Abstract
The analysis of cells frozen within the International Space Station (ISS) will provide crucial insights into the impact of the space environment on cellular functions and properties. The objective of this study was to develop a method for cryopreserving blood cells under the specific constraints of the ISS. In a ground experiment, mouse blood was directly mixed with a cryoprotectant and gradually frozen at −80 °C. Thawing the frozen blood sample resulted in the successful recovery of viable mononuclear cells when using a mixed solution of dimethylsulfoxide and hydroxyethyl starch as a cryoprotectant. In addition, we developed new freezing cases to minimize storage space utilization within the ISS freezer. Finally, we confirmed the recovery of major mononuclear immune cell subsets from the cryopreserved blood cells through a high dimensional analysis of flow cytometric data using 13 cell surface markers. Consequently, this ground study lays the foundation for the cryopreservation of viable blood cells on the ISS, enabling their analysis upon return to Earth. The application of this method in ISS studies will contribute to understanding the impact of space environments on human cells. Moreover, this method may find application in the cryopreservation of blood cells in situations where research facilities are inadequate.
Subject terms: Health care, Immunology, Preclinical research
Introduction
Throughout space missions, astronauts undergo an array of environmental changes that markedly differ from terrestrial conditions. These alterations encompass microgravity, exposure to high-energy space radiation, and psychological stress induced by confinement and fear, all of which may exert substantial influences on various physiological functions in human1,2. Several studies reported the impact of spaceflights on the immune systems1,3,4. For instance, various latent viruses were reportedly re-activated5. Moreover, analysis of blood samples from astronauts suggested that spaceflight influences the distribution of leukocytes6,7, natural killer cell function8, granulocyte and monocyte functions7,9, along with plasma cytokine levels6,10. Consequently, considering the implementation of short-term spaceflights for the public and long-term space exploration, including lunar and Mars missions, it is crucial to investigate the mechanisms through which spaceflights may affect immune function.
Single-cell RNA sequencing (scRNA-seq) technology has provided new insights into cell diversity and differentiation of immune cells11. In the NASA Twin Study12,13, scRNA-seq was employed to analyze the impact of spaceflight on gene expression and cell heterogeneity13. In this experiment, one twin served as the ground control and remained on Earth, while the other twin stayed aboard the ISS. For scRNA-seq, whole blood was collected into cell preparation tubes, and the microwell-based scRNA-seq analysis of peripheral blood mononuclear cells (PBMCs) was conducted. However, a limitation of this study is the absence of data collection during spaceflight; blood samples for scRNA-seq were obtained only before launch and after the return to Earth. Single-cell RNA-seq analysis of viable blood cells transported from the ISS may be compromised as the gene expression profile in these cells could be altered during the transportation to Earth. Whole blood cells in the cell preparation tube can be frozen for preservation, however, this freezing process induces cell lysis. Performing scRNA-seq analysis on a lysed blood cell mixture is extremely difficult, whereas bulk RNA-seq analysis of a mixed cell sample is feasible. Therefore, when generating scRNA-seq data from human PBMCs during spaceflight, it is crucial to preserve blood cells using a method that prevents their lysis14.
Cryopreservation is a widely-used method for generating viable cell stocks for long-term storage15–17. Density gradient centrifugation with Ficoll-Paque or cell preparation tubes is commonly employed for isolating PBMCs. In addition, erythrocytes are eliminated by the addition of a buffer solution containing ammonium chloride, enhancing purity and suitability for downstream analysis. For cryopreservation, cells precipitated by centrifuge are resuspended in suitable cryoprotectants containing organic solvents such as dimethylsulfoxide (DMSO). While these methods are relatively straightforward in a laboratory setting, executing them in the ISS poses unique challenges. Due to the higher requirement of biosafety in the ISS as compared to the normal laboratory on Earth, blood and organic solvents need to be handled within a closed system. Consequently, opening tubes with human blood and organic solvents need to be avoided. Therefore, despite the feasibility of centrifugation, the task of removing the solution through aspiration and subsequently resuspending precipitated blood cells in cryoprotectants containing organic solvents presents considerable difficulties during spaceflights and missions, even though techniques to isolate and preserve cells under microgravity conditions were reported18. Consequently, these constraints in handling human blood and organic solvents could potentially hinder the cryopreservation of blood samples onboard the ISS.
