Abstract
Plasma metabolites offer insights into aging processes and aging-related biomarkers. Here, the dietary effects of various functional foods on older adult mice were evaluated using metabolomic techniques. Fifty-week-old mice were divided into four groups (n = 4 each) and fed either a normal diet (AC) or the diets from Triticum aestivum sprout (TA), Schisandra chinensis (SZ), or Pisum sativum sprout (PS) extracts. Additionally, a group of 8-week-old mice fed a normal diet (YC; n = 5) was included for the comparison. The PS group had a significantly lower free fatty acid content and higher ornithine, proline, citric acid, and oxalic acid contents than the AC group. The PS group also showed reduced oxidative stress and muscle damage, suggesting the higher anti-aging efficacy of P. sativum sprouts than the other diets. These findings suggest plasma metabolite profiling is an effective tool to assess the anti-aging effects of functional foods.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01479-8.
Keywords: Aging, Pisum sativum, Triticum aestivum, Schisandra chinensis, Metabolomics
Introduction
Aging is represented by a progressive decline in the physical functions of an organism, during which various complex processes such as oxidative stress, mitochondrial damage, and cell aging are increased, leading to the manifestation of various aging-related diseases, such as Parkinson’s disease, Alzheimer’s disease, and arthritis (Bonomini et al., 2015). Middle age, at 40–60 years, is a transitional period from young to old age at which physical functions begin to decline (Kim et al., 2021). Most aging-related diseases can be efficiently detected early and prevented in middle age; therefore, research targeting middle age has gained interest (Kim et al., 2021). However, the medications against aging-related diseases currently in use exhibit limited efficacy or exert side effects. Nevertheless, the intake of functional foods rich in antioxidants and beneficial nutrients can help prolong life by preventing or reducing the occurrence of aging-related diseases (Singh et al., 2021). Therefore, the potency of natural foods containing physiologically active ingredients has been extensively explored to identify functional foods with anti-aging effects (Jędrusek-Golińska et al., 2020).
Physiologically active ingredients such as polyphenols, dietary fibers, and carotenoids are mainly present in plant-based functional foods (Jędrusek-Golińska et al., 2020), such as Pisum sativum sprouts (Borges-Martínez et al., 2021; Wu et al., 2023), Triticum aestivum sprouts (Lee et al., 2023), and Schisandra chinensis extracts (Yang and Yuan, 2021). Pisum sativum sprout contains a variety of phenolic compounds, including flavonoids such as quercetin glycosides and kaempferol glycosides, anthocyanins, and isoflavones. These compounds have been reported to increase antioxidant activity (Borges-Martínez et al., 2021; Wu et al., 2023). Triticum aestivum seedlings contain high concentrations of various bioactive metabolites, such as isoorientin, isoschaftoside, and isoscoparin (Lee et al., 2023). These compounds exert various health benefits, including radical scavenging activity (Cheel et al., 2005; Yuan et al., 2016), suggesting wheat seedlings as an important source of functional agents (Lee et al., 2023). Schisandrene, a dibenzocyclooctadiene lignan, is the major compound in Schisandra chinensis and shows strong antioxidant effects (Yang and Yuan, 2021). However, the anti-aging effects of these functional foods have not been explored yet.
Plasma metabolites analysis of an individual offers valuable insights into aging-related biomarkers, such as mitochondrial dysfunction, fatty acid oxidation, and athletic ability (Kondoh et al., 2020). Hao et al. (2022) demonstrated fatty acid and glucose metabolism in the blood of middle-aged mice are important metabolites in improving vascular remodeling and dysfunction due to aging. As metabolic challenges often emerge with aging, understanding the changes in plasma metabolites is crucial in studying aging-related diseases. Therefore, in this study, we aimed to evaluate the dietary effects of various functional foods on older adult mice using metabolomics techniques. We analyzed the alterations in plasma metabolites using gas chromatography–mass spectrometry (GC–MS)-based in 50-week-old mice, equivalent to 60–65 human years, fed with P. sativum sprouts, T. aestivum sprouts, or S. chinensis for 10 weeks.
Materials and methods
Preparation of diets
In this study, sprouts of T. aestivum and P. sativum were cultivated using the Korean cultivars Saekeumkang and Dacheong, respectively. These cultivars were grown in artificial soil in a growth chamber in 2022 under the following growth conditions: temperature, 18–20 °C; humidity, 60–70%; illumination, 5500 lx; light/dark cycle, 12 h of light followed by 12 h of darkness. Sprouts of T. aestivum and P. sativum were harvested 11 days after germination. The harvested samples were air-dried for 2 days at 50 °C to remove moisture, then freeze-dried at − 78 °C. The dried T. aestivum and P. sativum sprouts were extracted with 40% ethanol and 60% water (v/v) and 30% ethanol and 70% water (v/v), respectively. The selection of the extracts was based on the comparison of high-performance liquid chromatography (HPLC) chromatogram patterns, considering both the diversity of peaks and area values (Fig. S1). The extraction was performed at room temperature in a shaker for 18 h. Schisandra chinensis was purchased from Health & Co. (Gyeonggi-do, Korea). Dried S. chinensis (100 g) was extracted with 10 times the volume of water at 20–23 °C (2 h for each extraction) and then filtered. The water extract was then evaporated using a rotary vacuum evaporator at 45 °C, and the solvent was evaporated in vacuum to obtain an extract with a yield of 11.5% by weight. Each extract was then vacuum filtered using an 8 µm filter (1002-090, Whatman, Kent, UK), vacuum-concentrated, and freeze-dried at − 80 °C to obtain the dry powder (1 g S. chinensis water extract contains approximately 5 mg schizandrin).
