Abstract
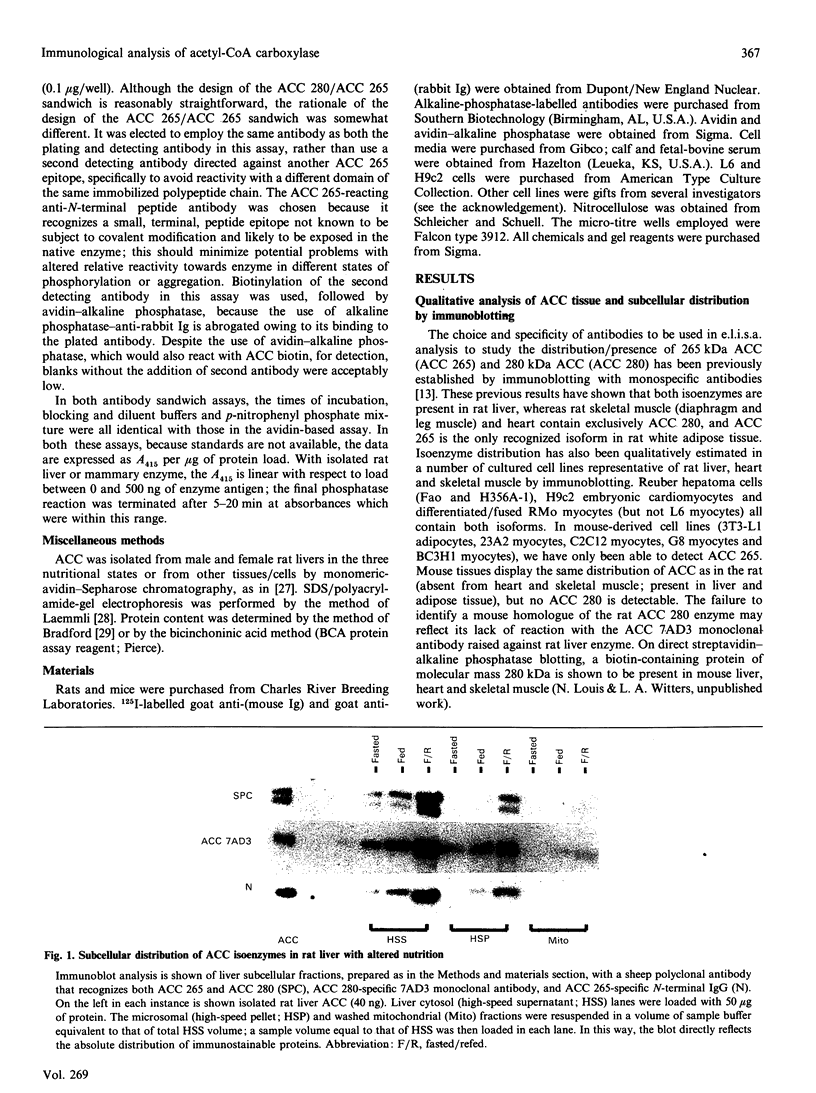
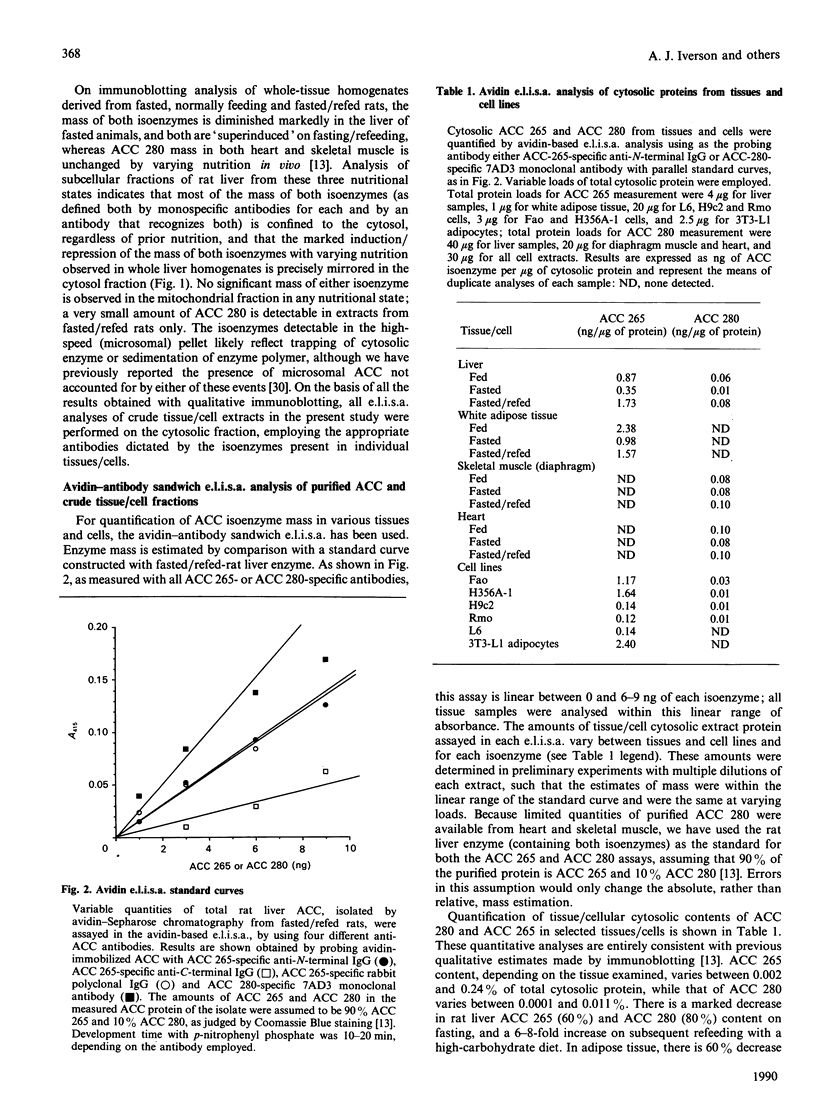
Changes in the mass and subunit structure of liver acetyl-CoA carboxylase (ACC) accompany altered nutrition in vivo. Enzyme activity in different tissues and cell lines is also, in part, determined by variations in both total mass and ACC isoenzyme composition. ACC isoenzyme mass and hetero/homo-isoenzyme association were quantified by three sandwich e.l.i.s.a. assays, i.e. an avidin-based assay that measured total isoenzyme mass and two antibody-sandwich assays which measure polypeptide association. Results from the avidin-based assay reveal that the two major isoenzymes, of molecular mass 265 kDa (ACC 265) and 280 kDa (ACC 280), are present in markedly variable concentration in several rat and mouse tissues and in cell lines of rat and mouse origin. Hepatic ACC mass has been reported to be distributed between mitochondrial and cytosolic fractions and to undergo only a change in subcellular distribution without alteration in total mass on induction/repression of activity in vivo [Roman-Lopez, Shriver, Joseph & Alfred (1989) Biochem. J. 260, 927-930]. However, in the present study, immunoblotting and e.l.i.s.a. analysis reveals that, in rat liver, the mass of both isoenzymes is predominantly cytosolic in distribution, is markedly diminished on fasting and rises 6-8-fold on refeeding of a high-carbohydrate diet. These data support the results of several other investigations of hepatic ACC mass, and are consistent with known nutritionally altered changes in ACC mRNA content. By the two antibody-sandwich e.l.i.s.a. assays, isoenzyme complexes either composed of both ACC 280 and 265 or with multiple copies of ACC 265 are detectable in rat liver enzyme; their concentration varies independently of total ACC mass with the nutritional state of the rat, being lowest in fasting and highest on fasting/refeeding. E.l.i.s.a. analysis, applicable to crude tissue/cell extracts, provides a simple, sensitive and quantitative measurement of ACC mass and subunit composition. Its use may permit needed quantitative insight into the role of variable total ACC and isoenzyme mass and of alterations in ACC subunit composition that occur in vivo or in isolated cells in response to a variety of hormonal and nutritional influences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Roman-Lopez C. R. Enzymatically inactive forms of acetyl-CoA carboxylase in rat liver mitochondria. Biochem J. 1988 May 1;251(3):881–885. doi: 10.1042/bj2510881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred J. B., Roman-Lopez C. R., Pope T. S., Goodson J. Dietary dependent distribution of acetyl CoA carboxylase between cytoplasm and mitochondria of rat liver. Biochem Biophys Res Commun. 1985 Jun 14;129(2):453–460. doi: 10.1016/0006-291x(85)90172-x. [DOI] [PubMed] [Google Scholar]