The gradual cryopreservation of cells is crucial for minimizing intracellular ice crystal formation15,16, thereby reducing the probability of cellular demise. In addition to programmable cell freezers, various containers with optimized features for efficient cell freezing have been developed. Typically, these cell containers facilitate a gradual freezing process in cryoprotectant at a controlled rate of −1 °C/min. These commercially available containers may impose significant spatial constraints within the ISS freezer and are also not applicable for cryopreservation using blood collection tubes. Consequently, it is important to establish a method for cryopreservation of blood cells within the ISS freezer without using a programmed cell freezer or commercially available containers.
We aim to establish a cryopreservation method suitable for execution aboard the ISS. In this study, we performed a ground experiment using murine blood samples and devices that can be used in the ISS environment. We found that freezing blood directly after adding a cryoprotectant containing dimethylsulfoxide and hydroxyethyl starch, without additional additives like serum and albumin, allowed for the recovery of viable cells after thawing. In addition, we report the development of a case designed for the cryopreservation of cells collected in a vacuum blood collection tube, which can be utilized in the ISS freezer. Overall, we propose that this procedure, along with a set of accompanying tools named the Biological sample MiXture device (BMX), would hold utility for the cryopreservation of viable blood mononuclear cells during missions on the ISS and thereby facilitate scRNA-seq and other analyses of PBMCs frozen aboard the ISS after their preservation and transport to Earth.
Results
Direct addition of cryoprotectant into EDTA-treated whole blood is applicable for cryopreservation of mononuclear cells
Due to the challenges of handling tubes containing both blood and organic solvents in the ISS environment, employing a standard cryopreservation method involving centrifugation, solution removal, and re-suspension in cryoprotectants becomes arduous. Therefore, we assessed a cryopreservation method involving the direct addition of cryoprotectants to an EDTA-treated blood sample19. For testing cryopreservation conditions, murine blood mononuclear cells were selected due to their phenotypic similarities to their human counterparts20. Moreover, due to the ethical constraints at the time, conducting experiments on mice was our most feasible option.
We first tested whether two general cryoprotectants can be used in this method. Dimethylsulfoxide (DMSO) is known as a popular and convenient cryoprotectant21. In addition, previous studies indicated that the use of a cryoprotectant mixture containing hydroxyethyl starch and DMSO enhanced the recovery of unfractionated bone marrow cells from cryopreservation22–24. In the use of this cryoprotectants, the mixture of 12% hydroxyethyl starch and 10% DMSO (hereafter referred as to the CP-1 solution) with 8% human serum albumin (HSA) in saline is added to cell suspension solution in equal volumes22–24. However, considering the richness of albumins in the mouse blood, we tested whether the addition of HSA could be omitted and replaced with phosphate-buffered saline. In all experiment conditions, equal volumes of cryoprotectant and mouse blood were mixed for the proper final concentration in a microcentrifuge tube. The tubes were enveloped with paper towels as insulators, a common bench-top method to facilitate a gradual cooling process without using cryogenic containers17, and then placed in a −80 °C freezer. After being placed for 3 days at −80 °C, frozen cells were thawed and recovered. Upon visual inspection after thawing, substantial evidence of spontaneous hemolysis was apparent, suggesting that the freezing and thawing process caused blood cell degradation. In contrast, the survival of mononuclear cells was confirmed by staining with propidium iodide (counting for dead cells) and acridine orange (counting total cells with nucleus). Cryopreservation using the CP-1 solution yielded an average 75% survival ratio of mononuclear cells after the recovery and seems to be better than DMSO (average 57%) (Fig. 1a). Notably, the survival ratio of recovered cells was above 60% for all trials using the CP-1 solution. In contrast, with DMSO, survival ratios in 3 trials among 5 trials were less than 50% (Fig. 1a). Accordingly, within the framework of this cryopreservation methodology, the using the CP-1 solution appears to be superior to DMSO. The addition of HSA in the CP-1 solution may slightly improve cell survival (Fig. 1a). Generally, it is recommended to mix HSA with the CP-1 solution immediately before adding them to cell suspensions. Therefore, considering the work efficiency in the ISS, we chose to use the CP-1 solution without HSA as the cryoprotectant in this method. Compared to the standard method of resuspending cell pellets in CP-1 solution after hemolysis, the survival ratio was slightly lower but still appears to be sufficient for downstream analysis (Supplementary Fig. 1).
Fig. 1. Cell survival ratio of recovered mononuclear cells in mouse blood.