Animals and sample collection
Male C57BL/6 J mice were obtained from the Jackson Laboratory (USA). The mice were divided into two groups: an aging group (50 weeks old, n = 20) and a young group (8 weeks old, n = 5). All mice were housed in individual cages under a 12 h light–dark cycle at 25 °C and 40–60% humidity. Young mice were used as non-aged controls (YC). However, several mice died in the aging group during the caging; therefore, the remaining 16 mice were randomly divided into four groups (n = 4 per group) based on their treatments. The mice in the control group (AC) were administered distilled water, and the other three groups were fed with extracts of P. sativum sprouts (PS group), T. aestivum sprouts (TA group), or S. chinensis (SZ group), at a dose of 200 mg/kg body weight per day. All mice were provided free access to tap water and a standard AIN-93G diet. Food consumption was recorded daily, and their water bottles were changed regularly. The test substances were administered orally at a volume of 10 mL/kg once daily for 10 weeks. During the experiment, forced swimming was conducted in a circular pool (diameter 100 cm, height 30 cm) containing warm water (temperature, 28 ± 1 °C) at a depth of 15 cm for 1 min a day, 30 min after administration. The daily food intake and body and tissue (liver, white adipose, and muscle) weights were measured in all groups to evaluate the effect of the three functional food diets. All animal experimental procedures were approved by the Animal Ethics Committee (approval number KNU 2022-0434).
At the end of the 10 weeks, the mice were fasted for 12 h and anesthetized via isoflurane inhalation. Afterward, the blood samples were collected from the inferior vena cava in heparin-treated tubes and centrifuged at 1200 × g for 10 min at − 4 °C. The supernatants (plasma samples) were collected and stored at − 80 °C until analysis.
Plasma extraction and analysis of metabolites
Low-molecular-weight metabolites (e.g., amino acids, organic acids, sugars, fatty acids, and sterols) from the plasma were analyzed with some modifications to the extraction methods reported by Kim et al. (2022). Briefly, for extraction of low-molecular weight metabolites such as amino acids and sugars, 500 µL methanol–chloroform (3:1, v/v) was added to a 2 mL tube containing 30 µL plasma sample. Subsequently, 30 µL l-2-chlorophenylalanine (0.3 mg/mL), used as an internal standard (IS), was added. After mixing for 10 s, the mixture was sonicated at 20 °C for 5 min and centrifuged at 14,000 × g at 4 °C for 15 min. The supernatant (500 µL) was transferred to a new tube and concentrated using a vacuum concentrator (CVE-3110, EYELA, Tokyo, Japan) for 2 h. The concentrated sample was derivatized for 90 min at 37 °C in 80 µL methoxyamine hydrochloride in pyridine (20 mg/mL) and then treated with 80 µL N,O-bis(trimethylsilyl) trifluoroacetamide containing 1% trimethylchlorosilane at 60 °C for 60 min. The mixture was then centrifuged at 14,000 × g for 15 min at room temperature. Subsequently, 160 µL extract was filtered through a 0.5 µm hydrophobic membrane filter and injected into GC–MS. In addition, 20 µL of each plasma sample was combined to create quality control (QC) samples. An auto-sampler AOC-20i and GCMS-QP2010 Ultra system (Shimadzu, Kyoto, Japan) were used to perform the GC–MS analysis. The injection parameter was in split mode with a split ratio of 1:10. Helium at a flow rate of 1.1 mL/min was used as the carrier gas, and the separation was carried out using a DB-5 column (30 m × 0.25 mm id, film thickness 1.0 µm; 122-5033, Agilent, Santa Clara, CA, USA). The initial temperature of the oven was set at 100 °C for 4 min until it was increased to 320 °C at a rate of 10 °C/min and then was held for 11 min. The MS range was set to 45–600 m/z. The peaks were identified by comparing the retention time and mass spectra of the GC–MS data with those of standard compounds, in-house and in MS libraries (NIST 7.0 and Wiley 9), respectively. The retention times of the peaks were corrected based on the retention time of a standard alkane series mixture (C-9 to C-33) using the Automatic Adjustment of Retention Time (AART) function of the Shimadzu GC–MS solution software (Table 1; Shiomi et al., 2011). To compare the metabolite contents in the five groups, the relative concentrations of amino acids, organic acids, carbohydrates, fatty acids, and sterols were quantitatively analyzed based on the ratio of each metabolite peak area to that of the IS (Shiomi et al., 2011; Kim et al., 2022).
Table 1.
