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4809–4813. doi: 10.1073/pnas.81.15.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Purification and characterization of heme-containing low-activity form of catalase from greening pumpkin cotyledons. Eur J Biochem. 1986 Sep 1;159(2):315–322. doi: 10.1111/j.1432-1033.1986.tb09870.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M. Purification of glyoxysomal catalase and immunochemical comparison of glyoxysomal and leaf peroxisomal catalase in germinating pumpkin cotyledons. Plant Physiol. 1984 Feb;74(2):261–267. doi: 10.1104/pp.74.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]