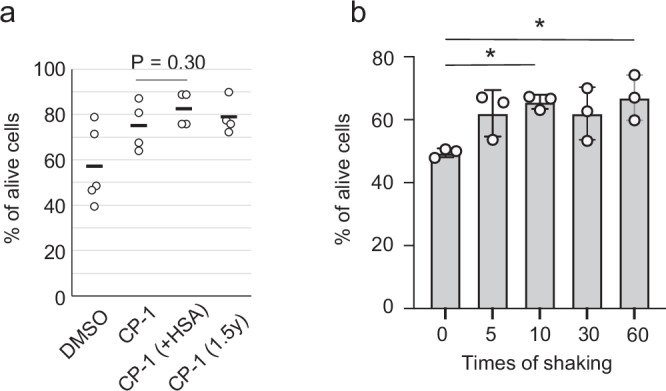
a Comparison in cryoprotectants among DMSO, the mixed solution of DMSO and hydroxyethyl starch (CP-1) in the presence and absence of human serum albumin (HSA), and CP-1 stored in a syringe for 1.5 year at room temperature (1.5 y). Samples (total 1 ml) were frozen in centrifuge tubes. N = 5 for DMSO, N = 4 for CP-1, N = 4 for CP-1 and HSA, and N = 2 for CP-1 for 6-month storage. Two-tailed and unpaired Student’s t-test. Bars indicate mean values. b Dependence of cell survival ratio on the mixing times after the cell recovery. After the addition of the cryoprotectant (CP-1) into the blood, the solution was mixed for the indicated time, wrapped with a paper towel, and frozen at −80 °C. After the recovery of the frozen cells, the viability was determined. Samples (total 4 ml) were frozen in blood collection tubes. N = 3. Mean values are shown as boxes, and bars indicate standard deviation. *P < 0.05. One-way ANOVA and Tukey’s test.
For the cryopreservation of blood during space missions, preserving cryoprotectants at ambient temperature for a long duration aboard the ISS would be preferable. We investigated the influence of long-term storage on the effectiveness of the CP-1 solution for cryopreservation. Following storage for 1.5 years in a plastic syringe at ambient temperature, the stored CP-1 solution underwent testing for blood cryopreservation. Data suggested that the storage for 1.5 years did not significantly influence the viability of recovered cells (Fig. 1a), implying that the stored CP-1 solution can be used for cryopreservation in relatively long-term space missions.
Furthermore, in addition to considering the shelf life of the CP-1 solution, cryopreserved blood samples may need to be stored at -80 °C for several months before analysis. We examined the influence of this long-term storage on cell viability. Our findings suggest that PBMCs can be cryopreserved in the CP-1 solution for up to 86 days with a survival rate exceeding 60%, and potentially preserved for up to 267 days (Supplementary Fig. 1).
Mingling two distinct solutions may pose challenges in the microgravity environment of the ISS. In addition, astronauts might not always possess expertise in biological experiments involving cells and blood. Therefore, we investigated the optimal shaking frequency required to maintain maximum viability. We selected 7 ml vacuum blood collection tubes with 2 ml of fill volume to ensure sufficient free volume for adding 2 ml of CP-1 in the same volume. After collecting 2 ml of murine blood in each tube, 2 ml of the CP-1 solution was added to a 7 ml blood collection tube. Subsequently, collection tubes underwent mixing 0, 5, 10, 30, and 60 times with a shaking rate of 2 times/second (Fig. 1b). To be slowly frozen, the tubes were enveloped in paper towels and placed in −80 °C for several days. Notably, shaking 10 times proved sufficient, yielding an average of 60% survival upon recovery. However, in some instances, the survival ratio was slightly lower. Data deviated from the initial trial using microcentrifuge tubes (Fig. 1a), likely due to an increase in total volume from 1 ml for the microcentrifuge tube to 4 ml for the blood collection tube. Survival rates consistently surpassed 50% with five or more shaking times, which would be deemed adequate for downstream analysis. However, in our following experiments, we shook the tubes 60 times to ensure sufficient mixing of the two solutions, considering the potential difficulty of mixing in microgravity conditions.
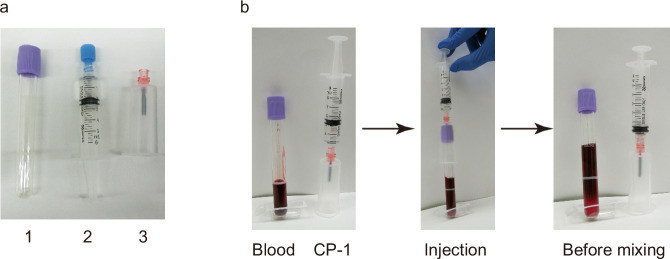
Our data suggest that directly adding the CP-1 solution as a cryoprotectant to the blood in the blood collection tubes is applicable for cryopreservation on the ISS. However, for biosafety reasons within the ISS laboratory, the possibility of splattering blood and organic solvents must be eliminated. Furthermore, the injection of the solution into a tube should be conducted safely and easily by astronauts. To address these concerns, we propose a procedure for injecting the CP-1 solution into the blood collection tube. As depicted in Fig. 2, the CP-1 solution is loaded into a syringe before launch, and then, in the ISS, the pre-loaded CP-1 is injected using a safeguarded needle, called a blood transfer device.
