Abstract
The aim of the study was to measure the degree of dentine surface roughness caused by five distinct lasers used to treat dentine hypersensitivity, as well as to evaluate the subsequent bacterial colonization on these irradiated surfaces. Sixty human maxillary premolar teeth without caries or restoration which were extracted for periodontal reasons were used in this study. Five different types of lasers were applied to the root dentin surface. Tested samples were divided into six groups of 10 samples each; control, diode (810 nm), diode (980 nm), Nd: YAG, Er: YAG, and Er, Cr: YSGG laser groups. The arithmetic mean of the surface roughness values (Ra) and the average roughness over a measurement area (Sa) were measured pre- and post-application using any of the laser types. Swab samples were then collected from the dentin surface. Following a 24-hour incubation period at 37 °C, the colony forming units were counted using a stereoscope. The results demonstrated a statistically significant difference in the surface roughness values pre- and post-application (Ra and Sa, respectively) in the Er, Cr: YSGG laser group (p = 0.037,p = 0.007). No significant difference was observed in the other groups (p > 0.05). There was no statistically significant difference in the number of bacterial colonies observed between the test and control groups. Diode and Nd: YAG lasers showed either a decrease or no change in surface roughness; however, the hard tissue lasers (Er: YAG, Er, Cr: YSGG) showed an increase. The Er: YAG and Nd: YAG laser groups exhibited decreased bacterial adhesion compared to the other groups.
Keywords: Dentin hypersensitivity; Bacterial adhesion; Diode laser; Nd:YAG laser; Er,Cr:YSGG laser
Introduction
Due to the increased awareness of oral and dental health in our world today, as well as the development of preventative and more accessible treatment methods, the longevity of teeth in the oral cavity has improved. Teeth that remain in the mouth for a longer period of time are subjected to increased abrasion, resulting in the exposure of the dentin surface. In addition, the dentin tubules become exposed to the oral environment as a result of parafunctional movements like clenching and grinding, improper tooth brushing habits, excessive consumption of acidic foods, and the use of mouthwashes. Dentin hypersensitivity, resulting from the exposure of dentinal tubules, can create physical and psychological issues in affected individuals and significantly reduces their quality of life. Under normal conditions, the dentin surface is protected by either the enamel or cementum and is not sensitive to direct stimulation; dentin hypersensitivity occurs when the dentin surface becomes exposed due to abrasion of the enamel or cementum [1]. Dentin hypersensitivity is characterized as an acute, sudden, sharp, and transient toothache that occurs on the dentin surface, which is caused by any stimuli and cannot be attributed to any underlying pathology [2]. Pain often arises from mechanical, thermal, or chemical stimuli on dentin-exposed surfaces, and ceases once the stimulus is no longer present.
Several theories have been proposed to explain the occurrence of dentin hypersensitivity. The prevailing consensus is that stimulus leads to the displacement of fluid within the dentinal tubules. The hydrodynamic theory explains that pain is caused by the movement of fluid within and outside the tubules, which stimulates the nerve endings at the pulp-dentin interface [3]. Therefore, the primary treatment for dentin hypersensitivity is to reduce fluid movement and dentin permeability. The closure of exposed dentin tubules can potentially decrease the occurrence of dentin hypersensitivity; however, several studies have indicated that dentin hypersensitivity may still persist despite successful closure of the tubules [4–6].
Various methods and materials have been developed to address dentin hypersensitivity. Traditional methods for addressing tooth sensitivity include use oftoothpastes, professional desensitizers, fluoride applications, varnishes. Typically, various agents that decrease or modify nerve impulses are used, either by expert application or local administration at home. Potassium salts, such as potassium fluoride, potassium chloride, and the most commonly used potassium nitrate, stand out as nerve modifiers [7]. Sodium fluoride gel (NaF) is the most commonly employed tubular occlusion agent [8]. The process relies on the mechanical occlusion caused by the precipitation of calcium fluoride crystals that are insoluble and do not adhere to the tubules. Therefore, it is unable to withstand the pressures of the oral environment and its efficacy diminishes over time [9]. Certain treatment options such as the addition of desensitizers to toothpastes, aim to occlude the dentinal tubules in order to reduce sensitivity.
Other alternatives in reducing dentin hypersensitivity include laser therapy. Studies have demonstrated the clinical efficacy from using various lasers for therapy [9–11]. Er: YAG and Er, Cr: YSGG lasers can occlude dentinal tubules by generating elevated temperatures in the tissue [12, 13]. Nd: YAG, GaAlAs and He-Ne lasers interfere with the Na+-K + ion pump in the cell membrane and prevent the transmission of the pain signals, thereby reducing pain symptoms [13, 14].
