Abstract
Patient: Male, 71-year-old
Final Diagnosis: Rhabdomyolysis secondary to Tribulus terrestris
Symptoms: Chest pain • myalgia
Clinical Procedure: —
Specialty: General and Internal Medicine
Objective:
Rare disease
Background:
Over-the-counter (OTC) supplement use is a very common practice within the United States. Supplements are not tightly regulated by the Food and Drug Administration. There are many case reports involving OTC supplement adverse effects and medication interactions, but there remains minimal clinical research regarding these subjects. Rhabdomyolysis is one interaction and adverse effect frequently documented in case reports among a variety of OTC supplements, although, to date, there is no documentation of rhabdomyolysis occurring from an interaction between the supplement Tribulus terrestris and atorvastatin.
Case Report:
A 71-year-old man presented to the Emergency Department in rhabdomyolysis with a mild transaminitis after taking the over-the-counter supplement Tribulus terrestris while on long-term atorvastatin. His rhabdomyolysis peaked at day 4 after cessation of the Tribulus and atorvastatin and aggressive fluid resuscitation with a normal saline bolus at admission followed by a D5 sodium bicarbonate drip later transitioned to a normal saline drip with subsequent down-trending of the creatinine phosphokinase levels.
Conclusions:
Tribulus terrestris is an herbal supplement used for erectile dysfunction and energy. Recent research suggests it to be a moderate CYP 3A4 inhibitor that plays a significant role in metabolism of statin and many other commonly prescribed medications. This may put patients at increased risk of developing serious adverse effects, including rhabdomyolysis and drug-induced liver injury. Screening patients for over-the-counter supplement use and educating them on the potential risks of their use is extremely important for inpatient and outpatient healthcare professionals to avoid dangerous medication interactions.
Key words: Cytochrome P-450 CYP3A, Dietary Supplements, Drug-Related Side Effects and Adverse Reactions, Rhabdomyolysis, Transaminases, Tribulus
Introduction
Rhabdomyolysis is a breakdown (“lysis”) of the muscle cells in the body, leading to a variety of symptoms and complications, including myalgias, hyperkalemia, and acute kidney injury [1–3]. This pathological process has many causes, with trauma and drug-induced being the most common. Drug-induced rhabdomyolysis is well described, especially among the statin medication class [1–3]. Rhabdomyolysis contributed to by herbal supplementation, including but not limited to licorice, red yeast rice, and jimson weed, is less well described, but there are reports found in the literature [4,5]. An area where there remains a paucity of research involves drug interactions between the statin medication class and herbal supplements. We describe what we believe to be the first known case of a medication-supplement-induced interaction between atorvastatin and the herbal supplement Tribulus terrestris, resulting in rhabdomyolysis.
Case Report
A 71-year-old man with a past medical history of coronary artery disease and hyperlipidemia presented to the Emergency Department for chest pain onset that morning. He woke up in his usual state of health but developed worsening cramping muscular pains in his extremities, which progressed into his chest. He denied any recent trauma or illnesses. He had no history of renal dysfunction or thyroidal illness. He admitted to drinking 3–4 beers on the weekend but denied any history of recreational drug use. He cycles 15 miles once a week for exercise but had not since the prior week. Medications included baby aspirin 81 mg daily, metoprolol succinate 100 mg daily, and long-term atorvastatin 40 mg daily. He had recently begun taking the supplement Tribulus terrestris 500 mg capsules once daily as directed per the bottle 2 weeks prior for energy. Physical examination revealed diffuse muscle tenderness. Laboratory test results demonstrated a creatine phosphokinase (CPK) of 7555 U/L, aspartate aminotransferase (AST) 152 U/L, alanine aminotransferase (ALT) 74 U/L, alkaline phosphatase (ALP) 74 U/L, total bilirubin 0.9 mg/dL, creatinine 0.83 mg/dL, and urinalysis with large amounts of blood and no red blood cells, consistent with rhabdomyolysis. His statin medication was held, and aggressive intravenous hydration was initiated with a 1-L bolus of normal saline at admission, followed by 150 meq sodium bicarbonate in D5 at 200 mL/hr for 24 h, then transitioned to normal saline at 75 mL/h until discharge. His CPK peaked on day 4 at 11 972 U/L before rapidly down-trending to 6610U/L the following 2 days (Figure 1). His AST also peaked at day 4 at 542 U/L (Figure 2). His ALT continued to show a slow increase up to his day of discharge at a level of 266 U/L (Figure 2). ALP and bilirubin never elevated above normal limits. Thankfully, during his admission he showed no evidence of renal dysfunction, as he had no elevation in creatinine past baseline or a decrease in urine output. He was discharged after a noted down-trend of the CPK and resolution of his symptoms. He was instructed to discontinue the use of atorvastatin and Tribulus terrestris and follow-up with his primary care physician for continued monitoring.