Fig. 2. A typical procedure for the addition of the cryoprotectant into the blood collected in the collection tube.
a A set of materials for introducing CP-1 into blood within a collection tube includes: (1) the blood collection tube, (2) CP-1 in a syringe, and (3) a blood transfer device. b A scheme for the procedure for adding CP-1 to blood in a collection tube. The CP-1 solution is pre-loaded into a syringe. After the blood collection in the tube, pre-loaded CP-1 is injected using a safeguarded needle (Injection). The needle is removed from the tube for shaking (Before mixing).
The ethylene propylene diene monomer (EPDM) elastomer is suitable for a case designed for the slow freezing of blood samples
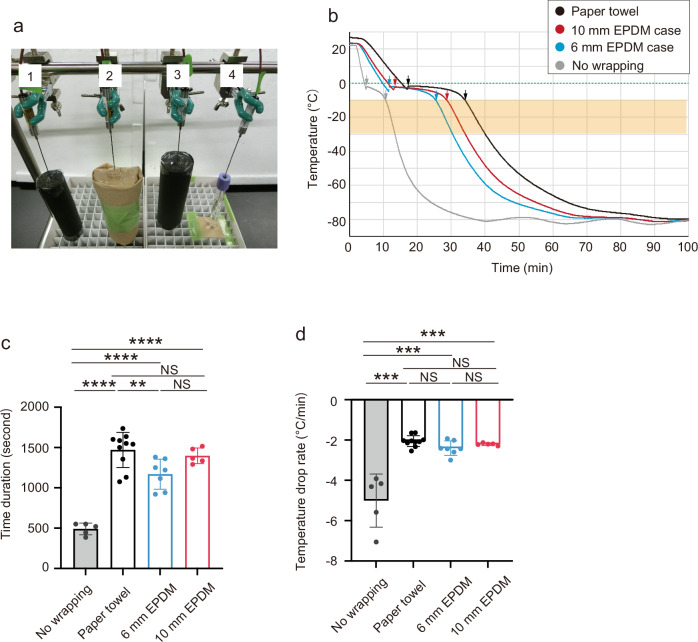
Freezing cells without using cryogenic containers or insulation materials like paper towels is a possible approach. However, the absence of packaging materials can lead to variations in the freezing rate of samples, influenced by their degree of contact with adjacent samples in the freezer. These variations may impact sample recovery, survival rates, and phenotypes of specimens. Therefore, we chose to use a suitable insulator to mitigate these risks. Although wrapping cryotubes with paper towels is a commonly used technique for slow freezing in cryopreservation17, it may be difficult for all astronauts to wrap tubes in the same way in the ISS. Moreover, commercially available containers do not accommodate the 7 ml vacuum blood collection tube. Therefore, we sought to design a specialized case specifically tailored for the slow freezing of blood in a standard blood collection tube within the ISS. As the material for the case, we chose ethylene propylene diene monomer rubber (EPDM), which is generally used as an insulator for sealing doors in freezers and refrigerators25. EPDM elastomers are light and maintain flexibility and elasticity even at low temperatures. Moreover, they resist degradation over time. These properties make them suitable for the materials of customized cases for freezing vacuum blood collection tubes in the ISS freezer. We utilized a tube-type of EPDM elastomer (6 mm and 10 mm thickness) that fits the blood collection tube (inner diameter, 16 mm) as the case for slow-freezing. We then assessed the feasibility of utilizing the EPDM elastomer tube as a slow-freezing case. Thus, we conducted a comparative study on the cooling kinetics of a solution (the 50% CP-1 solution) in blood collection tubes under 4 conditions: the tube placed in an EPDM elastomer case (6 mm or 10 mm thickness), the tube wrapped in paper towels, and the tube without any case or wrap. After being placed in the freezer (Fig. 3a), the solution initiated the freezing process after supercooling (Fig. 3b). The average duration from the freezing point to the linear cooling phase (durations between two arrows in Fig. 3b) was 490 s for the unwrapped tube (Fig. 3c). This duration was significantly longer for protected samples: 1169 s for the 6 mm EPDM elastomer case, 1397 s for the 10 mm EPDM elastomer case, and 1471 s for the paper towel (~10 mm thickness) (Fig. 3c). In the EPDM elastomer cases, the duration for the 10 mm case was comparable to that of the paper towel wrapping, whereas the duration for the 6 mm case was shorter. The cooling speeds of the steepest and most linear temperature change (around −10 °C to −30 °C) were also measured −2.40 °C/min for the 6 mm EPDM elastomer case, −2.20 °C/min for the 10 mm EPDM elastomer case, and −2.05 °C/min for the paper towel. The reduction rates of these cases were significantly slower than that of the unwrapped tube (−5.01 °C/min), and all conditions were practically comparable (Fig. 3b, d). Consequently, data suggested that the EPDM elastomer cases can be used for the slow freezing of the solution in a blood collection tube. Moreover, the 10 mm EPDM elastomer case may be more effective than the 6 mm EPDM elastomer case for slow freezing.
