Abstract
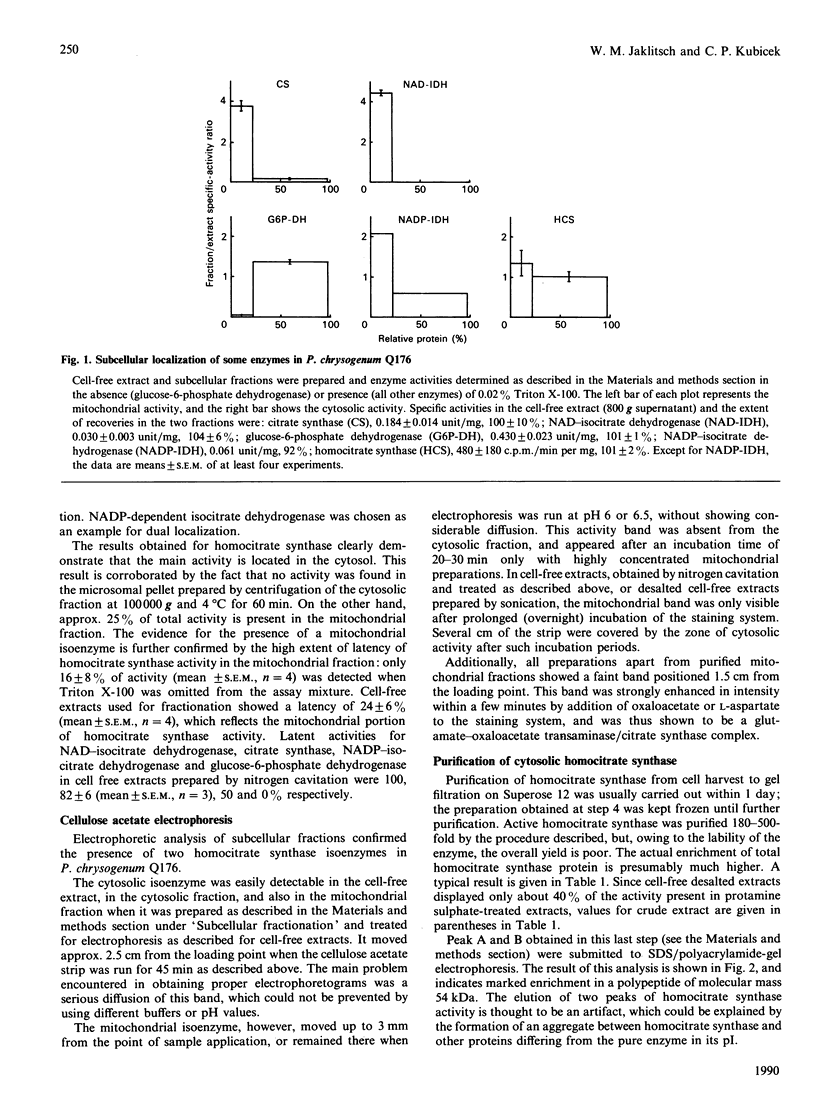
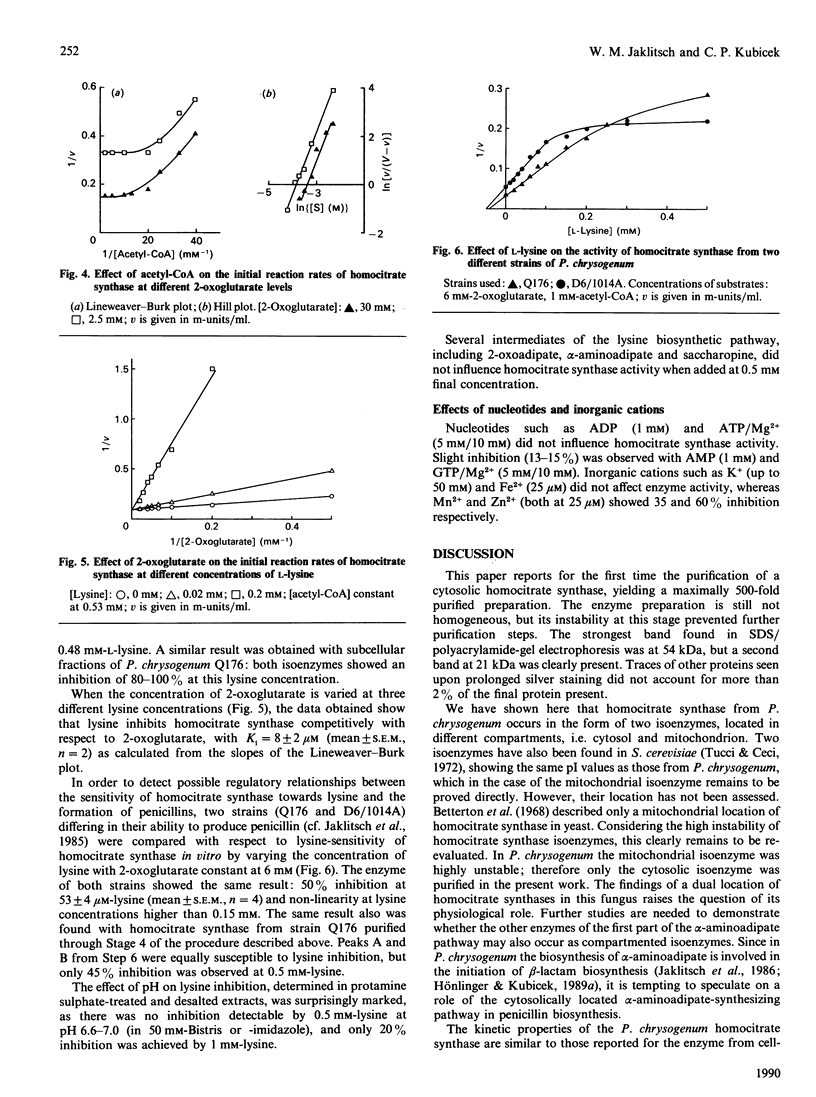
Subcellular fractionation of cell-free extracts obtained by nitrogen cavitation showed that Penicillium chrysogenum Q176 contains a cytosolic as well as a mitochondrial homocitrate synthase activity. The cytosolic isoenzyme was purified about 500-fold, and its kinetic and molecular properties were investigated. Native homocitrate synthase shows a molecular mass of 155 +/- 10 kDa as determined by gel filtration and a pH of 4.9 +/- 0.1 as determined by chromatofocusing. The kinetic behaviour towards 2-oxoglutarate is hyperbolic, with Km = 2.2 mM; with respect to acetyl-CoA the enzyme shows sigmoidal saturation kinetics, with [S]0.5 = 41 microM and h = 2.6. The enzyme was inhibited strongly by L-lysine (Ki = 8 +/- 2 microM; 50% inhibition by 53 microM at 6 mM-2-oxoglutarate), competitively with 2-oxoglutarate, in protamine sulphate-treated and desalted cell-free extracts and in partially purified preparations. The extent of this inhibition was strongly pH-dependent. Both isoenzymes are equally susceptible to inhibition by lysine. The same inhibition pattern is shown by the enzyme from strain D6/1014A, which is a better producer of penicillin than strain Q176.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affenzeller K., Jaklitsch W. M., Hönlinger C., Kubicek C. P. Lysine biosynthesis in Penicillium chrysogenum is regulated by feedback inhibition of alpha-aminoadipate reductase. FEMS Microbiol Lett. 1989 Apr;49(2-3):293–297. doi: 10.1016/0378-1097(89)90056-6. [DOI] [PubMed] [Google Scholar]