Figure 1.
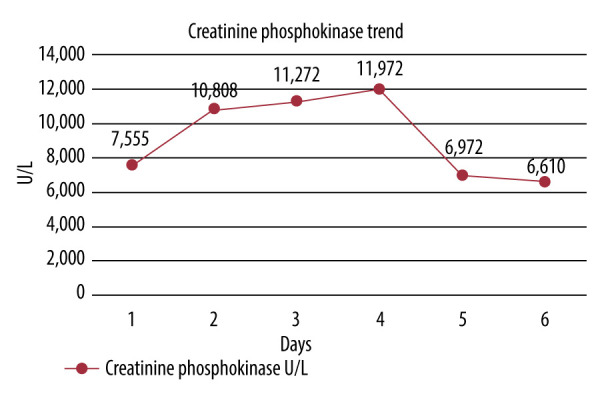
Trend of the creatinine phosphokinase levels from admission at day 1 up until discharge on day 6. Levels peaked on day 4 at 11 972U/L, then rapidly declined.
Figure 2.
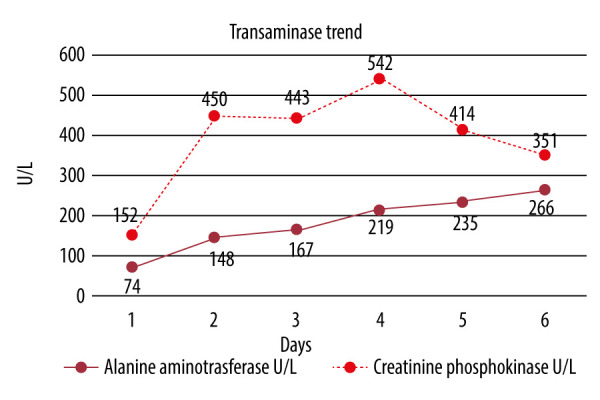
Trend of the alanine aminotransferase and aspartate aminotransferase levels from admission on day 1 up until discharge on day 6. AST followed a similar up-trend to the creatinine phosphokinase levels, peaking on day 4 and subsequently down-trending, which is often seen in rhabdomyolysis. ALT followed a slower up-trend and had not shown a peak, with evidence of down-trending prior to discharge. ALT commonly trails the AST in its peak and its down-trend. As the peak was not observed, the patient was directed to follow-up for repeat lab draws as an outpatient; however, this was either not completed or was not uploaded into the patient medical information system.
Discussion
While statin-induced myopathy is a commonly known adverse effect, with rhabdomyolysis being the most severe form, there is a low risk of it occurring. Clinical studies show the risk of serious muscle injury from statin use is <0.1%, with the highest risk occurring within the first year of treatment, increasing the dosage, or addition of an interacting drug [6]. Our patient had been on high-intensity atorvastatin for 6 years without complication until recent initiation of the herbal supplement Tribulus terrestris. Tribulus terrestris is sold commercially for erectile dysfunction and increased energy from its presumed testosterone-boosting effects. However, clinical trials continue to show varying results, with disagreements about the actual benefits the supplement provides. Often, the compounds tested vary significantly in their composition and biological properties as there is no standardization of its formulation. It is also marketed as being safe with minimal to no adverse effects, but information supporting this is lacking [7]. Recent research shows Tribulus terrestris to be a moderate inhibitor of cytochrome P450 3A4 (CYP 3A4), a significant factor in statin metabolism [8]. We hypothesize that the addition of Tribulus terrestris resulted in a medication interaction with atorvastatin, leading to an acute rise in serum levels, inducing rhabdomyolysis. Additionally, a case report of rhabdomyolysis contributed to by Tribulus terrestris use and clinical research demonstrating liver failure and muscle injury in sheep grazing on the natural plant suggests Tribulus terrestris drives its own process in causing rhabdomyolysis [9,10]. There are also 2 documented reports of severe nephrotoxicity from acute tubular necrosis contributed to by use of Tribulus terrestris in young male patients who had no prior renal disease [11,12]. The unregulated use of this supplement is concerning as it is known the cytochrome p450 metabolic pathway is foremost in a vast array of prescription medications, many of which can have adverse effects if serum levels rise unchecked. Further research regarding Tribulus terrestris’ adverse effects profile and its CYP inhibition is needed.