Laser therapy for dentin hypersensitivity is a convenient and quick method for patients. The impact is immediately noticeable following a single application. The effect can last for an average of 3–6 months with no reported side effects [15]. While laser desensitisation is an effective method, it does come with several drawbacks. It has been reported in the literature that lasers result in the roughening of the tooth surface [16, 17]. Bacteria colonise the tooth surface and form a biofilm through continuous adhesion. The initial adhesion of bacteria to the tooth surface is a crucial step in the development of bacterial plaque. The irregular geometry of a rough surface provides a better environment for bacteria to engage with the surface and establish a strong attachment [18, 19]. In contrast, a rough surface can result in the accumulation of bacteria, leading to the development oftooth decay and gum disease [20, 21]. The aim of this study was to assess the surface roughness and bacterial adhesion on dentin caused by different lasers used in the treatment of dentin hypersensitivity. The null hypothesis posited that there would be no changes in surface roughness and bacterial retention following dentine hypersensitivity treatments.
Materials and methods
Sample preparation
Prior to the commencement of the study, ethical approval was obtained from the Gaziantep University Clinical Research Ethics Committee (decision number: 2019/508). The minimum number of teeth required to achieve a statistically significant strong correlation (r = 0.80) between the amount of surface roughness and bacterial growth was found to be 9 (α = 0.05, 1-β = 0.80). Power analysis was performed using G-Power version 3.1.9.2. Sixty human maxillary premolar teeth without caries or restoration which were extracted for periodontal reasons were used in the study. The teeth were sectioned 1 mm below the cemento-enamel junction using a diamond saw (Isomet Diamond Wafering Blade, Buehler, Lake Bluff, IL, USA) in a low-speed, water-cooled cutting device. The root portion of the teeth were embedded in acrylic blocks to facilitate the execution of the treatments. Figure 1 shows that a total of 30 acrylic blocks were obtained by placing 2 teeth in each block. The fine abrasive paper (600, 800 and 1200 nm; 3 M ESPE, St. Paul, Minn) was used to smoothen one side of each sample by a single operator for 60 s. A standardised region with a diameter of 4 mm was established to facilitate the application of surface treatments and enable easier detection of surface roughness and bacterial adhesion.
Fig. 1.
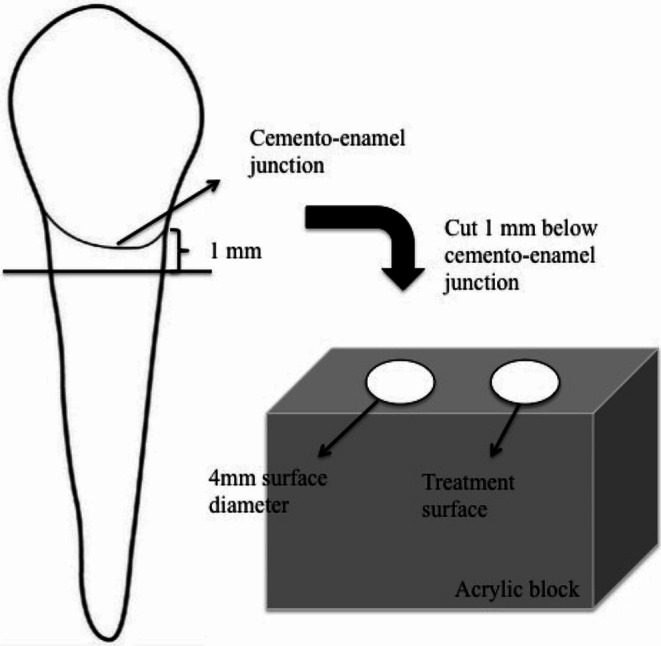
Diagram of the tooth set-up
Surface roughness test
The created blocks were immersed in distilled water. Each tooth was subsequently treated with a 24% ethylenediaminetetraacid (EDTA) solution for 60 s. Each sample was then purified using distilled water. This procedure opened the dentinal tubules, imitating the appearance of exposed dentin [20]. The surface roughness values were measured prior to any dental treatment. This measurement was done using an optical, non-contact, 3D surface profilometer (ZeeScope Optical Profilometer). Ra and Sa values were recorded. Following laser treatments, the surface roughness was reassessed using the same protocol.
Group formation and laser procedures
The blocks and teeth were randomly divided into six groups. The randomization process was conducted using the freely available ‘Research Randomizer’ website specifically developed for researchers. These groups are: control, diode (810 nm), diode (980 nm), Nd: YAG, Er: YAG, and Er, Cr: YSGG laser groups. The groups and processes for laser application are listed below and shown in Table 1.
Table 1.