Relative retention times (RRTs) and GC–MS data of hydrophilic and lipophilic compounds in the plasma samples of mice
| Compound | RT | RRT | RI | Quantitation ion (m/z)a |
|---|---|---|---|---|
| Pyruvic acid-meto-TMSb | 7.593 | 0.412 | 1046 | 174 |
| Lactic acid-2TMS | 7.862 | 0.427 | 1060 | 219 |
| Glycolic acid-2TMS | 8.118 | 0.441 | 1073 | 205 |
| Alanine-2TMS | 8.699 | 0.472 | 1103 | 190 |
| Glycine-2TMS | 8.973 | 0.487 | 1118 | 204 |
| Oxalic acid-2TMS | 9.401 | 0.510 | 1143 | 219 |
| 3HB-2TMSc | 9.711 | 0.527 | 1160 | 233 |
| Valine-2TMS | 10.758 | 0.584 | 1221 | 218 |
| Urea-2TMS | 10.931 | 0.593 | 1231 | 189 |
| 2-Aminoethanol-3TMS | 11.635 | 0.631 | 1275 | 174 |
| Leucine-2TMS | 11.649 | 0.632 | 1276 | 232 |
| Glycerol-3TMS | 11.626 | 0.631 | 1274 | 218 |
| Phosphoric acid-3TMS | 11.679 | 0.634 | 1278 | 314 |
| Isoleucine-2TMS | 12.017 | 0.652 | 1298 | 232 |
| Proline-2TMS | 12.149 | 0.659 | 1307 | 216 |
| Succinic acid-2TMS | 12.206 | 0.632 | 1311 | 247 |
| Glycine-3TMS | 12.287 | 0.667 | 1317 | 248 |
| Glyceric acid-3TMS | 12.523 | 0.680 | 1333 | 292 |
| Fumaric acid-2TMS | 12.663 | 0.687 | 1342 | 245 |
| Uracil-2TMS | 12.699 | 0.689 | 1344 | 255 |
| Nonanoic acid-TMS | 12.910 | 0.701 | 1359 | 215 |
| Serine-3TMS | 12.980 | 0.704 | 1363 | 306 |
| Threonine-3TMS | 13.402 | 0.727 | 1392 | 291 |
| Decanoic acid-TMS | 14.278 | 0.775 | 1456 | 229 |
| Malic acid-3TMS | 14.737 | 0.800 | 1489 | 233 |
| Methionine-2TMS | 15.271 | 0.829 | 1531 | 293 |
| 5-Oxoproline-2TMS | 15.347 | 0.833 | 1537 | 258 |
| Cysteine-3TMS | 15.726 | 0.853 | 1566 | 218 |
| Creatinine-3TMS | 15.875 | 0.862 | 1578 | 329 |
| Threonic acid-4TMS | 15.723 | 0.853 | 1566 | 292 |
| Phenylalanine-2TMS | 16.709 | 0.907 | 1646 | 218 |
| Glutamine-4TMS | 17.830 | 0.968 | 1651 | 227 |
| G3P-4TMSb | 18.146 | 0.985 | 1770 | 445 |
| Glutamine-3TMS | 18.268 | 0.991 | 1781 | 362 |
| L-2-chlorophenylalanined | 18.427 | 1.000 | 1795 | 218 |
| Ornithine-4TMS | 18.852 | 1.023 | 1834 | 174 |
| Citric acid-4TMS | 18.815 | 1.021 | 1831 | 363 |
| Myristic acid-1TMS | 18.988 | 1.030 | 1847 | 285 |
| Fructose-meto-5TMS(1) | 19.509 | 1.059 | 1895 | 307 |
| Fructose-meto-5TMS(2) | 19.602 | 1.064 | 1904 | 307 |
| Mannose-meto-5TMS | 19.678 | 1.068 | 1912 | 319 |
| Glucose-meto-5TMS(1) | 19.830 | 1.076 | 1927 | 319 |
| Lysine-4TMS | 19.930 | 1.082 | 1937 | 317 |
| Glucose-meto-5TMS(2) | 20.060 | 1.089 | 1949 | 319 |
| Tyrosine-3TMS | 20.137 | 1.093 | 1957 | 382 |
| Mannitol-6TMS | 20.286 | 1.101 | 1972 | 319 |
| Palmitoleic acid-TMS | 20.852 | 1.132 | 2029 | 311 |
| Palmitic acid-TMS | 21.005 | 1.140 | 2045 | 313 |
| Inositol-6TMS | 21.857 | 1.186 | 2134 | 318 |
| Linoleic acid-TMS | 22.640 | 1.229 | 2219 | 337 |
| Oleic acid-TMS | 22.664 | 1.230 | 2221 | 339 |
| Stearic acid-TMS | 22.852 | 1.240 | 2243 | 341 |
| Arachidonic acid-TMS | 24.095 | 1.308 | 2385 | 117 |
| Cholesterol-TMS | 33.215 | 1.803 | 3235 | 458 |
RT retention time (min), RRT relative retention time (retention time of analyte/retention time of l-2-chlorophenylalanine), RI retention index
aMass ion used for quantification
bTMS, trimethylsilylation
c3HB, 3-hydroxybutyric acid and G3P, glycerol-3-phosphate
dInternal standard
Statistical analysis
After the GC–MS data were normalized (unit variance scaling), principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed. Soft independent modeling was performed for PCA, PLS-DA, and OPLS-DA using the SIMCA package 14.1 software (Umetrics, Ume, Sweden). R2Y and Q2 were used to measure the goodness of fit and predictability of the OPLS-DA model, respectively. The R2Y and Q2 values range between 0 and 1, and a value higher than 0.5 between the two groups indicated a significant metabolic difference. The Student's t-test and analysis of variance (ANOVA) were performed using Prism GraphPad 9.0. (GraphPad, San Diego, CA, USA) to statistically evaluate the data. A p-value less than 0.05 indicated a statistically significant difference between the means of the groups being compared.
Results and discussion
Animal characteristic data
Compared to the YC group, the four older adult mice groups (AC, TA, SZ, and PS) had significantly higher body weights at the start of the experiment. However, at the end of the experiment, no significant differences in body weight were observed among the five groups (Table S1). Food intake and the weights of the liver and white adipose tissue significantly increased in the AC group compared to the YC group, while muscle weight showed a significant decrease in the AC group. Among the old age mice groups, TA supplementation decreased liver tissue weight while it increased muscle weight compared to those of other diets. Furthermore, the liver weight was decreased in the SZ group compared to that in the AC group.
