Abstract
OBJECTIVES
Extracorporeal cardiopulmonary resuscitation (ECPR) has emerged as a rescue for refractory cardiac arrest, of which acute coronary syndrome is a common cause. Data on the coronary revascularization strategy in patients receiving ECPR remain limited.
METHODS
The ECPR databases from two referral hospitals were screened for patients who underwent emergent revascularization. The baseline characteristics were matched 1:1 using propensity score between patients who underwent coronary artery bypass grafting (CABG) and those who received percutaneous coronary intervention (PCI). Outcomes, including success rate of weaning from extracorporeal membrane oxygenation (ECMO), hospital survival, and midterm survival in hospital survivors, were compared between CABG and PCI.
RESULTS
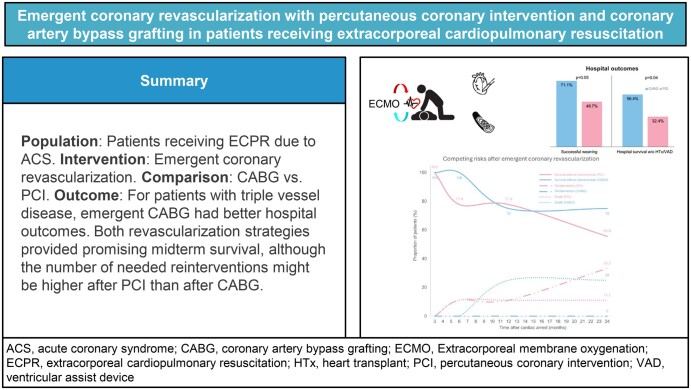
After matching, most of the patients (95%) had triple vessel disease. Compared with PCI (n = 40), emergent CABG (n = 40) had better early outcomes, in terms of the rates of successful ECMO weaning (71.1% vs 48.7%, P = 0.05) and hospital survival (56.4% versus 32.4%, P = 0.04). After a mean follow-up of 2 years, both revascularization strategies were associated with favourable midterm survival among hospital survivors (75.3% after CABG vs 88.9% after PCI, P = 0.49), with a trend towards fewer reinterventions in patients who underwent CABG (P = 0.07).
CONCLUSIONS
In patients who received ECPR because of triple vessel disease, the hospital outcomes were better after emergent CABG than after PCI. More evidence is required to determine the optimal revascularization strategy for patients who receive ECPR.
Keywords: Acute coronary syndrome, Cardiogenic shock, Coronary artery bypass grafting, Extracorporeal cardiopulmonary resuscitation, Percutaneous coronary intervention
The prognosis of cardiac arrest depends primarily on timely cardiopulmonary resuscitation (CPR) and therapeutic interventions for the underlying cause of the arrest.
Graphical abstract
INTRODUCTION
The prognosis of cardiac arrest depends primarily on timely cardiopulmonary resuscitation (CPR) and therapeutic interventions for the underlying cause of the arrest. For patients experiencing refractory cardiac arrest, extracorporeal cardiopulmonary resuscitation (ECPR) has emerged as an effective life-saving intervention [1]. Driven by the encouraging results of various observational studies and recent randomized clinical trials, the use of EPCR has significantly increased over the last few decades [2–6]. According to data from the Extracorporeal Life Support Organization (ELSO), ECPR has a 30% average survival to hospital discharge.
Acute coronary syndrome (ACS) is a common cause of cardiogenic shock and was reported to account for approximately 85% of cases of refractory cardiac arrest [3]. The groundbreaking Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial, which was conducted two decades ago, demonstrated a late survival benefit with early revascularization, compared with initial medical stabilization, in patients with acute myocardial infarction complicated by cardiogenic shock (AMICS) [7]. Moreover, studies have shown that early and aggressive intraarrest percutaneous coronary intervention (PCI) was associated with improved outcomes [8, 9]. Subsequently, guidelines have recommended immediate coronary intervention as part of post-resuscitation care for patients receiving ECPR, unless a noncardiac cause is evident [1]. Given the historically reported increased accessibility of primary PCI and the poor outcome of emergency coronary artery bypass grafting (CABG), PCI has become the preferred approach for early revascularization in cases of AMICS [10, 11].
However, the significant advances in revascularization techniques, periprocedural care, and mechanical circulatory support (MCS) make it necessary to reevaluate the outcomes of emergent coronary intervention [12]. Hence, this study aimed to report the outcomes of emergent coronary revascularization in patients who received ECPR and to provide valuable insights into their management.