Lasers sytems and parameters
| Lasers | Parameters | Manufacturers |
|---|---|---|
| Diode (810 nm) | 300 micron diameter fibre, a power of 0.2 W, continuous mode, 30 s. | (Cheese dental laser; Wuhan Gigaa Optronics Technology Co. Ltd., Wuhan, China) |
| Diode (980 nm) | 300 micron diameter fibre, a power of 0.5 W, continuous mode, 60 s, T on at 100 ms and T off at 100 ms, | (Cheese dental laser; Wuhan Gigaa Optronics Technology Co. Ltd., Wuhan, China) |
| Er, Cr: YSGG | A Z6 sapphire tip, 6 mm length, 600 μm diameter, frequency 20 Hz, pulse width 140–200 µs, a power of 0.25 W, 60 s. | (Waterlase, Biolase Technology, Irvine, CA) |
| Er: YAG | A R02-handpiece, non-contact mode, a power of 0.2 W, energy per pulse 80 mJ/pulse, frequency 3 Hz, short pulse mode, 60 s | (Fotona Laser AT, Fidelis Plus III, Ljubljana, Slovenia) |
| Nd: YAG | A 300 μm Nd: YAG fibre, a power of 1 W, the frequency 10 Hz, short pulse mode, 60 s. | (Fotona Laser AT, Fidelis Plus III, Ljubljana, Slovenia) |
Group 1: Control group, no surface treatment following tooth preparation.
Group 2: Diode laser (810 nm wavelength) (Cheese dental laser; Wuhan Gigaa Optronics Technology Co. Ltd., Wuhan, China) was applied to surface using a 300 micron fibre optic cable with spot mode and a power of 0.2 W in continuous mode for 30 s. [22].
Group 3: Diode laser (980 nm wavelength) (Cheese dental laser; Wuhan Gigaa Optronics Technology Co. Ltd., Wuhan, China). A power of 0.5 W was applied in continuous mode for 60 s, T on at 100 ms and T off at 100 ms, using a 300 micron diameter fibre tip [23].
Group 4: Nd: YAG laser (1064 nm wavelength) (Fotona Laser AT, Fidelis Plus III, Ljubljana, Slovenia)was applied according to the parameters recommended for Fotona laser devices and based on previous investigations in the literature [23].The power indicator was set to 1 W, the frequency was set to 10 Hz, and the device was set to short pulse mode (pulse duration, 180 µs). A 300 μm Nd: YAG fibre was used for 60 s (the distance between tissue and fibre tip was 2 mm), applied slowly and evenly to the 4 mm surface [24].
Group 5: Er: YAG laser (2940 nm wavelength) (Fotona Laser AT, Fidelis Plus III, Ljubljana, Slovenia) was applied according to the parameters recommended for Fotona laser devices and studies in the literature [23]. An R02-handpiece (Fotona AT, Fidelis III, Ljubljana, Slovenia) was positioned 6 mm away from the tissue and used for laser application for 60 s with power display 0.2 W, energy per pulse 80 mJ/pulse, frequency 3 Hz and the device set to short pulse mode (pulse duration 300 µs). The treatment was administered gradually and evenly with water irrigation (25 mL/min, to protect from thermal changes) to the surface (surface diameter, 4 mm) [24].
Group 6: Er, Cr: YSGG laser (2780 nm wavelength) (Waterlase, Biolase Technology, Irvine, CA). The application procedure: An Er, Cr: YSGG (A Z6 sapphire tip, 6 mm length, 600 μm diameter) laser is applied perpendicularly to the surface with a 1-mm defocused beam over an area of 4 mm for 60 s with scanning movements, 0% water, and 0% air [25]. The samples were subjected to the following parameters; frequency 20 Hz, pulse width 140–200 µs, and power 0.25 W [25].
Following the laser operations, the samples were rinsed with distilled water for 10 s and were subsequently preserved in sterile distilled water.
Bacterial adhesion test
First, the samples were sterilised. The bacterial adhesion test utilized Streptococcus mutans that was cultivated in sucrose medium. Dentin samples were placed in sterile cell culture plates and inoculated overnight with standard cultures in the same medium supplemented with 5% sucrose. The samples were then incubated at 37 °C for 24 h and covered with 1.5 mL of Brain Heart infusion.
Following the incubation period, the dentin fragments were washed in sterile distilled water to remove non-adherent microorganisms. Swab samples were then collected from the dentin surface and evenly distributed onto the surface of agar plates supplemented with sucrose and sheep’s blood. Following a 24-hour incubation period at 37 °C, colony forming units (CFU) were counted using a stereoscope and results were expressed in CFU/mL.
Statistical analysis
Kolmogorov-Smirnov and Shapiro-Wilk tests were used to test if the distribution of the data deviated from the normal distribution. Kruskal-Wallis and Dunn multiple comparison tests were used to compare non-normally distributed data across the six groups. A Wilcoxon test was performed to compare initial and final measurements. The statistical analysis was performed using SPSS for Windows version 24.0 and p < 0.05 was considered statistically significant.