Plasma metabolite profiling
Metabolomics is a powerful tool for studying the effects of anti-aging functional foods on the body. It provides a comprehensive interpretation of biochemical pathways and processes altered by dietary consumption (May et al., 2013). Wang et al. (2022) demonstrated that metabolomics analysis in mice fed with plant-based diets revealed changes in the levels and types of plasma metabolites. Furthermore, metabolomics can be used to identify changes in the levels of amino acids, lipids, and other small molecules involved in energy metabolism, antioxidant defense, and other physiological processes. Together, these findings indicate metabolomics analysis can provide insights into the mechanisms by which functional foods exert their effects and help identify novel biomarkers.
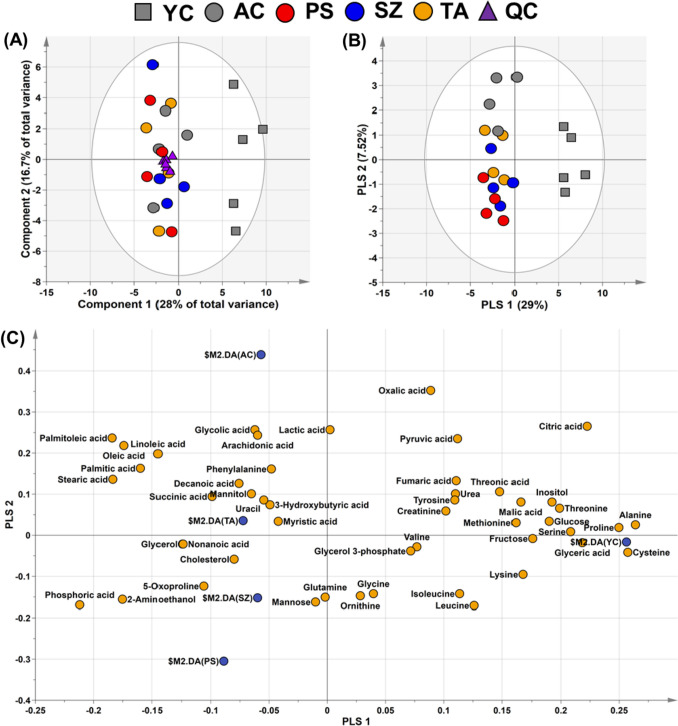
In this study, we identified 49 metabolites comprising 16 amino acids, 10 organic acids, 3 sugars, 2 sugar alcohols, 1 steroid, 1 pyrimidine, 9 fatty acids, and 7 other compounds in the plasma of the five mice groups (Table S2). The unsupervised statistical method, PCA, is useful for identifying variations between metabolic phenotypes (Park et al., 2023). In experiments with various data, the QC collected for each group increases the reliability of the statistical results (Godzien et al., 2015). In this study, unsupervised PCA revealed the aggregation of all QC samples of all the diet groups (n = 6 in each) in the center of the plot (Fig. 1A), which confirmed the reliability and reproducibility of the metabolic analysis performed in this study. However, the samples in different age groups [old-age (TA, SZ, and PS) and young-age (YC) groups] formed distinct clusters. Nevertheless, PCA is not ideal for predicting the distinct separation between variables (Park et al., 2023). In contrast, PLS-DA is frequently used to identify disparities among groups in various categories and is a suitable method for confirming differences in plasma metabolites resulting from various dietary interventions (Yoon et al., 2019). Therefore, we employed PLS-DA to visualize the variations in plasma metabolites among the five groups. In the PLS-DA model, PLS 1 and 2 of the score plot represented 36.5% of the total variance (PLS 1, 29.0%; PLS 2, 7.5%; Fig. 1B). PLS 1 separated the old and young mice groups (Fig. 1B). In addition, notable differences in the metabolite separation were observed in the normal diet old group (AC) and the functional diet old groups (TA, SZ, and PS; Fig. 1B). Metabolites that affected the separation were identified using the loading plot (Fig. 1C). Among the metabolites that contributed most to the separation, the contents of amino acids, such as alanine, cysteine, proline, and serine, were higher in the YC group than those in the old mice groups. Their eigenvector values were 0.264, 0.257, 0.250, and 0.208, respectively. The content of free fatty acids, such as palmitoleic, stearic, oleic, and palmitic acids (eigenvector values were − 0.184, − 0.183, − 0.174, and − 0.160, respectively), increased in the AC group compared to those in the other groups. Consistent with these findings, those in previous studies have demonstrated a relatively high content of amino acids and tricarboxylic acid cycle intermediates in the plasma samples of young mice (Adachi et al., 2018; Kim et al., 2022). In contrast, the plasma samples from the old mice showed relatively low levels of amino acids and high levels of free fatty acids (Kim et al., 2022). Furthermore, the PLS-DA results revealed that each group was divided according to aging and diets, and the functional diet groups (TA, SZ, and PS) exhibited differences in their metabolite profiles compared with the YC and AC groups.
Fig. 1.