MATERIALS AND METHODS
Patients
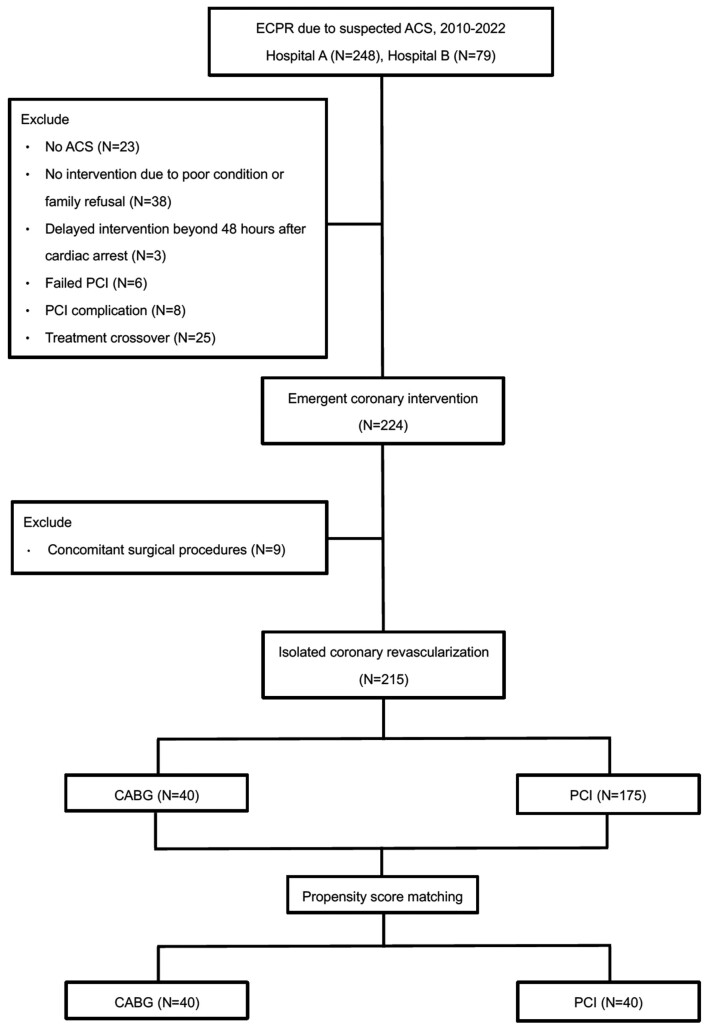
The extracorporeal membrane oxygenation (ECMO) databases of two referral hospitals, including the National Taiwan University Hospital (Hospital A) and the National Taiwan University Hospital Hsin-Chu Branch (Hospital B), were reviewed. The study focused on patients who received ECPR because of ACS and emergent coronary intervention from 2010 to 2022. Patient enrolment fully complied with the ELSO definition of ECPR, and our ECPR approach has been standardized over the years [2]. In all cases, ACS was confirmed by coronary angiography. Emergent coronary intervention was defined as revascularization within 48 h of cardiac arrest. Information on the patient baseline characteristics, procedural details of revascularization, and outcomes was collected. Thereafter, the eligible patients were grouped based on the method of coronary revascularization.
Ethical statement
The Research Ethics Committee of National Taiwan University Hospital granted approval for this study and a waiver of informed consent (institutional review board number: 202307142RIND; date of approval: 2023/08/08).
Patients who did not receive any intervention because of family refusal or poor medical condition, received delayed intervention beyond 48 h of cardiac arrest, underwent CABG after a failed or complicated PCI, or received PCI as a bridge to CABG during preparation of the operating theatre were excluded. Furthermore, we excluded patients with mechanical complications, such as rupture of the ventricular septum, papillary muscle, or free wall, and those who needed concomitant valve or ablation surgeries. These exclusions were made to ensure a direct comparison between CABG and PCI in patients who received ECPR.
Emergent coronary intervention
The selection of emergent coronary revascularization procedure was left to the discretion of the responsible interventional cardiologist and cardiovascular surgeon. Typically, PCI was prioritized for single-lesion or culprit-oriented approach, whereas CABG was considered in cases with a SYNTAX score of >32, if the PCI approach was challenging, or if there was an ongoing myocardial ischaemia.
Anticoagulation/antiplatelet management
Protocol-driven anticoagulation management during ECMO and/or intra-aortic balloon counter pulsation (IABP) support was implemented in all patients with low risk of bleeding. Unfractionated heparin was continuously infused to achieve an activated clotting time of 160–180 s or an activated partial thromboplastin time of 50–70 s. In addition to heparin, dual antiplatelet therapy was administered after coronary stent placement, as recommended by the guidelines. For patients who underwent surgical revascularization, single antiplatelet therapy with aspirin was preferred during the first 48–72 h after the operation. A P2Y12 inhibitor was added until the surgical bleeding decreased.