Fig. 3. Development of an elastomer case used for slow cell freezing.
a A photograph for slow freezing of solutions using EPDM elastomer or paper towel wrapping in −80 °C freezer. Label 1, 6 mm EPDM elastomer case; label 2, paper towel; label 3, 10 mm EPDM elastomer case; label 4, without case and wrapping. b A typical plot of decreasing rate in the temperature of the solution in tubes of the 6 mm EPDM elastomer case (blue), the 10 mm elastomer case (red), paper towels with ~10 mm thickness (black), or without wrapping (gray) at −80 °C. A green dot line indicates 0 °C. The orange shade indicates the temperature range exhibiting nearly linear cooling rates. Arrows indicate the time points at which freezing starts and the linear cooling phase begins. c Summary of durations during which the temperature changed from the freezing point to the linear cooling phase (durations between two arrows in Fig. 3b). One-way ANOVA and Tukey’s test. ***P < 0.001. NS indicates not significant. N = 5 for no wrapping, N = 10 for paper towel wrapping, N = 7 for 6 mm EPDM elastomer, N = 5 for 10 mm EPDM elastomer. Average values are shown as boxes, and bars indicate standard deviation. d Summary of cooling rates in the nearly linear temperature range. One-way ANOVA and Tukey’s test. ***P < 0.001. NS indicates not significant. N = 5 for no wrapping, N = 10 for paper towel wrapping, N = 7 for 6 mm EPDM elastomer, N = 5 for 10 mm EPDM elastomer. Average values are shown as boxes, and bars indicate standard deviation.
Cell-freezing by the direct CP-1 addition method causes a limited impact on the frequency of blood mononuclear cells
Our data suggested that the combination of the pre-loaded CP-1 solution in the syringe, blood transfer device, and collection tube, along with the EPDM elastomer case, may be used for the cryopreservation of blood in the ISS. This combination is named as the Biological sample MiXture device (hereafter referred as to BMX) with 6 mm (BMX-6) or 10 mm (BMX-10) thickness. We next tested that the BMXs are indeed appropriate for the recovery of viable blood mononuclear cells. Thus, the CP-1 solution was directly added to EDTA-treated mouse blood in the 7 ml blood collection tube. After mixing 60 times to complete the mixing, the tube was inserted into either case and placed in a −80 °C freezer. Frozen cells in the tubes were thawed after 3 days, and the cell survival ratio was determined (Fig. 4a). BMX-10 demonstrated a cell viability of ~60%, comparable to the method using paper towels. In contrast, BMX-6 exhibited a slightly lower cell viability of around 50%. Moreover, these viabilities were slightly lower than that observed with the conventional method, which involves cryopreservation with CP-1 solution after eliminating red blood cells using ammonium chloride solution. Consistently, the use of BMXs resulted in a slight reduction in the total number of live cells per ml of blood compared to both the paper towel wrapping method and the conventional method (Fig. 4b). Overall, these data suggested that both BMX-10 and BMX-6 enable the recovery of viable mononuclear cells from frozen blood and can be used for various downstream analyses such as scRNA-seq.