A Score plot of the PCA models based on the 49 metabolites in the plasma samples in the five groups (young group, square; aging groups, circle), including the QC samples (quality control, triangle). This plot shows the clustering of QC samples, demonstrating that metabolic analysis has good stability, reliability, and reproducibility. B Score and C loading plots of the PLS-DA models based on the 49 metabolites in the plasma of five groups (young group, square; aging groups, circle). These plots show the relationship between the metabolites and the classification of the groups based on their plasma profiles
Metabolites comparison of different diets
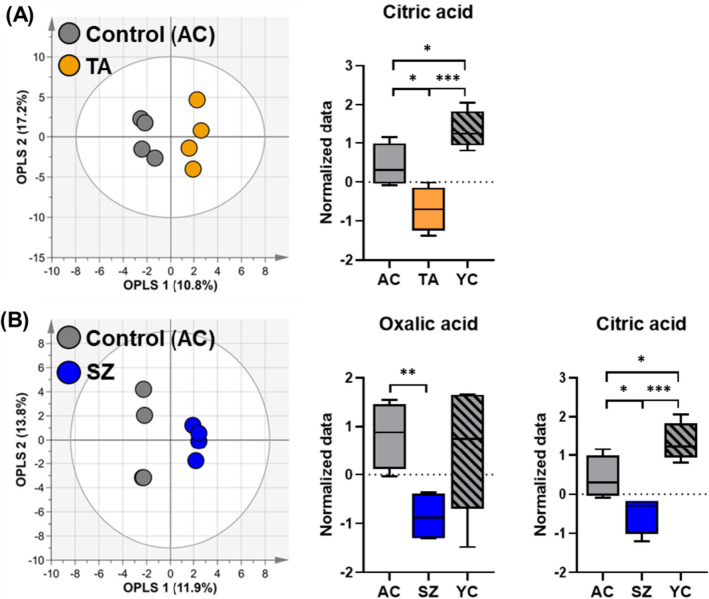
Next, we performed OPLS-DA analysis to compare each functional diet group with the normal diet group (AC), which allowed for graphical visualization of the differences and similarities between groups (Fig. 2). OPLS-DA is a supervised statistical technique that separates variables related to class identifiers (Blasco et al., 2015). Contrary to PLS-DA, OPLS-DA facilitated the identification of metabolic differences between the AC and each extract-fed group (TA, SZ, and PS).
Fig. 2.
A Score plot of the OPLS-DA models based on the 49 metabolites obtained in the plasma of AC and TA groups. Box plots show significant differences in ANOVA based on 49 metabolites identified in the plasma of AC, TA, and YC groups. B Score plot of the OPLS-DA models based on the 49 metabolites identified in the plasma of AC and SZ groups. Box plots show significant differences in ANOVA based on 49 metabolites in the plasma samples of AC, SZ and YC groups. The OPLS-DA model represents the difference between the two groups. *p < 0.05, **p < 0.01, and ***p < 0.001. AC, TA, and SZ groups were fed with 200 mg/kg/day normal, Triticum aestivum sprout extract, and Schisandra chinensis extract diets for 10 weeks, respectively
The OPLS-DA model showed no significant difference between the TA and AC groups, with a Q2 value of − 0.349 and an R2Y value of 0.96 (Fig. 2A). Nevertheless, only citric acid was identified as a significant metabolite (Fig. 2A; Student’s t-test). Moreover, ANOVA revealed elevated levels of citric acid in the YC group (Fig. 2A). Citric acid serves as an intermediate in the tricarboxylic acid cycle, suggesting a higher energy metabolic rate in young mice than that in old mice, consistent with previous findings (Kim et al., 2022). Furthermore, the OPLS-DA model between the SZ and AC groups had a Q2 value of 0.213 and an R2Y value of 0.995; however, it was difficult to clarify whether the two groups were completely separated (Fig. 2B). The ANOVA results showed that citric acid and oxalic acid were significant metabolites that distinguished the groups (Fig. 2B). In particular, oxalic acid had a p-value of less than 0.01, and relatively less oxalic acid was detected in the SZ group compared to those in the YC and AC groups.
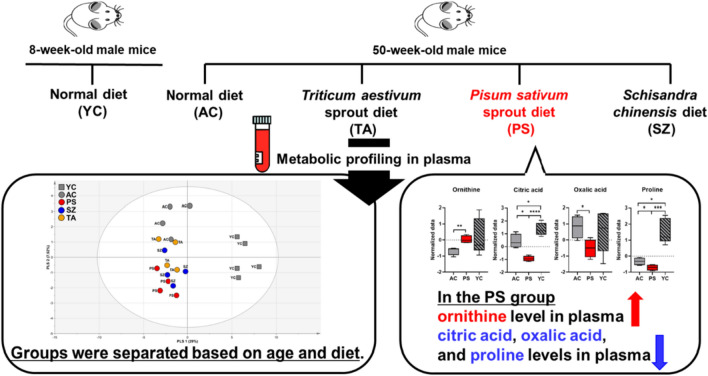
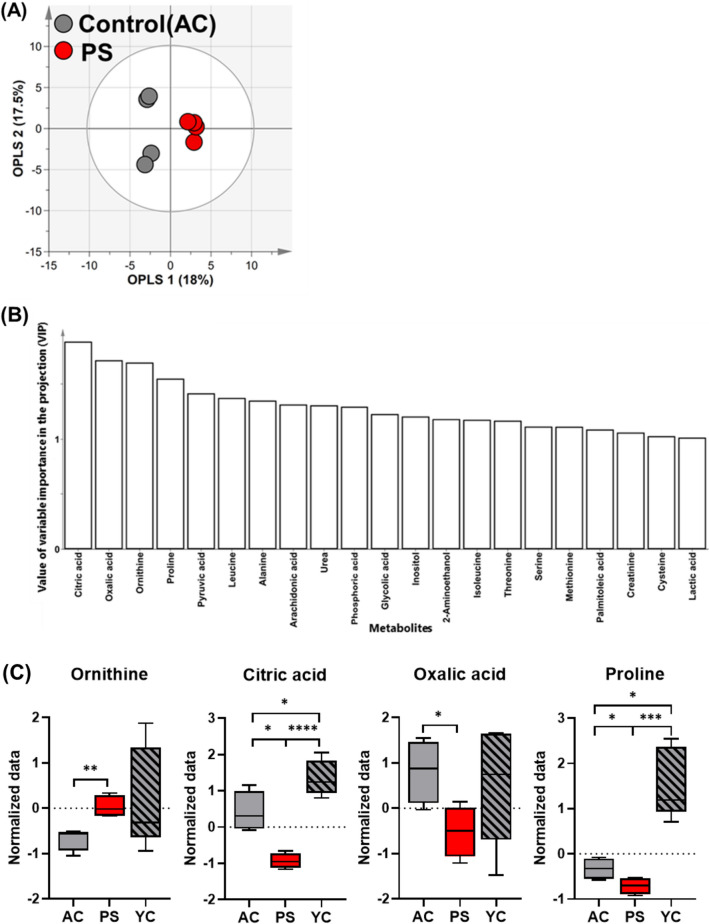
Unlike the previous comparison, the OPLS-DA model between the PS and AC groups had Q2 and R2Y values of 0.659 and 0.984, respectively. This indicated a statistically significant difference between the two groups (Fig. 3A). To investigate plasma metabolites that significantly influenced clustering patterns in the OPLS-DA models, the variable importance in projection (VIP) plots were constructed. The VIP value represents the importance of each contributing metabolite relative to other metabolites. Components with VIP values higher than 1.0 are considered significant in the grouping of subjects (Yoon et al., 2019). In this study, the VIP plots of the AC and PS groups for 21 metabolites showed significant VIP values (> 1.0). The highest VIP values were identified for organic acids, such as citric, oxalic, and pyruvic acids, as well as for amino acids, such as ornithine, proline, and leucine (Fig. 3B). ANOVA was performed on plasma metabolites with a VIP value of > 1.0 from mice fed with P. sativum sprout, which confirmed the significant difference (p < 0.05; Fig. 3C). Ornithine and citric acid showed the most significant differences (p < 0.01) between groups. A previous study has shown that citrulline can modify body composition and positively influence lipid metabolism in older adult mice (Moinard et al., 2015). Ornithine is a