Outcome measurements
The primary outcomes included the rates of ECMO weaning success, which was defined as survival for >48 h after decannulation without reinitiating MCS, and hospital survival without the need for heart transplantation (HTx) or durable ventricular assist device (VAD). The secondary outcome was a composite of major complications, including continuous renal replacement therapy, intracranial haemorrhage, major bleeding, or tracheostomy before discharge. Major bleeding referred to surgical or cannulation site bleeding that required surgical re-exploration. For hospital survivors, the HTx/VAD-free survival and cumulative risk of reintervention were analysed. The prespecified cardiac causes of reintervention included coronary angioplasty, reinitiation of MCS, or HTx.
Statistical analysis
Categorical variables were reported as frequency and compared using the chi-squared test, when the expected number of observations was more than five, or the Fisher’s exact test, when the minimum expected cell size assumption was not met [13–15]. Continuous variables were presented as median with interquartile ranges (IQR) and compared using the Mann–Whitney U-test. To control the heterogeneity between the two treatment groups and the potential confounders of the exposure–outcome association, a propensity score-matched (PSM) analysis was conducted. A propensity score was generated for each patient using a multivariate logistic regression model that incorporated important demographic and resuscitation variables (Table 1). Each patient in the CABG group was paired with one patient in the PCI group using greedy 1:1 matching, with a calibre width of 0.20 standard deviation of the logit of the propensity score. Quality of the matching was assessed by calculating the standardized mean differences for each variable, with a cutoff value of <0.2 accepted as an adequate balance. The HTx/VAD-free survival and cumulative risk of reintervention in hospital survivors were calculated using the Kaplan–Meier survival analysis and competing risks analysis, respectively [16]. All statistical analyses were performed using the STATA® 13.0 statistical package (StataCorp MP, College Station, TX), with statistical significance indicated by a P-value of <0.05.
Table 1:
Baseline and resuscitation characteristics
| Baseline and resuscitation characteristics | Before PSM matching |
After PSM matching |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CABG (n = 40) | PCI (n = 175) | P-value | SMD | CABG (n = 40) | PCI (n = 40) | P-value | SMD | ||
| Age, years (median, IQR) | 61.6 (54.55–67.63) | 60.1 (51.3–67.9) | 0.33 | 0.16 | 61.6 (54.55–67.63) | 62.45 (56.95–67.3) | 0.68 | −0.13 | |
| Male sex, no. (%) | 34 (85%) | 157 (89.7%) | 0.41 | 0.14 | 34 (85%) | 36 (90%) | 0.63 | −0.15 | |
| Hypertension, no. (%) | 23 (65.7%) | 95 (59.0%) | 0.46 | 0.14 | 23 (65.7%) | 26 (65%) | 0.96 | 0.01 | |
| Diabetes, no. (%) | 17 (48.6%) | 74 (46.0%) | 0.78 | 0.05 | 17 (48.6%) | 25 (62.5%) | 0.38 | −0.28 | |
| Previous MI, no. (%) | 6 (17.1%) | 22 (13.7%) | 0.60 | 0.09 | 6 (17.1%) | 10 (25%) | 0.55 | −0.19 | |
| CAD, no. (%) | 10 (28.6%) | 65 (40.4%) | 0.19 | −0.25 | 10 (28.6%) | 24 (60%) | 0.05 | −0.67 | |
| CVA, no. (%) | 3 (8.6%) | 7 (4.3%) | 0.39 | 0.18 | 3 (8.6%) | 1 (2.5%) | 0.40 | 0.27 | |
| PAD, no. (%) | 1 (2.9%) | 12 (7.5%) | 0.47 | −0.21 | 1 (2.9%) | 2 (5%) | 0.73 | 0.11 | |
| NYHA FC III or IV, no. (%) | 3 (8.6%) | 32 (19.9%) | 0.11 | −0.33 | 3 (8.6%) | 13 (32.5%) | 0.08 | −0.62 | |
| CKD stage III, no. (%) | 3 (8.6%) | 27 (16.8%) | 0.22 | −0.25 | 3 (8.6%) | 9 (22.5%) | 0.24 | −0.39 | |
| ESRD, no. (%) | 3 (8.6%) | 20 (12.4%) | 0.59 | −0.12 | 3 (8.6%) | 5 (12.5%) | 0.69 | 0.13 | |
| OHCA, no. (%) | 15 (37.5%) | 86 (49.4%) | 0.17 | −0.24 | 15 (37.5%) | 13 (32.5%) | 0.74 | 0.1 | |
| Initial rhythm VT/VF, no. (%) | 25 (65.8%) | 102 (68.9%) | 0.71 | −0.07 | 25 (65.8%) | 24 (64.9%) | 0.95 | 0.02 | |
| CPR duration, min (median, IQR) | 41.0 (27.0–57.0) | 45.0 (30.0–60.0) | 0.38 | −0.17 | 41 (27–57) | 41 (30–54) | 0.87 | −0.05 | |
| Lactate level before ECMO, mmol/l (median, IQR) | 10.77 (6.79–13.59) | 10.92 (7.46–14.98) | 0.33 | −0.21 | 10.77 (6.79–13.59) | 9.95 (5.75–14) | 0.99 | <0.01 | |
| Severity of coronary artery diseasea | 1VD, 2VD, no. (%) | 2 (5.0%) | 65 (37.1%) | <0.001 | 0.86 | 2 (5%) | 2 (5%) | 1.00 | 0 |
| 3VD, no. (%) | 38 (95.0%) | 110 (62.9%) | 38 (95%) | 38 (95%) | |||||
| SAPS 3 (median, IQR) | 53 (47–61) | 55 (47–63) | 0.77 | 0.02 | 53 (47–61) | 53 (47–62) | 0.77 | −0.11 | |
Isolated left main disease was classified as a two-vessel disease, regardless of the status of the left anterior descending artery and left circumflex coronary artery.