- Bai D. H., Pape M. E., López-Casillas F., Luo X. C., Dixon J. E., Kim K. H. Molecular cloning of cDNA for acetyl-coenzyme A carboxylase. J Biol Chem. 1986 Sep 15;261(26):12395–12399. [PubMed] [Google Scholar]
- Bianchi A., Evans J. L., Iverson A. J., Nordlund A. C., Watts T. D., Witters L. A. Identification of an isozymic form of acetyl-CoA carboxylase. J Biol Chem. 1990 Jan 25;265(3):1502–1509. [PubMed] [Google Scholar]
- Borthwick A. C., Edgell N. J., Denton R. M. Use of rapid gel-permeation chromatography to explore the inter-relationships between polymerization, phosphorylation and activity of acetyl-CoA carboxylase. Effects of insulin and phosphorylation by cyclic AMP-dependent protein kinase. Biochem J. 1987 Feb 1;241(3):773–782. doi: 10.1042/bj2410773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Kim K. H. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. Arch Biochem Biophys. 1974 Oct;164(2):478–489. doi: 10.1016/0003-9861(74)90058-7. [DOI] [PubMed] [Google Scholar]
- Evans J. L., Witters L. A. Quantitation by immunoblotting of the in vivo induction and subcellular distribution of hepatic acetyl-CoA carboxylase. Arch Biochem Biophys. 1988 Jul;264(1):103–113. doi: 10.1016/0003-9861(88)90575-9. [DOI] [PubMed] [Google Scholar]
- Goodson J., Pope T. S., Allred J. B. Molecular weights of subunits of acetyl CoA carboxylase in rat liver cytoplasm. Biochem Biophys Res Commun. 1984 Jul 31;122(2):694–699. doi: 10.1016/s0006-291x(84)80089-3. [DOI] [PubMed] [Google Scholar]
- Guy P. S., Hardie D. G. Regulation of mammalian acetyl-CoA carboxylase: limited proteolysis mimics dephosphorylation. FEBS Lett. 1981 Sep 14;132(1):67–70. doi: 10.1016/0014-5793(81)80428-0. [DOI] [PubMed] [Google Scholar]
- Jacobs R., Kilburn E., Majerus P. W. Acetyl coenzyme A carboxylase. The effects of biotin deficiency on enzyme in rat liver and adipose tissue. J Biol Chem. 1970 Dec 10;245(23):6462–6467. [PubMed] [Google Scholar]
- Jamil H., Madsen N. B. Phosphorylation state of acetyl-coenzyme A carboxylase. I. Linear inverse relationship to activity ratios at different citrate concentrations. J Biol Chem. 1987 Jan 15;262(2):630–637. [PubMed] [Google Scholar]
- Jamil H., Madsen N. B. Phosphorylation state of acetyl-coenzyme A carboxylase. II. Variation with nutritional condition. J Biol Chem. 1987 Jan 15;262(2):638–642. [PubMed] [Google Scholar]