Results
The bacterial colonization and surface roughness values of the tested groups were subjected to statistical analysis. The surface roughness values were summarized in Table 2 using descriptive statistics.
Table 2.
Descriptive statistics for surface roughness values
| Surface Roughness (Ra) | Surface Roughness (Sa) | |||||
|---|---|---|---|---|---|---|
| Initial | Final | P | Initial | Final | P | |
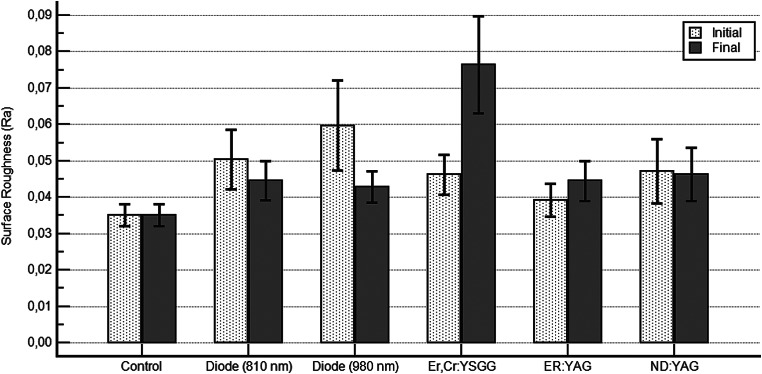
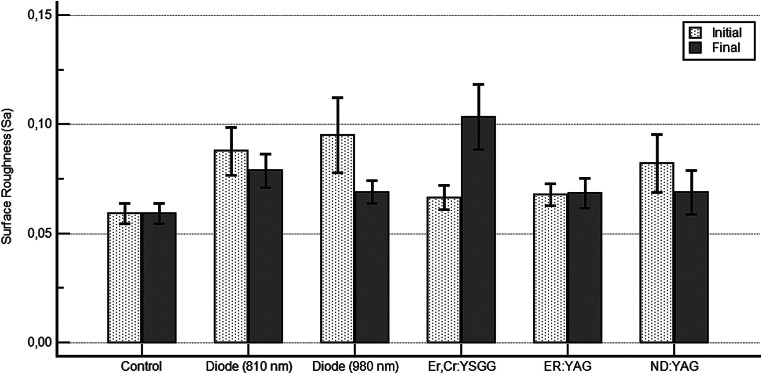
| Control | 0.04 ± 0.01 | 0.04 ± 0.01 | 1.000 | 0.06 ± 0.01 | 0.06 ± 0.01 | 1.000 |
| Diode(810 nm) | 0.05 ± 0.03 | 0.04 ± 0.02 | 0.445 | 0.09 ± 0.03 | 0.08 ± 0.02 | 0.445 |
| Diode(980 nm) | 0.06 ± 0.04 | 0.04 ± 0.01 | 0.139 | 0.1 ± 0.05 | 0.07 ± 0.02 | 0.169 |
| Er, Cr: YSGG | 0.05 ± 0.02 | 0.08 ± 0.04 | 0.037* | 0.07 ± 0.02 | 0.1 ± 0.05 | 0.007* |
| Er: YAG | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.241 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.799 |
| Nd: YAG | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.646 | 0.08 ± 0.04 | 0.07 ± 0.03 | 0.203 |
| P* | 0.397 | 0.010* | 0.213 | 0.021* | ||
*Significant at 0.05 level; Wilcoxon test for within group, Kruskal Wallis test for between group comparisons
The results showed a statistically significant difference in the surface roughness values (Ra and Sa) between the pre and post-application stages in the Er, Cr: YSGG laser group (p = 0.037,p = 0.007). No statistically significant difference was seen in the other groups (p > 0.05).
The Ra values resulting from the laser applications were compared and summarised in Fig. 2. Statistically significant differences were observed between the control and Er, Cr: YSGG laser groups (p = 0,001); the Nd: YAG and Er, Cr: YSGG laser groups (p = 0,009); the diode (980 nm) and Er, Cr: YSGG laser groups (p = 0,011); the Er: YAG and Er, Cr: YSGG laser groups (p = 0,015); and the diode (810 nm) and Er, Cr: YSGG laser groups (p = 0,015).
Fig. 2.
Comparison of final RA values between groups (Dunn’s test)
The Sa values resulting from the laser applications were compared and summarised in Fig. 3. Statistically significant differences were observed between the control and Er, Cr: YSGG laser groups (p = 0,001); the control and diode (810 nm) laser groups (p = 0,034); the Nd: YAG and Er, Cr: YSGG laser groups (p = 0,007); the diode (980 nm) and Er, Cr: YSGG laser groups (p = 0,037); and the Er: YAG and Er, Cr: YSGG laser groups (p = 0,042).