CABG: coronary artery bypass grafting; CAD: coronary artery disease; CKD: chronic kidney disease; CPR: cardiopulmonary resuscitation; CVA: cerebrovascular accident; ECMO: extracorporeal membrane oxygenation; ESRD: end stage renal disease; IQR: interquartile range; MI: myocardial infarction; NYHA FC: New York Heart Association functional classification; OHCA: out-of-hospital cardiac arrest; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; PSM: propensity score matching; SAPS 3: Simplified Acute Physiology Score III; SMD: standardized mean difference; 1/2/3VD: one-/two-/three-vessel disease; VF: ventricular fibrillation; VT: ventricular tachycardia.
RESULTS
Baseline and resuscitation characteristics
Of the 327 patients screened, 215 patients were found eligible for our study; of these, 40 underwent CABG and 175 received PCI (Fig. 1). Most of the patients in the study cohort were middle-aged men (median age, 61.6 years in the CABG group vs 60.1 years in the PCI group, P = 0.33). Compared with the PCI group, the CABG group comprised a higher proportion of patients with severe coronary artery disease (95% vs 62.9%, P < 0.001, Supplementary Material, Fig. S1).
Figure 1:
Flow diagram of the study. Patients who underwent ECPR because of ACS and received emergent coronary intervention from 2010 to 2022 at the two participating institutions were screened for eligibility. A total of 215 patients were included in the analysis. A 1:1 propensity score matching paired 40 patients in the CABG group with 40 patients in the PCI group. ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; ECPR: extracorporeal cardiopulmonary resuscitation; PCI: percutaneous coronary intervention.
A total of 40 patients in the CABG group were matched with 40 patients in the PCI group. The cardiac arrest location and myocardial territories supplied by the diseased coronary arteries, which might greatly influence the outcome of cardiac arrest and the choice of revascularization strategy, were ideally balanced between the CABG and PCI groups. Remarkably, up to 95% of the matched patients had 3-vessel disease (Table 1, Supplementary Material, Fig. S2).
Procedural details of emergent coronary intervention
Compared with the PCI group, the CABG group had longer ECMO-to-revascularization time (280 min vs 90 min, P = 0.005) but similar frequencies of IABP use (75% vs 70%, respectively, P = 0.59). Three patients in the CABG group underwent advanced left ventricle (LV) unloading, including surgical LV vent, pulmonary artery drainage, and left atrial drainage through a percutaneous atrial septostomy (Table 2).
Table 2:
Procedural details
| CABG (n = 40) | PCI (n = 40) | P-value | |
|---|---|---|---|
| ECMO-to-revascularisation time, min (median, IQR) | 280 (176.5–350.5) | 90 (51–153.5) | 0.005 |
| IABP, no. (%) | 28 (70%) | 30 (75%) | 0.593 |
| LV unloading, no. (%) | 3 (7.5%) | 0 (0%) | – |
CABG: coronary artery bypass grafting; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon counter pulsation; IQR: interquartile range; LV: left ventricle; PCI: percutaneous coronary intervention.
Hospital outcomes
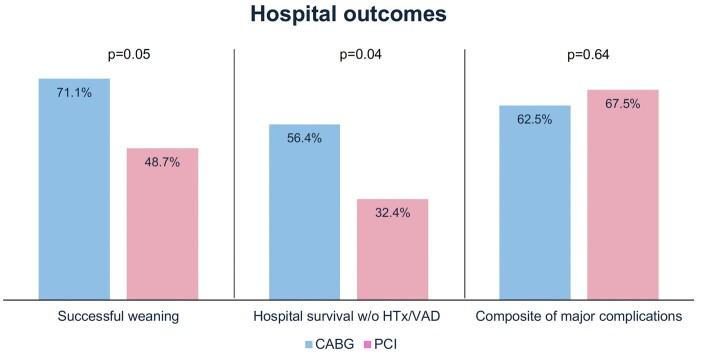
There were four patients who underwent HTx during the index admission (one in the CABG group and three in the PCI group). Compared with the PCI group, the CABG group had higher rates of successful ECMO weaning (71.1% vs 48.7%, P = 0.05) and hospital survival without the need for HTx or durable VAD (56.4% vs 32.4%, P = 0.04). The incidence of composite major complications was comparable between the CABG and PCI groups (62.5% vs 67.5%, respectively, P = 0.64; Fig. 2). Subgroup analysis of patients with out-of-hospital and in-hospital cardiac arrest showed similar trends of better outcomes after CABG than after PCI (Supplementary Material, Table S1).