- Betterton H., Fjellstedt T., Matsuda M., Ogur M., Tate R. Localization of the homocitrate pathway. Biochim Biophys Acta. 1968 Dec 23;170(2):459–461. doi: 10.1016/0304-4165(68)90036-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee J. K. alpha-Aminoadipate pathway for the biosynthesis of lysine in lower eukaryotes. Crit Rev Microbiol. 1985;12(2):131–151. doi: 10.3109/10408418509104427. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Masurekar P. S. Lysine inhibition of in vivo homocitrate synthesis in Penicillium chrysogenum. J Gen Microbiol. 1974 May;82(1):143–151. doi: 10.1099/00221287-82-1-143. [DOI] [PubMed] [Google Scholar]
- Friedrich C. G., Demain A. L. Uptake and metabolism of alpha-aminoadipic acid by Penicillium chrysogenum Wis 54-1255. Arch Microbiol. 1978 Oct 4;119(1):43–47. doi: 10.1007/BF00407926. [DOI] [PubMed] [Google Scholar]
- Gaillardin C. M., Poirier L., Heslot H. A kinetic study of homocitrate synthetase activity in the yeast Saccharomycopsis lipolytica. Biochim Biophys Acta. 1976 Feb 13;422(2):390–406. doi: 10.1016/0005-2744(76)90150-9. [DOI] [PubMed] [Google Scholar]
- Hogg R. W., Broquist H. P. Homocitrate formation in Neurospora crassa. Relation to lysine biosynthesis. J Biol Chem. 1968 Apr 25;243(8):1839–1845. [PubMed] [Google Scholar]
- Hönlinger C., Kubicek C. P. Regulation of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine and isopenicillin N biosynthesis in Penicillium chrysogenum by the alpha-aminoadipate pool size. FEMS Microbiol Lett. 1989 Nov;53(1-2):71–75. doi: 10.1016/0378-1097(89)90368-6. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W. M., Hampel W., Röhr M., Kubicek C. P., Gamerith G. alpha-Aminoadipate pool concentration and penicillin biosynthesis in strains of Penicillium chrysogenum. Can J Microbiol. 1986 Jun;32(6):473–480. doi: 10.1139/m86-087. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., López M. J., Villanueva J. R., Martín J. F. Inhibition and repression of homocitrate synthase by lysine in Penicillium chrysogenum. J Bacteriol. 1980 Dec;144(3):869–876. doi: 10.1128/jb.144.3.869-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., Villanueva J. R., Martín J. F. Lysine regulation of penicillin biosynthesis in low-producing and industrial strains of Penicillium chrysogenum. J Gen Microbiol. 1979 Nov;115(1):207–211. doi: 10.1099/00221287-115-1-207. [DOI] [PubMed] [Google Scholar]
- Masurekar P. S., Demain A. L. Insensitivity of homocitrate synthase in extracts of Penicillium chyrosogenum to feedback inhibition by lysine. Appl Microbiol. 1974 Aug;28(2):265–270. doi: 10.1128/am.28.2.265-270.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Scrutton M. C. The sub-cellular localisation and regulatory properties of pyruvate carboxylase from Rhizopus arrhizus. Eur J Biochem. 1985 Feb 15;147(1):119–128. doi: 10.1111/j.1432-1033.1985.tb08727.x. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Scrutton M. C. The sub-cellular localisation of pyruvate carboxylase and of some other enzymes in Aspergillus nidulans. Eur J Biochem. 1983 Jul 1;133(3):551–560. doi: 10.1111/j.1432-1033.1983.tb07499.x. [DOI] [PubMed] [Google Scholar]
- Tracy J. W., Kohlhaw G. B. Reversible, coenzyme-A-mediated inactivation of biosynthetic condensing enzymes in yeast: a possible regulatory mechanism. Proc Natl Acad Sci U S A. 1975 May;72(5):1802–1806. doi: 10.1073/pnas.72.5.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci A. F., Ceci L. N. Homocitrate synthase from yeast. Arch Biochem Biophys. 1972 Dec;153(2):742–750. doi: 10.1016/0003-9861(72)90393-1. [DOI] [PubMed] [Google Scholar]