precursor for citrulline synthesis; therefore, an increase in ornithine levels may positively affect citrulline synthesis and its associated benefits (Marini, 2012). In addition, citric acid is a known biomarker of aging that can distinguish between aging states (Yue et al., 2022). Cardiovascular disease is a typical aging-related disease closely associated with the citric acid cycle (North and Sinclair, 2012). Cardiac muscle mitochondria play an important role in ATP production in the citric acid cycle. As the cardiac muscle ages, mitochondria alter the cycle flux, reduce ATP production, and accumulate citric acid metabolites in the plasma. Therefore, high levels of citric acid in the plasma are closely related to aging and can act as a predictive biomarker for cardiovascular disease (Santos et al., 2023). Oxalic acid and proline levels between the PS and AC groups were also significantly different (p < 0.05). The presence of a high concentration of oxalic acid in the plasma can be an indicator of oxidative stress, which impairs antioxidant defense mechanisms (Korol et al., 2021). Oxalic acid can also contribute to urea formation and exacerbate oxidation (Mydlik and Derzsiova, 2010). The link between oxidative stress and major aging-related diseases is well-established (Liguori et al., 2018). Reactive oxygen species are byproducts of biological oxidation and cause progressive oxidative damage, leading to cell dysfunction and death (Bonomini et al., 2015). In addition, a high concentration of proline in the plasma is associated with sarcopenia and cognitive impairment (Toyoshima et al., 2017). Sarcopenia and proline levels in the plasma are closely related because muscle atrophy and proline metabolism increase (Ilaiwy et al., 2016). High concentrations of proline can induce oxidative damage to proteins, lipids, and DNA (Ferreira et al., 2014). Additionally, it prevents the uptake of glutamine, a neurotransmitter, in the cerebral cortex and hippocampus of mice (Delwing et al., 2007). Furthermore, patients with Alzheimer’s disease and amnestic mild cognitive impairment have high plasma proline concentrations (Wang et al., 2014). This finding suggests a link between increased proline levels and cognitive decline. In addition, ANOVA results showed that the contents of oxalic acid, ornithine, citric acid, and proline in YC plasma were higher than those in AC and PS groups (Fig. 3C). This could be the result of an increased metabolic rate in younger age groups (Kim et al., 2022). Taken together, the plasma metabolite profiling data indicated that mice ingested with P. sativum sprout had a significant metabolic difference compared to those ingested with other diets.
Fig. 3.
A Score and B VIP plots of the OPLS-DA models were obtained from 49 metabolites in the plasma of AC and PS. The OPLS-DA represents the difference between the two groups. C Box plots showing significant differences in ANOVA based on 49 metabolites identified in the plasma samples of AC, PS, and YC groups. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. AC and PS groups were fed with 200 mg/kg/day normal and Pisum sativum sprout extract diets for 10 weeks, respectively
In conclusion, the dietary effects of functional foods (T. aestivum sprout, S. chinensis, and P. sativum sprout) on the plasma metabolites of old mice were successfully evaluated using metabolomics techniques. After 10 weeks of feeding, metabolic differences were identified using multivariate analyses. In particular, the OPLS-DA model showed a statistically significant difference between the PS and AC groups. The PS group had lower levels of citric acid, oxalic acid, and proline and higher levels of ornithine than the AC group. These results indicate that consumption of P. sativum sprout extract can potentially prevent or ameliorate the symptoms of aging-related diseases. The results of this study highlight the importance of plasma metabolite profiling in evaluating aging-related biomarkers and suggest the need for further research on various functional foods.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a Grant from the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. RS-2022-RD010283)” funded by the Rural Development Administration (RDA), Republic of Korea and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1A6A3A01087368), Republic of Korea.