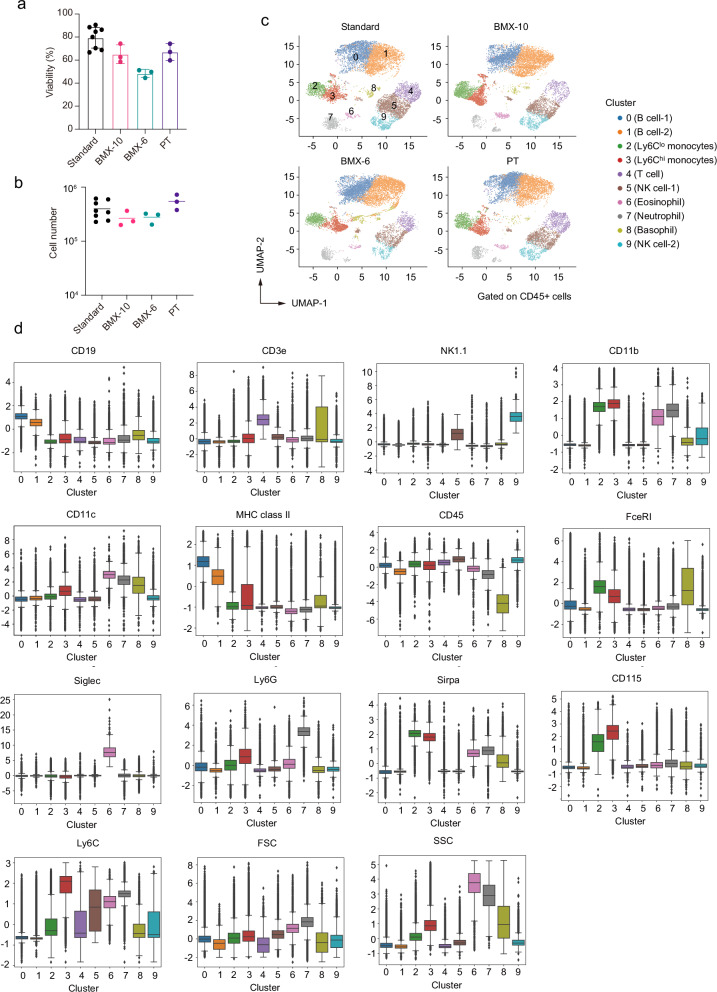
Fig. 4. High-dimensional analysis of flow cytometric data.
a Cell survival ratio of recovered cells in mouse blood using the Biological sample MiXture device (BMX). Triplicate experiments for each BMX condition were conducted using murine blood pooled from 16 mice. Average values are shown as boxes, and bars indicate standard deviation. “PT” indicates the method of using paper towels instead of BMXs for wrapping 7 ml blood collection tubes. For PT, the data in Fig. 1b (60 s) are shown again for comparison. “Standard” indicates the conventional method involving cryopreservation using the CP-1 solution in a 1.5 ml centrifuge tube, following the elimination of red blood cells with ammonium chloride solution. Paper towels were used for wrapping 1.5 ml tubes. In each experiment, the standard method was conducted as a control, and data (a total of 8 samples) were accumulated. b Recovered alive cell number after each cryopreservation method. Bars indicate average values. c Uniform manifold approximation and production (UMAP) plot of flow cytometric data from mouse blood mononuclear cells after the recovery from cryopreservation. Cell clusters are separated by colors and numbers in the plot. All samples were integrated for the analysis, and dots exhibit 10,000 cells randomly selected from each dataset. d Expression level of surface markers in each cluster. The box is drawn from the first quartile to the third quartile. Bars indicate values of the minimum, the maximum, and the sample median. Outliers are shown as dots.
To investigate the influence of using the BMXs for cryopreservation on the frequency of cell populations in blood mononuclear cells, we conducted a flow cytometric analysis on recovered cells, using 13 surface markers to identify major populations of mononuclear cells26 (Supplementary Fig. 2). For flow cytometric data analysis, we employed a high-dimensional approach based on the expression levels of each marker. Specifically, we used UMAP dimensional reduction27 and k-means clustering28 to separate cell types (Fig. 4c). The optimal number of clusters for the k-means algorithm was determined to be 10 using the elbow method29, which assists in identifying a suitable number of clusters based on the within-cluster sum of squares. We assigned these clusters based on the expression levels of surface markers (Fig. 4d). Clusters 0 and 1, exhibiting high expressions of CD19 and MHC class II, were identified as B cells. Cluster 4, showing high expression of CD3e, was designated as T cells, while clusters 5 and 9, expressing high levels of NK1.1, were categorized as NK cells. B cell clusters were further separated by their expression levels of CD19 and MHC class II, which may reflect slight differences in the maturation stage. NK clusters were also divided into two groups, likely due to variations in the expression levels of NK1.1 and CD11b30. In addition to these lymphoid lineage cells, clusters 2, 3, 6, 7, and 8 were categorized as myeloid lineage cells based on expression of other markers. Cluster 8, with lower levels of CD45 and higher levels of FceR1, was identified as basophils. Cluster 6 was designated as eosinophils due to the expression of Siglec, while cluster 7 exhibited high expression of Ly6G, consistent with neutrophils. Clusters 2 and 3 were identified as monocytes, with cluster 2 characterized as Ly6Clo and cluster 3 as Ly6Chi monocytes31.