Figure 2:
Hospital outcomes. Compared with patients who received PCI, those who underwent CABG have superior rates of ECMO weaning success and hospital survival. Both revascularisation strategies are associated with similar risks of major complications. CABG: coronary artery bypass grafting; ECMO: extracorporeal membrane oxygenation; HTx: heart transplantation; PCI: percutaneous coronary intervention; VAD: ventricular assist device.
Midterm follow-up of hospital survivors
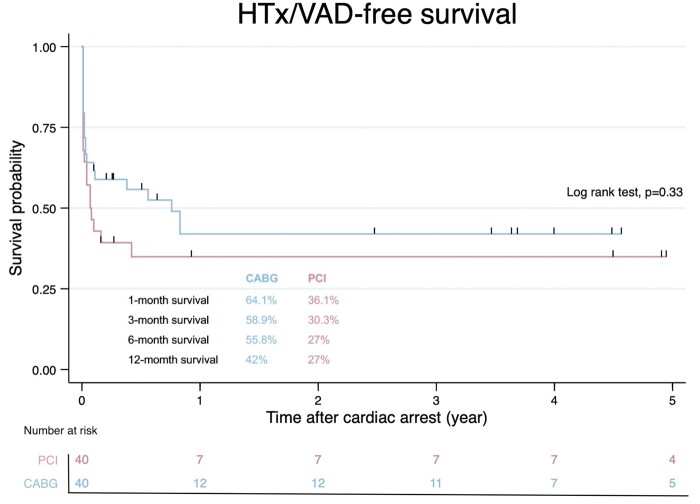
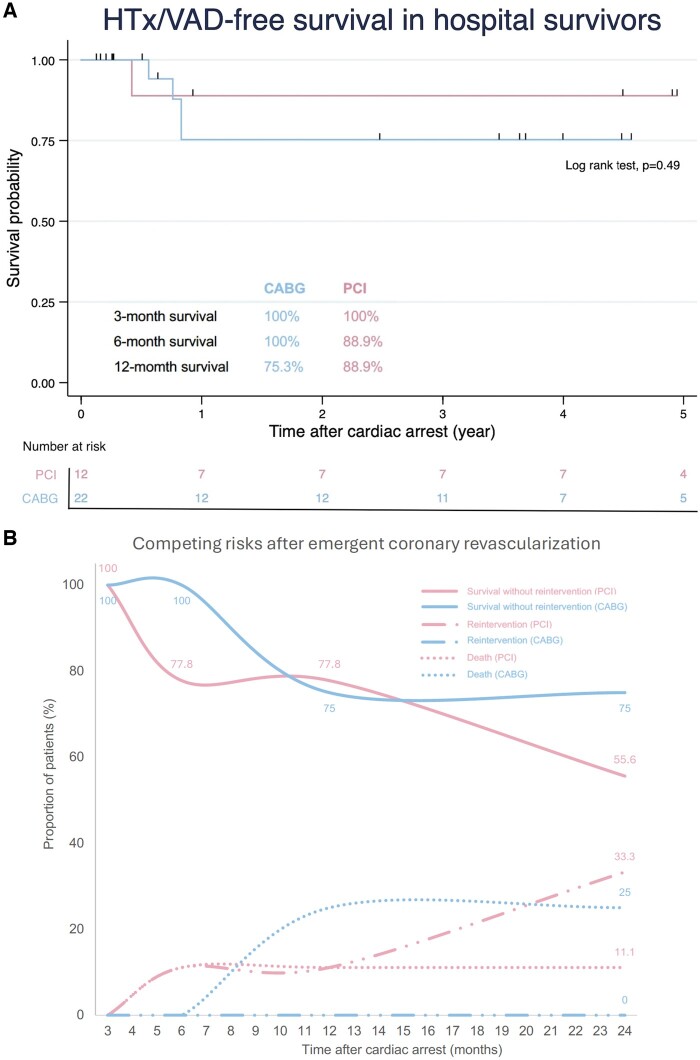
After a median follow-up of 3.61 years (IQR 0.52–5.95 years), there was no significant difference in the HTx/VAD-free survival between the CABG and PCI groups (42% vs 27%, respectively, P = 0.33; Fig. 3). For patients who survived the emergent coronary revascularization, the HTx/VAD-free survival was similarly encouraging in both groups (75.3% in the CABG group vs 88.9% in the PCI group, P = 0.49, Fig. 4A). Notably, there was an observable albeit insignificant difference in the risk of reintervention between the two groups (P = 0.07, Fig. 4B).