Author contributions
SHY: Data curation, writing review and editing. YJK: Formal analysis, writing review and editing. HGL: Investigation, Resources. WDS: Methodology, Resources. EYK: Conceptualization, Writing—original draft. JKK: Conceptualization, supervision, writing review & editing.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
So Hwi Yang and Ye Jin Kim contributed equally to this work as co-first authors.
Contributor Information
Eun Young Kwon, Email: eykwon@knu.ac.kr.
Jae Kwang Kim, Email: kjkpj@inu.ac.kr.
References
- Adachi Y, Ono N, Imaizumi A, Muramatsu T, Andou T, Shimodaira Y, Nagao K, Kageyama Y, Mori M, Noguchi Y, Hashizume N, Nukada H. Plasma amino acid profile in severely frail elderly patients in Japan. International Journal of Gerontology. 12: 290-293 (2018) 10.1016/j.ijge.2018.03.003 [DOI] [Google Scholar]
- Blasco H, Błaszczyński J, Billaut JC, Nadal-Desbarats L, Pradat PF, Devos D, Moreau C, Andres CR, Emond P, Corcia P, Słowiński R. Comparative analysis of targeted metabolomics: dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. Journal of Biomedical Informatics. 53: 291-299 (2015) 10.1016/j.jbi.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging and Disease. 6: 109 (2015) 10.14336/AD.2014.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges-Martínez E, Gallardo-Velázquez T, Cardador-Martínez A, Moguel-Concha D, Osorio-Revilla G, Ruiz-Ruiz JC, Martínez CJ. Phenolic compounds profile and antioxidant activity of pea (Pisum sativum L.) and black bean (Phaseolus vulgaris L.) sprouts. Food Science and Technology. 42: e45920 (2021) [Google Scholar]
- Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.). Journal of Agricultural and Food Chemistry. 53: 2511-2517 (2005) 10.1021/jf0479766 [DOI] [PubMed] [Google Scholar]
- Delwing D, Delwing D, Sanna RJ, Wofchuk S, Wyse AT. Proline promotes decrease in glutamate uptake in slices of cerebral cortex and hippocampus of rats. Life Sciences. 81: 1645-1650 (2007) 10.1016/j.lfs.2007.09.031 [DOI] [PubMed] [Google Scholar]
- Ferreira AG, Scherer EB, da Cunha AA, Manfredini V, Biancini GB, Vanzin CS, Vargas CR, Wyse AT. Hyperprolinemia induces DNA, protein and lipid damage in blood of rats: antioxidant protection. The International Journal of Biochemistry and Cell Biology. 54: 20-25 (2014) 10.1016/j.biocel.2014.05.027 [DOI] [PubMed] [Google Scholar]
- Godzien J, Alonso-Herranz V, Barbas C, Armitage EG. Controlling the quality of metabolomics data: new strategies to get the best out of the QC sample. Metabolomics. 11: 518-528 (2015) 10.1007/s11306-014-0712-4 [DOI] [Google Scholar]
- Hao Z, Xu G, Yuan M, Tan R, Xia Y, Liu Y, Yin X. Leucine supplementation in middle-aged male mice improved aging-induced vascular remodeling and dysfunction via activating the Sirt1-Foxo1 axis. Nutrients. 14: 3856 (2022) 10.3390/nu14183856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilaiwy A, Quintana MT, Bain JR, Muehlbauer MJ, Brown DI, Stansfield WE, Willis MS. Cessation of biomechanical stretch model of C2C12 cells models myocyte atrophy and anaplerotic changes in metabolism using non-targeted metabolomics analysis. The International Journal of Biochemistry and Cell Biology. 79: 80-92 (2016) 10.1016/j.biocel.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jędrusek-Golińska A, Górecka D, Buchowski M, Wieczorowska-Tobis K, Gramza-Michałowska A, Szymandera-Buszka K. Recent progress in the use of functional foods for older adults: a narrative review. Comprehensive Reviews in Food Science and Food Safety. 19: 835-856 (2020) 10.1111/1541-4337.12530 [DOI] [PubMed] [Google Scholar]
- Kim Y-T, Jeon H, Kim S, Heo K, Shim J, Lee J, Yang D, Kang SC. Fermented antler recovers stamina, muscle strength and muscle mass in middle-aged mice. Applied Sciences. 12: 106 (2021) 10.3390/app12010106 [DOI] [Google Scholar]
- Kim YJ, Park BS, Song N, Tu TH, Lee S, Kim JK, Kim JG. Metabolic profiling in the hypothalamus of aged mice. Biochemical and Biophysical Research Communications. 599: 134-141 (2022) 10.1016/j.bbrc.2022.02.042 [DOI] [PubMed] [Google Scholar]
- Kondoh H, Kameda M, Yanagida M. Whole blood metabolomics in aging research. International Journal of Molecular Sciences. 22: 175 (2020) 10.3390/ijms22010175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol L, Stepanova N, Vasylchenko V, Snisar L, Lebid L, Kolesnyk M. Plasma oxalic acid as a trigger for oxidative processes in end-stage renal disease patients. Ukrainian Journal of Nephrology and Dialysis. 1: 46-53 (2021) 10.31450/ukrjnd.1(69).2021.07 [DOI] [Google Scholar]