Fig. 3.
Comparison of final SA values between groups (Dunn’s test)
The average CFU/mL obtained in all the test groups are shown in Table 3. There was no statistically significant difference in the number of bacterial colonies seen between the test groups and the control group. The Er: YAG and Nd: YAG laser groups presented reduced bacterial adhesion compared to the other groups.
Table 3.
The average CFU/mL obtained in all test groups
| Colony amount (cfu/ml) | P | |
|---|---|---|
| Control | 17,000 ± 10593,5 | 0.133 |
| Diode(810 nm) | 8000 ± 14757,3 | |
| Diode(980 nm) | 8000 ± 13165,61 | |
| Er, Cr: YSGG | 9000 ± 12866,84 | |
| Er: YAG | 7000 ± 9486,83 | |
| Nd: YAG | 7000 ± 6749,49 |
Discussion
Laser systems used to treat dentin hypersensitivity are an effective treatment option, but they have a tendency to induce tooth surface roughness, which can lead to bacterial retention. In this study, 5 different types of lasers with varying wavelengths were compared and only the Er, Cr: YSGG laser resulted in an increase in roughness on the root dentin surface. Since the wavelength of the Er, Cr: YSGG laser (2780 nm) is close to the wavelength of the Er: YAG laser (2940 nm), there was an increase in surface roughness in the Er: YAG laser group. However, this increase was not deemed statistically significant.
Laser treatments are the preferred method over chemical agents for treating dentin hypersensitivity due to their ease of application and longer duration of action [17]. It has been reported that chemical agents used in conventional dentin hypersensitivity treatments have been found to cause surface roughness [20]. Nogueira et al. reported that roughness and bacterial adhesion were significantly higher on flouride varnish-treated surfaces compared to laser-treated surfaces [21]. In a study, it was reported that all chemical agents and laser treatments that were examined were found to decrease dentin permeability [17]. Upon evaluating studies investigating the impact of conventional treatments and lasers on surface roughness, it can be concluded that chemical agent applications cause more roughness. The results of this study indicate that only the Er, Cr: YSGG laser increased the surface roughness compared to the pre-application condition.
When considering treatments for dentin hypersensitivity, it is important to evaluate both the benefits and the potential risks. An inherent danger of dentin hypersensitivity treatments is the possibility of causing dentin surface roughness and associated caries formation. Consequently, in vitro studies are gaining significance in this context. Several in vitro studies have have investigated the impact of chemical agents and laser systems on tooth surface roughness. However, there is no precise regarding the level of surface roughness induced by different laser systems and the types of bacteria and biofilm that adhere to teeth [16, 20, 21]. In this study, only the Er, Cr: YSGG laser exhibited a significantly increase in surface roughness and bacterial colonisation in comparison to the control and other tested groups. This finding was related to the fact that hard tissue lasers can cause morphological and chemical changes in dental hard tissue. There is a lack of comparative research in the existing literature that examine all types of lasers. A comparative investigation on high intensity lasers reported that Er, Cr: YSGG laser application resulted in more surface roughness compared to diode and Nd: YAG lasers, which aligns with our own findings [21]. A recent study examined the efficacy of Er: YAG laser by applying at different intensities [16]. The study found that as the energy density increased, the roughness of the enamel surface also increased [16]. Additionally, the study reported that higher surface roughness leads to greater bacterial adhesion [16]. Similarly, in our study, bacterial colonisation was highest in the Er, Cr: YSGG laser group in which the surface roughness increased the most.
Clinical success with laser treatment of dentin hypersensitivity has been reported in many in vivo studies [15, 22, 25]. Low-intensity lasers are known to be effective in reducing dentin hypersensitivity, but the effect of high-intensity lasers is still a subject of debate. The Er: YAG laser’s high wavelength absorption in water can cause the evaporation of the dentinal fluid and smear layer [26, 27]. Studies evaluating the efficacy of Er: YAG and Er, Cr: YSGG lasers have reported that these lasers are highly effective in treating dentinal sensitivity. They exhibit the best clinical performance, do not damage the pulp, and remain effective for 6 months [27, 28]. High-intensity lasers are used to treat dentin tubules by coagulation, protein precipitation, or the formation of insoluble calcium complexes, which reduce or close the diameter of the tubules. Clinically, the outcome may be deemed satisfactory. However, based on the findings of our laboratory investigation, it can be concluded that high-intensity lasers result in greater surface roughness, whereas low-intensity lasers are more reliable in clinical use.