Figure 3:
Midterm survival. After a mean follow-up of 2 years, the HTx/VAD-free survival is comparable between patients who underwent CABG and those who received PCI. The numbers of patients at risk are shown below the graph. CABG: coronary artery bypass grafting; HTx: heart transplantation; PCI: percutaneous coronary intervention; VAD: ventricular assist device.
Figure 4:
Outcomes in hospital survivors. (A) Both CABG and PCI are associated with favourable midterm survival. (B) After 2 years of follow-up, 75% of patients were alive without reintervention and no patients received reintervention in the CABG group, while 55.6% of patients were alive without reintervention and 33.3% of patients needed reintervention in the PCI group (P-value for cumulative risk of reintervention = 0.07). CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
DISCUSSION
The present study compared the early and midterm outcomes between emergent CABG and PCI in exclusive ECPR patients. For patients with triple vessel disease, CABG had higher rates of successful ECMO weaning and hospital survival and showed a tendency for fewer reinterventions.
Emergent revascularization for refractory cardiac arrest
Most of the published studies on the revascularization strategy for AMICS excluded patients who received ECPR. Unstable haemodynamics, impaired consciousness, end organ failure, coagulopathy, and overwhelming systemic inflammatory response often manifest in the acute post-resuscitation period and might complicate the emergent coronary revascularization.
Following the SHOCK trial, Kagawa et al. investigated the outcomes of intraarrest PCI with ECMO support in patients with refractory cardiac arrest. With rates of 59% for weaning from ECMO and 36% for 30-day survival, they concluded that ECMO plus intraarrest PCI was feasible and associated with improved outcomes [8]. Meanwhile, Acharya et al. analysed the Society of Thoracic Surgeons National Database and reported an operative mortality rate of 53.3% for emergent salvage CABG [17]. A Nordic multicentre study by Axelsson et al. reported a hospital mortality of 41% for salvage CABG [18]. These studies enrolled a heterogeneous group of patients with AMI and varying shock severity and MCS utilization. In contrast, our study focused on the outcomes of emergent revascularization with either CABG or PCI in patients who developed an extreme stage of AMICS complicated by refractory cardiac arrest (i.e. shock stage EA of the Society for Cardiovascular Angiography and Interventions classification) and were exclusively on ECMO support [19].
Comparison between CABG and PCI
The optimal coronary revascularization approach for patients receiving ECPR remains uncertain [12]. In the secondary analysis of the SHOCK trial, the prevalence of diabetes was higher and the coronary disease was worse in patients who underwent CABG than in those who received PCI. Nevertheless, the survival rates at 30 days and 12 months were similar between the two methods of revascularization [20]. Furthermore, the relatively long delay from symptom onset to revascularization is an inherent drawback of CABG. Based on our secondary analysis, it appeared that a prolonged ECMO-to-revascularization time did not compromise the hospital outcomes in patients who underwent CABG (Supplementary Material, Table S2). These paradoxes suggested a potential benefit of CABG over PCI in patients with cardiogenic shock or receiving ECPR.
Several plausible mechanisms in favour of CABG have been proposed. One significant factor is the ease of achieving complete revascularization [21]. Although the CULPRIT-SHOCK trial showed a higher 30-day composite risk of death or severe renal failure after immediate multivessel PCI than after culprit-only PCI, the difference in mortality decreased beyond 30 d; moreover, revascularization and rehospitalization for heart failure were more frequent after culprit-only PCI [22]. Immediate multivessel PCI at the time of cardiogenic shock could be hazardous because of the relatively high dose of contrast medium used and the delay in correcting haemodynamic and metabolic derangements at the catheterization laboratory [22, 23]. However, these unfavourable consequences of multivessel PCI might be offset by the prompt use of MCS, which was underreported in the CULPRIT-SHOCK trial [24]. In fact, large nationwide registries demonstrated the more pronounced early survival benefits of multivessel PCI in AMICS cases requiring MCS [25, 26]. Presumably, a similar benefit in patients receiving ECPR can be expected from the relatively high rate of complete revascularization after CABG.
Myocardial stunning is not uncommon during the early post-resuscitation period and might be aggravated by the increased afterload during ECMO support [27]. Early active LV unloading was found to enhance coronary flow, reduce infarct size, and improve early outcomes in patients with AMICS and on ECMO support [28–30]. IABP is the most commonly used modality for LV decompression. However, we found no significant difference in the early survival between patients with IABP and those without IABP (Supplementary Material, Fig. S3). Cardiopulmonary bypass could offer simultaneous full circulatory support and effective ventricular unloading during CABG. Moreover, with conventional CABG, myocardial oxygen consumption can be further reduced by cardioplegic arrest [31]. Therefore, CABG should be expected to play a beneficial role, especially in patients receiving ECPR.