- Lee H, Yang JY, Ra JE, Ahn HJ, Lee MJ, Kim HY, Song S-Y, Kim DH, Lee JH, Seo WD. Elucidation of phenolic metabolites in wheat seedlings (Triticum aestivum L.) by NMR and HPLC-Q-Orbitrap-MS/MS: changes in isolated phenolics and antioxidant effects through diverse growth times. Food Chemistry X. 17: 100557 (2023) 10.1016/j.fochx.2022.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Francesco C, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clinical Interventions in Aging. 13: 757-772 (2018) 10.2147/CIA.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. The Journal of Nutrition. 142: 572-580 (2012) 10.3945/jn.111.153825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May DH, Navarro SL, Ruczinski I, Hogan J, Ogata Y, Schwarz Y, Levy L, Holzman T, McIntosh MW, Lampe JW. Metabolomic profiling of urine: response to a randomised, controlled feeding study of select fruits and vegetables, and application to an observational study. British Journal of Nutrition. 110: 1760-1770 (2013) 10.1017/S000711451300127X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moinard C, Le Plenier S, Noirez P, Morio B, Bonnefont-Rousselot D, Kharchi C, Ferry A, Neveux N, Cynober L, Raynaud-Simon A. Citrulline supplementation induces changes in body composition and limits age-related metabolic changes in healthy male rats. The Journal of Nutrition. 145: 1429-1437 (2015) 10.3945/jn.114.200626 [DOI] [PubMed] [Google Scholar]
- Mydlik M, Derzsiova K. Oxalic acid—important uremic toxin. Vnitrni Lekarstvi. 56: 695-701 (2010) [PubMed] [Google Scholar]
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circulation Research. 110: 1097-1108 (2012) 10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Kim YJ, Park SU, Kim HY, Yang JY, Song SY, Lee MJ, Seo WD, Kim JK. Lipids and volatile organic compounds in sesame seeds and their relationships with environmental temperature-induced stress. Food Research International. 169: 112831 (2023) 10.1016/j.foodres.2023.112831 [DOI] [PubMed] [Google Scholar]
- Santos JL, Ruiz-Canela M, Razquin C, Clish CB, Guasch-Ferré M, Babio N, Corella D, Gómez-Gracia E, Fiol M, Estruch R, Lapetra J, Fitó M, Aros F, Serra-Majem L, Liang L, Martínez MÁ, Toledo E, Salas-Salvadó J, Hu FB, Martínez-González MA. Circulating citric acid cycle metabolites and risk of cardiovascular disease in the PREDIMED study. Nutrition, Metabolism and Cardiovascular Diseases. 33: 835-843 (2023) 10.1016/j.numecd.2023.01.002 [DOI] [PubMed] [Google Scholar]
- Shiomi Y, Nishiumi S, Ooi M, Hatano N, Shinohara M, Yoshie T, Kondo Y, Furumatsu K, Shiomi H, Kutsumi H, Azuma T, Yoshida M. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflammatory Bowel Diseases. 17: 2261-2274 (2011) 10.1002/ibd.21616 [DOI] [PubMed] [Google Scholar]
- Singh JP, Singh B, Kaur A. Nutraceuticals and functional foods in aging and aging-associated diseases. Nutrition, Food and Diet in Ageing and Longevity. 14: 221-238 (2021) 10.1007/978-3-030-83017-5_12 [DOI] [Google Scholar]
- Toyoshima K, Nakamura M, Adachi Y, Imaizumi A, Hakamada T, Abe Y, Kaneko E, Takahashi S, Shimokado K. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE. 12: e0185206 (2017) 10.1371/journal.pone.0185206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhou Y, Huang FJ, Tang HD, Xu XH, Liu JJ, Wang Y, Deng YL, Ren RJ, Xu W, Ma JF, Zhang YN, Zhao AH, Chen SD, Jia W. Plasma metabolite profiles of Alzheimer’s disease and mild cognitive impairment. Journal of Proteome Research. 13: 2649-2658 (2014) 10.1021/pr5000895 [DOI] [PubMed] [Google Scholar]
- Wang F, Baden MY, Guasch-Ferré M, Wittenbecher C, Li J, Li Y, Wan Y, Bhupathiraju SN, Tobias DK, Clish CB, Mucci LA, Eliassen AH, Costenbader KH, Karlson EW, Ascherio A, Rimm EB, Manson JE, Liang L, Hu FB. Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes. Diabetologia. 65: 1119-1132 (2022) 10.1007/s00125-022-05692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DT, Li WX, Wan JJ, Hu YC, Gan RY, Zou L. A comprehensive review of pea (Pisum sativum L.): chemical composition, processing, health benefits, and food applications. Foods. 12: 2527 (2023) 10.3390/foods12132527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yuan C. Schisandra chinensis: a comprehensive review on its phytochemicals and biological activities. Arabian Journal of Chemistry. 14: 103310 (2021) 10.1016/j.arabjc.2021.103310 [DOI] [Google Scholar]
- Yoon D, Choi BR, Ma S, Lee JW, Jo IH, Lee YS, Kim GS, Kim S, Lee DY. Metabolomics for age discrimination of ginseng using a multiplex approach to HR-MAS NMR spectroscopy, UPLC–QTOF/MS, and GC × GC–TOF/MS. Molecules. 24: 2381 (2019) 10.3390/molecules24132381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Wang J, Wu W, Liu Q, Liu X. Effect of isoorientin on intracellular antioxidant defence mechanisms in hepatoma and liver cell lines. Biomedicine and Pharmacotherapy. 81: 356-362 (2016) 10.1016/j.biopha.2016.04.025 [DOI] [PubMed] [Google Scholar]
- Yue T, Tan H, Shi Y, Xu M, Luo S, Weng J, Xu S. Serum metabolomic profiling in aging mice using liquid chromatography–mass spectrometry. Biomolecules. 12: 1594 (2022) 10.3390/biom12111594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.