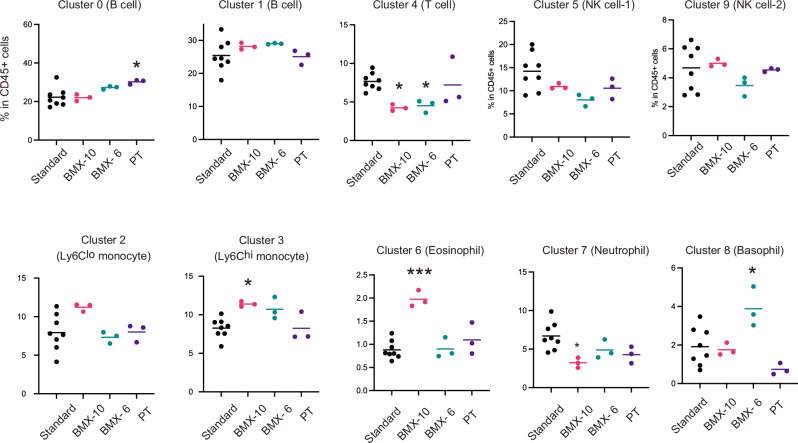
We proceeded to compare the influence of cryopreservation using BMXs on the frequency of these cell subsets. The data suggested that all major subtypes of mononuclear cells in the blood were present in the samples under all cryopreservation conditions (Fig. 5). However, the ratio of some subsets in CD45+ cells was significantly altered when BMXs were utilized. Reductions in the ratio of T cells for both BMXs, an increase in basophil for BMX-6, and increases in Ly6Chi monocytes and eosinophils for the BMX-10 were observed. Despite these limitations, since all major subsets are successfully recovered, we propose that the direct addition of the CP-1 solution to the whole blood using BMX can be employed for freezing blood in the ISS.
Fig. 5. Comparison in the impact of cryopreservation methods on the ratios of cell clusters in recovered CD45+ cells.
The conditions of Standard, BMX-10, BMX-6, and PT indicate are described in the legend of Fig. 4. *P < 0.05, ***P < 0.001, indicating a significance in the difference from the standard method. One-way ANOVA and Tukey’s test. Labels are the same with that of Fig. 4.
Discussion
In this study, we developed a procedure wherein the CP-1 solution was directly added to EDTA-treated whole blood in a vacuum blood collection tube. During our investigation, Satpathy et al. reported the SENSE method, which involves freezing whole blood directly by adding a solution containing 80% heat-inactivated fetal bovine serum (FBS) and 20% DMSO at a 1:1 ratio19. In contrast to the SENSE method, our method does not use FBS but rather hydroxyethyl starch, which is widely employed as an artificial colloid plasma volume expander32. Given the extended storage of pre-loaded cryoprotectants at room temperature during the ISS mission, using a solution containing FBS in the ISS may not be feasible. We confirmed that the prolonged storage (1.5 year) of CP-1 at ambient temperature does not affect its efficacy as a cryoprotectant. Consequently, BMXs appear to hold promise as tools for cryopreserving blood on the ISS.
In microgravity, liquids exhibit different behaviors compared to Earth: liquids are held together by surface tension. These characteristics of liquids in microgravity may hinder the mixing of two distinct solutions aboard the ISS. In this study, we examined the shaking times required for mixing the CP-1 solution with whole blood in 7 ml blood collection tubes. Our data suggest that 10 shaking cycles may be enough to mix them in this tube. However, given the specific behaviors of liquids in microgravity, we propose using 60 shaking cycles for mixing the CP-1 solution and whole blood in 7 ml vacuum blood collection tubes in the ISS. Notably, the 60-time shaking did not reduce the cell viability of recovered cells, suggesting this shaking speed (2 times/second) did not cause physical lethal damage to blood mononuclear cells.
When freezing starts after the blood and cryoprotective solution have been mixed in the tube, a temperature gradient is expected between the periphery and the interior of the tube. On Earth, these temperature gradients can induce buoyancy-driven and Marangoni convection, which may affect the efficiency of solution mixing. In microgravity, the absence of buoyancy-driven convection may alter the freezing rate. Conversely, Marangoni convection still occurs in microgravity due to gradients in surface tension caused by temperature variations, thereby influencing the freezing rate. However, the complex composition of the blood and cryoprotectant mixture, which contains various components such as proteins, lipids, and cellular debris in addition to an organic solvent, can affect the formation and stability of surface tension gradients, potentially limiting the effectiveness of Marangoni convection.