Midterm outcome
Data on the extended comparison of revascularization strategies for patients with AMICS had been lacking. In this study, a favourable midterm survival in patients who survived emergent revascularization was demonstrated. Despite the similar survival rates between CABG and PCI, more reinterventions might be needed by patients who received PCI than those who underwent CABG. All these reinterventions were coronary angioplasty, and no patient received VAD support or HTx during follow-up. Rather than staged PCI, unplanned PCI after clinical events comprised most of the coronary reinterventions after discharge.
Study limitations
Our study was subject to the common limitations that are inherent to all retrospective cohort studies. To maintain statistical power, only important demographic and resuscitation variables were included in the PSM; therefore, there might have been residual uncontrolled confounders that affected the exposure–outcome association. The choice between CABG and PCI was left to the discretion of the duty interventionist and surgeon. Finally, the small sample size might have compromised the internal and external validity of our study.
CONCLUSIONS
In patients who received ECPR because of triple vessel disease, emergent CABG was associated with better hospital outcomes, compared with those of PCI. The midterm survival rates of CABG and PCI were similarly reassuring for hospital survivors, although more reinterventions might be needed after PCI than after CABG. More evidence is needed to determine the optimal revascularization strategy for patients who receive ECPR.
Supplementary Material
Glossary
ABBREVIATIONS
- ACS
Acute coronary syndrome
- AMICS
Acute myocardial infarction complicated by cardiogenic shock
- CABG
Coronary artery bypass grafting
- ECMO
Extracorporeal membrane oxygenation
- ECPR
Extracorporeal cardiopulmonary resuscitation
- HTx
Heart transplantation
- IABP
Intra-aortic balloon counter pulsation
- IQR
Interquartile ranges
- LV
Left ventricle
- MCS
Mechanical circulatory support
- PCI
Percutaneous coronary intervention
- PSM
Propensity score-matched
- SHOCK
Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock
- VAD
Ventricular assist device
Contributor Information
Hsun-Yi Fu, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital Hsinchu Branch, Hsinchu, Taiwan.
Yih-Sharng Chen, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
Hsi-Yu Yu, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
Nai-Hsin Chi, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
Ling-Yi Wei, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
Kevin Po-Hsun Chen, School of Medicine, Auckland Univ ersity, Auckland, New Zealand.
Heng-Wen Chou, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
Nai-Kuan Chou, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
Chih-Hsien Wang, Division of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
This work was supported by the National Science and Technology Council, Taiwan [112-2314-B-002-239 to C.-H.W.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly for the privacy of the individuals who were included in the study. The data will be shared upon reasonable request to the corresponding author. The authors thank NTUH-ECMO team members for their excellent database maintaining. The NTUH datasets in this study were retrospectively collected in the NTUH-ECMO database following the regulation of NTUH-IRB-201002034R.
Author contributions
Hsun-Yi Fu: Investigation; Writing—original draft. Yih-Sharng Chen: Conceptualization; Investigation; Methodology; Resources; Supervision; Validation; Visualization; Writing—review and editing. Hsi-Yu Yu: Conceptualization; Investigation; Methodology; Supervision; Validation; Visualization. Nai-Hsin Chi: Supervision; Visualization. Ling-Yi Wei: Visualization. Kevin Po-Hsun Chen: Visualization. Heng-Wen Chou: Visualization. Nai-Kuan Chou: Visualization. Chih-Hsien Wang: Conceptualization; Funding acquisition; Investigation; Methodology; Supervision; Validation; Visualization; Writing—review and editing
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Antonio Maria Calafiore, Michele Gallo and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L. et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO J 2021;67:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT. et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372:554–61. [DOI] [PubMed] [Google Scholar]
- 3. Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A. et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol 2017;70:1109–17. [DOI] [PubMed] [Google Scholar]
- 4. Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA. et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020;396:1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M. et al. ; Prague OHCA Study Group. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA 2022;327:737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suverein MM, Delnoij TSR, Lorusso R, Brandon Bravo Bruinsma GJ, Otterspoor L, Elzo Kraemer CV. et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med 2023;388:299–309. [DOI] [PubMed] [Google Scholar]
- 7. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD. et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341:625–34. [DOI] [PubMed] [Google Scholar]
- 8. Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M. et al. Should we emergently revascularize occluded coronaries for cardiac arrest? Rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation 2012;126:1605–13. [DOI] [PubMed] [Google Scholar]