- Kim K. H., López-Casillas F., Bai D. H., Luo X., Pape M. E. Role of reversible phosphorylation of acetyl-CoA carboxylase in long-chain fatty acid synthesis. FASEB J. 1989 Sep;3(11):2250–2256. doi: 10.1096/fasebj.3.11.2570725. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- Merrill G. F. Clonal derivation of a rat muscle cell strain that forms contraction-competent myotubes. In Vitro Cell Dev Biol. 1989 May;25(5):471–476. doi: 10.1007/BF02624635. [DOI] [PubMed] [Google Scholar]
- Moss J., Lane M. D. Acetyl coenzyme A carboxylase. 3. Further studies on the relation of catalytic activity to polymeric state. J Biol Chem. 1972 Aug 25;247(16):4944–4951. [PubMed] [Google Scholar]
- Pape M. E., Lopez-Casillas F., Kim K. H. Physiological regulation of acetyl-CoA carboxylase gene expression: effects of diet, diabetes, and lactation on acetyl-CoA carboxylase mRNA. Arch Biochem Biophys. 1988 Nov 15;267(1):104–109. doi: 10.1016/0003-9861(88)90013-6. [DOI] [PubMed] [Google Scholar]
- Roman-Lopez C. R., Goodson J., Allred J. B. Determination of the quantity of acetyl CoA carboxylase by [14C]methyl avidin binding. J Lipid Res. 1987 May;28(5):599–604. [PubMed] [Google Scholar]
- Roman-Lopez C. R., Shriver B. J., Joseph C. R., Allred J. B. Mitochondrial acetyl-CoA carboxylase. Time course of mobilization/activation in liver of refed rats. Biochem J. 1989 Jun 15;260(3):927–930. doi: 10.1042/bj2600927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thampy K. G., Wakil S. J. Regulation of acetyl-coenzyme A carboxylase. II. Effect of fasting and refeeding on the activity, phosphate content, and aggregation state of the enzyme. J Biol Chem. 1988 May 5;263(13):6454–6458. [PubMed] [Google Scholar]
- Tipper J. P., Witters L. A. In vitro phosphorylation and inactivation of rat liver acetyl-CoA carboxylase purified by avidin affinity chromatography. Biochim Biophys Acta. 1982 Apr 13;715(2):162–169. doi: 10.1016/0304-4165(82)90354-3. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Friedman S. A., Bacon G. W. Microsomal acetyl-CoA carboxylase: evidence for association of enzyme polymer with liver microsomes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3639–3643. doi: 10.1073/pnas.78.6.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Watts T. D., Daniels D. L., Evans J. L. Insulin stimulates the dephosphorylation and activation of acetyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5473–5477. doi: 10.1073/pnas.85.15.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Watts T. D., Gould G. W., Lienhard G. E., Gibbs E. M. Regulation of protein phosphorylation by insulin and an insulinomimetic oligosaccharide in 3T3-L1 adipocytes and Fao hepatoma cells. Biochem Biophys Res Commun. 1988 Jun 30;153(3):992–998. doi: 10.1016/s0006-291x(88)81326-3. [DOI] [PubMed] [Google Scholar]