Currently, dentin hypersensitivity treatment involves the utilization of different types of lasers, different wavelengths, chemical agents, or a combination of these approaches. A recent systematic review has determined that both the Nd: YAG and the diode lasers are effective in the treatment of dentin hypersensitivity [29]. Furthermore, according to our study findings, Nd: YAG and diode lasers had the least effect on surface roughness. Therefore, it can be said that these two types of laser are more preferable in clinical conditions and are the least harmful options for the tooth.
Laser effects are influenced by several parameters such as wavelength, output power, duration and mode of emission, beam profile, and spot size [30]. Typically, diode lasers utilize a wavelength range of 810–980 nm. While 808–810 nm wavelength was used in some studies [31–35], 980 nm wavelength was used in other studies [23, 36–38]. However, we do not have clear evidence regarding the optimal wavelength for diode lasers or the best approach to achieve photobiomodulatory effects or superficial melting and dentinal tubule occlusion. Therefore, diode lasers with both wavelengths were used in our study. There was no difference in the surface roughness and bacterial colonisation between the two diode lasers.
In surface roughness analysis, the Ra value, typically representing the arithmetic mean, has been considered. Cury et al. [20] measured the Sa value of surface roughness. The Ra value (roughness average) represents the mean of the surface heights along a measurement path. Sa value is the average roughness within a specified measurement area. Therefore, in order to obtain more accurate data, we measured both Ra and Sa values in our study. Similar data were obtained for both values.
The present study was carried out on extracted human teeth inside a controlled laboratory environment. The aim of the study is to measure surface roughness, and it is not possible to measure surface roughness in the oral environment. However, bacterial colonisation in the oral environment could differ under in vitro conditions, and this may be considered a limitation of the study.
Conclusions
The present study was conducted using five different laser types and wavelengths. It was concluded that only the Er, Cr: YSGG laser resulted in an increased surface roughness and bacterial colonisation. There was also an increase in surface roughness for the Er: YAG laser group, but it was not statistically significant. The Er: YAG and Nd: YAG laser groups exhibited lower bacterial adhesion compared to the other groups. Diode lasers and Nd: YAG laser showed a decrease or absence of alteration in surface roughness, while hard tissue lasers (Er: YAG, Er, Cr: YSGG) demonstrated an increase. Results of this in-vitro study will be useful for clinicians to evaluate the selection of the appropriate laser type for the treatment of dentin hypersensitivity.
Author contributions
OPO formed the hypothesis and idea of the research. OPO, ND and IK3 provided the organization for the design, execution of the study. IK3, YZ and OP prepared the experimental environment and made the measurements. IK2 wrote and prepared the manuscript. All of the authors approved the final version.
Funding
The authors did not receive support from any organization for the submitted work.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Clinical Research Ethics Committee of Gaziantep University, approval number 2019/508.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Canadian Advisory Board on Dentin Hypersensitivity (2003) Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc 69(4):221–226 [PubMed] [Google Scholar]
- 2.Liu XX, Tenenbaum HC, Wilder RS et al (2020) Pathogenesis, diagnosis and management of dentin hypersensitivity: an evidence-based overview for dental practitioners. BMC Oral Health 20:220. 10.1186/s12903-020-01199-z 10.1186/s12903-020-01199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brännström M (1966) Sensitivity of dentine. Oral Surg Oral Med Oral Pathol 21(4):517–526. 10.1016/0030-4220(66)90411-7 10.1016/0030-4220(66)90411-7 [DOI] [PubMed] [Google Scholar]
- 4.Hsu PJ, Chen JH, Chuang FH et al (2006) The combined occluding effects of fluoride-containing dentin desensitizer and Nd-YAG laser irradiation on human dentinal tubules: an in vitro study. Kaohsiung J Med Sci 22(1):24–29. 10.1016/S1607-551X(09)70216-5 10.1016/S1607-551X(09)70216-5 [DOI] [PubMed] [Google Scholar]