Conclusions
Herbal supplementation is a common practice with minimal regulation or safety profile research, which opens the door for dangerous adverse effects and medication interactions among unsuspecting patients. Patients often do not readily mention over-the-counter supplements when asked about their home medications, so it is the physician’s responsibility to actively screen patients regarding supplement use, both inpatient and outpatient, and provide the necessary education to prevent harm. Physicians and other healthcare professionals need to be aware of the commonly used and emerging supplements such as Tribulus terrestris to warn their patients and be prepared to prevent and manage complications of their use when they occur.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Cabral BMI, Edding SN, Portocarrero JP, Lerma EV. Rhabdomyolysis. Dis Mon. 2020;66(8):101015. doi: 10.1016/j.disamonth.2020.101015. [DOI] [PubMed] [Google Scholar]
- 2.Hohenegger M. Drug induced rhabdomyolysis. Curr Opin Pharmacol. 2012;12(3):335–39. doi: 10.1016/j.coph.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma U. Statin-induced delayed rhabdomyolysis. BMJ Case Rep. 2019;12(9):e231125. 30–31. doi: 10.1136/bcr-2019-231125. [Corrected in: Drug Ther Bull. 2020;58(2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373–82. doi: 10.1055/s-0029-1240006. [DOI] [PubMed] [Google Scholar]
- 5.Allkanjari O, Menniti-Ippolito F, Ippoliti I, et al. A descriptive study of commercial herbal dietary supplements used for dyslipidemia-Sales data and suspected adverse reactions. Phytother Res. 2022;36(6):2583–604. doi: 10.1002/ptr.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38–e81. doi: 10.1161/ATV.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 7.Ștefănescu R, Tero-Vescan A, Negroiu A, et al. A Comprehensive review of the phytochemical, pharmacological, and toxicological properties of Tribulus terrestris L. Biomolecules. 2020;10(5):752. doi: 10.3390/biom10050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Shi C, Feng L, et al. Potent inhibition of human cytochrome P450 3A4 by biflavone components from Ginkgo biloba and Selaginella tamariscina. Front Pharmacol. 2022;13:856784. doi: 10.3389/fphar.2022.856784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A, Lim B, Chaya C. Bulgarian tribulus side effect mimicking liver disease. Am J Gastroenterol. 2013;108:S353. [Google Scholar]
- 10.Aslani MR, Movassaghi AR, Mohri M, et al. Experimental Tribulus terrestris poisoning in sheep: Clinical, laboratory and pathological findings. Vet Res Commun. 2003;27(1):53–62. doi: 10.1023/a:1022010707704. [DOI] [PubMed] [Google Scholar]
- 11.Ryan M, Lazar I, Nadasdy GM, et al. Acute kidney injury and hyperbilirubinemia in a young male after ingestion of Tribulus terrestris. Clin Nephrol. 2015;83(3):177–83. doi: 10.5414/CN108324. [DOI] [PubMed] [Google Scholar]
- 12.Talasaz A. Tribulus terrestris-induced severe nephrotoxicity in a young healthy male. Nephrol Dial Transplant. 2010;25(11):3792–93. doi: 10.1093/ndt/gfq457. [DOI] [PubMed] [Google Scholar]


