Abstract
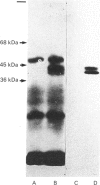
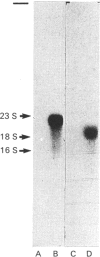
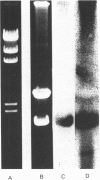
A cDNA clone for cystathionine gamma-lyase was isolated from a rat cDNA library in lambda gt11 by screening with a monospecific antiserum. The identity of this clone, containing 600 bp proximal to the 3'-end of the gene, was confirmed by positive hybridization selection. Northern-blot hybridization showed the expected higher abundance of the corresponding mRNA in liver than in brain. Two further cDNA clones from a plasmid pcD library were isolated by colony hybridization with the first clone and were found to contain inserts of 1600 and 1850 bp. One of these was confirmed as encoding cystathionine gamma-lyase by hybridization with two independent pools of oligodeoxynucleotides corresponding to partial amino acid sequence information for cystathionine gamma-lyase. The other clone (estimated to represent all but 8% of the 5'-end of the mRNA) was sequenced and its deduced amino acid sequence showed similarity to those of the Escherichia coli enzymes cystathionine beta-lyase and cystathionine gamma-synthase throughout its length, especially to that of the latter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allington W. B., Cordry A. L., McCullough G. A., Mitchell D. E., Nelson J. W. Electrophoretic concentration of macromolecules. Anal Biochem. 1978 Mar;85(1):188–196. doi: 10.1016/0003-2697(78)90289-0. [DOI] [PubMed] [Google Scholar]
- Beatty P. W., Reed D. J. Involvement of the cystathionine pathway in the biosynthesis of glutathione by isolated rat hepatocytes. Arch Biochem Biophys. 1980 Oct 1;204(1):80–87. doi: 10.1016/0003-9861(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Faibes D., Uren J. R., Livingston D. M. Immunologic analyses of mouse cystathionase in normal and leukemic cells. Biochemistry. 1978 Nov 28;17(24):5181–5187. doi: 10.1021/bi00617a018. [DOI] [PubMed] [Google Scholar]
- Bikel I., Pavlatos T. N., Livingston D. M. Purification and subunit structure of mouse liver cystathionase. Arch Biochem Biophys. 1978 Feb;186(1):168–174. doi: 10.1016/0003-9861(78)90476-9. [DOI] [PubMed] [Google Scholar]
- Braunstein A. E., Goryachenkova E. V. The beta-replacement-specific pyridoxal-P-dependent lyases. Adv Enzymol Relat Areas Mol Biol. 1984;56:1–89. doi: 10.1002/9780470123027.ch1. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dwivedi C. M., Ragin R. C., Uren J. R. Cloning, purification, and characterization of beta-cystathionase from Escherichia coli. Biochemistry. 1982 Jun 22;21(13):3064–3069. doi: 10.1021/bi00256a005. [DOI] [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Fearon C. W., Rodkey J. A., Abeles R. H. Identification of the active-site residue of gamma-cystathionase labeled by the suicide inactivator beta, beta, beta-trifluoroalanine. Biochemistry. 1982 Aug 3;21(16):3790–3794. doi: 10.1021/bi00259a011. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Enzymatic synthesis of homocysteine or methionine directly from O-succinyl-homoserine. Biochim Biophys Acta. 1967 Mar 15;132(2):400–405. doi: 10.1016/0005-2744(67)90158-1. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen K. Studies on cystathionase activity in rat liver and brain during development. Effects of hormones and amino acids in vivo. Biochem J. 1973 Dec;136(4):1011–1015. doi: 10.1042/bj1361011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Ogura M., Suda M. Control mechanism in the rat liver enzyme system converting L-methionine to L-cystine. 3. Noncompetitive inhibition of cystathionine synthetase-serine dehydratase by elemental sulfur and competitive inhibition of cystathionase-homoserine dehydratase by L-cysteine and L-cystine. J Biochem. 1966 Jan;59(1):40–48. doi: 10.1093/oxfordjournals.jbchem.a128256. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A. Proteins transferred to nitrocellulose for use as immunogens. Anal Biochem. 1985 Jun;147(2):285–288. doi: 10.1016/0003-2697(85)90273-8. [DOI] [PubMed] [Google Scholar]
- Kraus J. P. Cystathionine beta-synthase (human). Methods Enzymol. 1987;143:388–394. doi: 10.1016/0076-6879(87)43068-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Link D., Drebing C., Glode L. M. Cystathionase: a potential cytoplasmic marker of hematopoietic differentiation. Blut. 1983 Jul;47(1):31–39. doi: 10.1007/BF00321048. [DOI] [PubMed] [Google Scholar]
- Martel A., Bouthier de la Tour C., Le Goffic F. Pyridoxal 5'phosphate binding site of Escherichia coli beta cystathionase and cystathionine gamma synthase comparison of their sequences. Biochem Biophys Res Commun. 1987 Sep 15;147(2):565–571. doi: 10.1016/0006-291x(87)90968-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Maxwell F., Hahn W. E. Use of CH3HgOH-agarose gels for the electrophoresis of heterogeneous nuclear RNA and messenger RNA from mammalian cells. Anal Biochem. 1979 Oct 15;99(1):146–160. doi: 10.1016/0003-2697(79)90056-3. [DOI] [PubMed] [Google Scholar]
- McPhaul M., Berg P. Identification and characterization of cDNA clones encoding two homologous proteins that are part of the asialoglycoprotein receptor. Mol Cell Biol. 1987 May;7(5):1841–1847. doi: 10.1128/mcb.7.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Obaru K., Nomiyama H., Shimada K., Nagashima F., Morino Y. Cloning and sequence analysis of mRNA for mouse aspartate aminotransferase isoenzymes. J Biol Chem. 1986 Dec 25;261(36):16976–16983. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pühler G., Leffers H., Gropp F., Palm P., Klenk H. P., Lottspeich F., Garrett R. A., Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. doi: 10.1073/pnas.86.12.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K., Kushiya E., Obinata M., Odani S., Takahashi Y. Molecular cloning and the nucleotide sequence of cDNA for neuron-specific enolase messenger RNA of rat brain. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7453–7457. doi: 10.1073/pnas.82.21.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K., Yoshida Y., Nabeshima Y., Takahashi Y. Biosynthesis of the brain-specific 14-3-2 protein in a cell-free system from wheat germ extract directed with poly(A)-containing RNA from rat brain. J Neurochem. 1980 Mar;34(3):687–693. doi: 10.1111/j.1471-4159.1980.tb11198.x. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Stipanuk M. H., Beck P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982 Aug 15;206(2):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren J. R., Ragin R., Chaykovsky M. Modulation of cysteine metabolism in mice--effects of propargylglycine and L-cyst(e)ine-degrading enzymes. Biochem Pharmacol. 1978;27(24):2807–2814. doi: 10.1016/0006-2952(78)90194-6. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]