- 5.Corona SA, Nascimento TN, Catirse AB et al (2003) Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 30(12):1183–1189. 10.1111/j.1365-2842.2003.01185.x 10.1111/j.1365-2842.2003.01185.x [DOI] [PubMed] [Google Scholar]
- 6.Kumar NG, Mehta DS (2005) Short-term assessment of the nd:YAG laser with and without sodium fluoride varnish in the treatment of dentin hypersensitivity–a clinical and scanning electron microscopy study. J Periodontol 76(7):1140–1147. 10.1902/jop.2005.76.7.1140 10.1902/jop.2005.76.7.1140 [DOI] [PubMed] [Google Scholar]
- 7.Hong JY, Lim HC, Herr Y (2016) Effects of a mouthwash containing potassium nitrate, sodium fluoride, and cetylpyridinium chloride on dentin hypersensitivity: a randomized, double blind, placebo controlled study. J Periodontal Implant Sci 46(1):46–56. 10.5051/jpis.2016.46.1.46 10.5051/jpis.2016.46.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orchardson R, Gillam DG (2000) The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain 14(1):9–19 [PubMed] [Google Scholar]
- 9.Lee SY, Jung HI, Jung BY et al (2015) Desensitizing efficacy of nano-carbonate apatite dentifrice and er, Cr:YSGG laser: a randomized clinical trial. Photomed Laser Surg 33:9–14. 10.1089/pho.2014.3787 10.1089/pho.2014.3787 [DOI] [PubMed] [Google Scholar]
- 10.Sgolastra F, Petrucci A, Severino M et al (2013) Lasers for the treatment of dentin hypersensitivity: a meta-analysis. J Dent Res 92:492–499. 10.1177/0022034513487212 10.1177/0022034513487212 [DOI] [PubMed] [Google Scholar]
- 11.Rosa RR, Calazans FK, Nogueira RD et al (2016) Effects of different desensitizing treatments on root dentin permeability. Braz Oral Res 10;30(1):e111. 10.1590/1807-3107BOR-2016.vol30.0111 [DOI] [PubMed]
- 12.Gholami GA, Fekrazad R, Esmaiel-Nejad A et al (2011) An evaluation of the occluding effects of Er;cr:YSGG, nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg 29(2):115–121. 10.1089/pho.2009.2628 10.1089/pho.2009.2628 [DOI] [PubMed] [Google Scholar]
- 13.Al-Saud LM, Al-Nahedh HN (2012) Occluding effect of nd:YAG laser and different dentin desensitizing agents on human dentinal tubules in vitro: a scanning electron microscopy investigation. Oper Dent 37(4):340–355. 10.2341/10-188-L 10.2341/10-188-L [DOI] [PubMed] [Google Scholar]
- 14.Orchardson R, Gillam DG (2006) Managing dentin hypersensitivity. J Am Dent Assoc 137(7):990–998. 10.14219/jada.archive.2006.0321 10.14219/jada.archive.2006.0321 [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E et al (2011) Clinical evaluation of Er,Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. J Dent 39(3):249–254. 10.1016/j.jdent.2011.01.003 10.1016/j.jdent.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 16.Contreras-Bulnes T-CB, Scougall-Vilchis R RJ et al (2019) Effect of Er:YAG laser irradiation on deciduous enamel roughness and bacterial adhesion: an in vitro study. Microsc Res Tech 82(11):1869–1877. 10.1002/jemt.23354 10.1002/jemt.23354 [DOI] [PubMed] [Google Scholar]
- 17.Öncü E, Karabekiroğlu S, Ünlü N (2017) Effects of different desensitizers and lasers on dentine tubules: an in-vitro analysis. Microsc Res Tech 80(7):737–744. 10.1002/jemt.22859 10.1002/jemt.22859 [DOI] [PubMed] [Google Scholar]
- 18.Yuzugullu B, Celik C, Ozcelik TB et al (2016) The effect of different polishing sequences on the adhesion of Streptococcus mutans to feldspathic porcelain. J Adhes 92:939–949. 10.1080/00218464.2015.1059763 10.1080/00218464.2015.1059763 [DOI] [Google Scholar]
- 19.Lee DH, Mai HN, Thant PP et al (2019) Effects of different surface finishing protocols for zirconia on surface roughness and bacterial biofilm formation. J Adv Prosthodont 11(1):41–47. 10.4047/jap.2019.11.1.41 10.4047/jap.2019.11.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cury MS, Silva CB, Nogueira RD et al (2018) Surface roughness and bacterial adhesion on root dentin treated with diode laser and conventional desensitizing agents. Lasers Med Sci 33(2):257–262. 10.1007/s10103-017-2356-x 10.1007/s10103-017-2356-x [DOI] [PubMed] [Google Scholar]
- 21.Nogueira RD, Silva CB, Lepri CP et al (2017) Evaluation of surface roughness and bacterial adhesion on tooth enamel ırradiated with high ıntensity lasers. Braz Dent J 28(1):24–29. 10.1590/0103-6440201701190 10.1590/0103-6440201701190 [DOI] [PubMed] [Google Scholar]