- 9. Kuroki N, Abe D, Iwama T, Suzuki K, Sugiyama K, Akashi A. et al. Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation 2017;114:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS, NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448–54. [DOI] [PubMed] [Google Scholar]
- 11. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM. et al. ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;145:e18–e114. [DOI] [PubMed] [Google Scholar]
- 12. Henry TD, Tomey MI, Tamis-Holland JE, Thiele H, Rao SV, Menon V et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Cardiovascular and Stroke Nursing. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2021;143:e815–e29. [DOI] [PubMed] [Google Scholar]
- 13. Yates D, Moore MD, McCabe G.. The Practice of Statistics (1st Ed.). New York: W.H. Freeman, 1999. [Google Scholar]
- 14. Bewick V, Cheek L, Ball J.. Statistics review 8: qualitative data—tests of association. Crit Care 2003;8:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bland M, An Introduction to Medical Statistics. 4th Ed., Oxford University Press, 2015. ISBN: 9780199589920 [Google Scholar]
- 16. Blackstone EH, Lytle BW.. Competing risks after coronary bypass surgery: the influence of death on reintervention. J Thorac Cardiovasc Surg 2000;119:1221–30. [DOI] [PubMed] [Google Scholar]
- 17. Acharya D, Gulack BC, Loyaga-Rendon RY, Davies JE, He X, Brennan JM. et al. Clinical characteristics and outcomes of patients with myocardial infarction and cardiogenic shock undergoing coronary artery bypass surgery: data from The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Axelsson TA, Mennander A, Malmberg M, Gunn J, Jeppsson A, Gudbjartsson T.. Is emergency and salvage coronary artery bypass grafting justified? The Nordic Emergency/Salvage coronary artery bypass grafting study. Eur J Cardiothorac Surg 2016;49:1451–6. [DOI] [PubMed] [Google Scholar]
- 19. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD. et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 20. White HD, Assmann SF, Sanborn TA, Jacobs AK, Webb JG, Sleeper LA. et al. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: results from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial. Circulation 2005;112:1992–2001. [DOI] [PubMed] [Google Scholar]
- 21. Davierwala PM, Leontyev S, Verevkin A, Rastan AJ, Mohr M, Bakhtiary F. et al. Temporal trends in predictors of early and late mortality after emergency coronary artery bypass grafting for cardiogenic shock complicating acute myocardial infarction. Circulation 2016;134:1224–37. [DOI] [PubMed] [Google Scholar]
- 22. Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G. et al. ; CULPRIT-SHOCK Investigators. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med 2018;379:1699–710. [DOI] [PubMed] [Google Scholar]
- 23. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R. et al. ; CULPRIT-SHOCK Investigators. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017;377:2419–32. [DOI] [PubMed] [Google Scholar]
- 24. Lemor A, Basir MB, Patel K, Kolski B, Kaki A, Kapur NK. et al. ; National Cardiogenic Shock Initiative Investigators. Multivessel versus culprit-vessel percutaneous coronary intervention in cardiogenic shock. JACC Cardiovasc Interv 2020;13:1171–8. [DOI] [PubMed] [Google Scholar]
- 25. Omer MA, Brilakis ES, Kennedy KF, Alkhouli M, Elgendy IY, Chan PS et al Multivessel versus culprit-vessel percutaneous coronary intervention in patients with non-ST-segment elevation myocardial infarction and cardiogenic shock. JACC Cardiovasc Interv 2021;14:1067–78. [DOI] [PubMed] [Google Scholar]
- 26. Choi KH, Yang JH, Park TK, Lee JM, Song YB, Hahn JY. et al. Culprit-only versus immediate multivessel percutaneous coronary intervention in patients with acute myocardial infarction complicating advanced cardiogenic shock requiring venoarterial-extracorporeal membrane oxygenation. J Am Heart Assoc 2023;12:e029792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW. et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–83. [DOI] [PubMed] [Google Scholar]
- 28. Schrage B, Sundermeyer J, Blankenberg S, Colson P, Eckner D, Eden M. et al. Timing of active left ventricular unloading in patients on venoarterial extracorporeal membrane oxygenation therapy. JACC Heart Fail 2023;11:321–30. [DOI] [PubMed] [Google Scholar]
- 29. Swain L, Reyelt L, Bhave S, Qiao X, Thomas CJ, Zweck E. et al. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J Am Coll Cardiol 2020;76:684–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapur NK, Alkhouli MA, DeMartini TJ, Faraz H, George ZH, Goodwin MJ. et al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction. Circulation 2019;139:337–46. [DOI] [PubMed] [Google Scholar]
- 31. Smilowitz NR, Alviar CL, Katz SD, Hochman JS.. Coronary artery bypass grafting versus percutaneous coronary intervention for myocardial infarction complicated by cardiogenic shock. Am Heart J 2020;226:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly for the privacy of the individuals who were included in the study. The data will be shared upon reasonable request to the corresponding author. The authors thank NTUH-ECMO team members for their excellent database maintaining. The NTUH datasets in this study were retrospectively collected in the NTUH-ECMO database following the regulation of NTUH-IRB-201002034R.