- 22.Tabatabaei MH, Chiniforush N, Hashemi G et al (2018) Efficacy comparison of nd:YAG laser, diode laser and dentine bonding agent in dentine hypersensitivity reduction: a clinical trial. Laser Ther 31(4):265–270. 10.5978/islsm.27_18-OR-24 10.5978/islsm.27_18-OR-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umberto R, Claudia R, Gaspare P et al (2012) Treatment of dentine hypersensitivity by diode laser: a clinical study. Int J Dent 2012(1):1–8. 10.1155/2012/858950 10.1155/2012/858950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurt S, Kırtıloğlu T, Yılmaz NA et al (2018) Evaluation of the effects of Er: YAG laser, Nd: YAG laser, and two different desensitizers on dentin permeability: in vitro study. Lasers Med Sci 2018;33(9):1883–1890. 10.1007/s10103-018-2546-1 [DOI] [PubMed]
- 25.Aranha AC, Eduardo Cde P (2012) Effects of Er:YAG and Er,Cr:YSGG lasers on dentine hypersensitivity. Short-term clinical evaluation. Lasers Med Sci 27(4):813–818. 10.1007/s10103-011-0988-9 10.1007/s10103-011-0988-9 [DOI] [PubMed] [Google Scholar]
- 26.Hibst R, Keller U (1989) Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg Med 9(4):338–344. 10.1002/lsm.1900090405 10.1002/lsm.1900090405 [DOI] [PubMed] [Google Scholar]
- 27.Schwarz F, Arweiler N, Georg T, Reich E (2002) Desensitizing effects of an Er:YAG laser on hypersensitive dentine. J Clin Periodontol 29:211–215. 10.1034/j.1600-051x.2002.290305.x 10.1034/j.1600-051x.2002.290305.x [DOI] [PubMed] [Google Scholar]
- 28.Badran Z, Boutigny H, Struillou X, Baroth S, Laboux O, Soueidan A (2011) Tooth desensitization with an Er:YAG laser: in vitro microscopical observation and a case report. Lasers Med Sci 26:139–142. 10.1007/s10103-010-0835-4 10.1007/s10103-010-0835-4 [DOI] [PubMed] [Google Scholar]
- 29.Cattoni F, Ferrante L, Mandile S et al (2023) Comparison of lasers and desensitizing agents in dentinal hypersensitivity therapy. Dent J 11(3):63. 10.3390/dj11030063 10.3390/dj11030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fornaini C, Merigo E, Sozzi M et al (2016) Four different diode lasers comparison on soft tissues surgery: a preliminary ex vivo study. Laser Ther Jun 29(2):105–114. 10.5978/islsm.16-OR-08 10.5978/islsm.16-OR-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes AO, de Paula Eduardo C, Aranha ACC (2017) Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 32(5):1023–1030. 10.1007/s10103-017-2203-0 10.1007/s10103-017-2203-0 [DOI] [PubMed] [Google Scholar]
- 32.Moura GF, Zeola LF, Silva MB et al (2019) Four-session protocol effectiveness in reducing cervical dentin hypersensitivity: a 24-week randomized clinical trial. Photobiomodul Photomed Laser Surg 37(2):117–123. 10.1089/photob.2018.4477 10.1089/photob.2018.4477 [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz H, Yilmaz S, Cengiz E (2011) Long-term effect of diode laser irradiation compared to sodium fluoride varnish in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized controlled clinical study. J Photomed Laser Surg 29(11):721–725. 10.1089/pho.2010.2974 10.1089/pho.2010.2974 [DOI] [PubMed] [Google Scholar]
- 34.Sicilia A, Cuesta-Frechoso S, Suárez A et al (2009) Immediate efficacy of diode laser application in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized clinical trial. J Clin Periodontol 36(8):650–660. 10.1111/j.1600-051X.2009.01433.x 10.1111/j.1600-051X.2009.01433.x [DOI] [PubMed] [Google Scholar]
- 35.Naghsh N, Kachuie M, Kachuie M et al (2020) Evaluation of the effects of 660-nm and 810-nm low-level diode lasers on the treatment of dentin hypersensitivity. J Lasers Med Sci 11(2):126–131. 10.34172/jlms.2020.22 10.34172/jlms.2020.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raut CP, Sethi KS, Kohale B et al (2018) Evaluation of diode laser and stannous flouride in the treatment of root sensitivity after access flap surgery: Randomized controlled clinical trial. J Ind Soc Periodontol 22(2):158–163. 10.4103/jisp.jisp_2_18 10.4103/jisp.jisp_2_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabibzadeh Z, Fekrazad R, Esmaeelnejad A et al (2018) Effect of combined application of high- and low-intensity lasers on dentin hypersensitivity: a randomized clinical trial. J Dent Res Dent Clin Dent Prospects 12(1):49–55. 10.15171/joddd.2018.008 10.15171/joddd.2018.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pourshahidi S, Ebrahimi H, Mansourian A et al (2019) Comparison of Er,Cr:YSGG and diode laser effects on dentin hypersensitivity: a split-mouth randomized clinical trial. Clin Oral Investig 23(11):4051–4058. 10.1007/s00784-019-02841-z 10.1007/s00784-019-02841-z [DOI] [PubMed] [Google Scholar]