After thawing and retrieval of blood cells from cryopreservation, typically 60% of mononuclear cells survived, indicating that around 40% mononuclear cells were dead due to the thawing and retrieval procedure. However, analysis of flow cytometric data suggests that this cryopreservation procedure enables the successful recovery of the majority of lymphoid or myeloid lineage cell populations, similar to the normal freezing procedure, albeit with a little more pronounced impact on T cell frequency, possibly due to high sensitivity of some T cell subsets to freezing-induced apoptosis33,34. Overall, despite some limitations, our method is suitable for convenient cryopreservation in the ISS, where specialized laboratory equipment is not fully accessible.
Data suggested that the use of BMXs resulted in a decrease in the frequency of T cells following their recovery from cryopreservation. However, the underlying cause of this reduction remains unidentified. Given the diverse subtypes of T cells, further investigation is warranted to elucidate whether the cryopreservation by BMXs selectively influences specific subsets of T cells. Moreover, it should be noted that murine blood was used in this study. Previous studies have indicated similarities in global gene expression among immune cells in mice and humans20. Nevertheless, notable differences persist in the phenotypic characteristics of immune cells between the two species35. For instance, the proportion of lymphocytes in peripheral blood is substantially higher in mice compared to humans. Therefore, it is important to confirm that BMXs are applied for cryopreservation of human blood in the future.
In addition to this procedure, we developed a new type of case for cryopreservation of blood in the 7 ml vacuum blood collection tube. Our data suggested that wrapping by paper towel yielded the highest recovery ratio of viable cells after the cryopreservation. However, it is pertinent to acknowledge the possible variability in the wrapping technique, which may stem from individual differences. Consequently, the execution of this task by astronauts, notwithstanding their expertise, may encounter challenges, particularly in the microgravity environment of the ISS. Given the possible influence of wrapping methods on cell viability, adopting a simpler approach is recommended. Notably, the EPDM elastomer case presents itself as an uncomplicated and standardized alternative, ensuring consistency across users. The 10 mm thickness of the case may be preferred over the 6 mm thickness due to data indicating a slightly better survival ratio when using BMX-10. However, the difference between these two was relatively small; therefore, the selection of these two tubes may depend on the allowed space in a freezer for each mission. Consequently, we propose that both BMX-6 and BMX-10 can be used for freezing viable blood cells under the constraints of the ISS.
The developed cryopreservation method and BMXs may hold significant promise for diverse applications in closed and microgravity environments such as the ISS. For instance, in standard scRNA-seq analysis, it is essential to use viable cells or cells that have been freshly fixed. The fixation of PBMCs using a fixative solution, such as paraformaldehyde may be difficult under the specific constraints of the ISS. Moreover, given that all blood cells, including red blood cells, could be fixed, only PBMCs need to be sorted from a pool of fixed blood cells containing a large number of fixed erythrocytes, which would present an additional challenge. Therefore, it could be beneficial to explore the cryopreservation of viable PBMCs and their return to Earth for scRNA-seq analysis to determine the status of PBMCs in humans aboard the ISS. The BMXs that we developed in this study may be useful for such purposes. Moreover, by using BMX, biological fluid samples collected in tubes can be pretreated by adding some reagents and other substances in closed and microgravity environments such as the ISS without opening the tubes.
In addition to usage in space missions, BMXs may be applicable to terrestrial research settings, facilitating the cryopreservation of blood cells for other medical studies. This would offer a potential breakthrough in preserving blood samples when research facilities are limited or unavailable, proving beneficial for medical research and diagnostics in resource-constrained environments. Consequently, BMXs and the cryopreservation technique developed for the ISS not only advance space-based research but also have far-reaching applications in medical science, healthcare, and emergency scenarios, making it a versatile and impactful innovation with broad-reaching potential.
Methods
Mouse
Animal experiments were approved by the Institutional Animal Care and Use Committee of RIKEN, Yokohama Branch (2018-075), and performed following its guidelines. C57BL/6JJcl mice (CLEA Japan) were maintained in standard controlled conditions with a 12-h lighting cycle and access to chow and water ad libitum. Animals were randomly assigned to each group, and there were no exclusion data points. Eight to 10-week-old male mice were used for preparing blood samples. Mice were used for blood sample preparation immediately after purchase from the vendor and were not acclimatized.
Antibodies and reagents
Antibodies and reagents employed included FITC-anti-mouse NK1.1 (BioLegend, clone PK136, Cat#108705), PerCP-eFluor710-anti-mouse CD172a (eBioscience, clone P84, Cat# 46-1721-80), Alexa Fluor 647-anti-mouse Siglec F (BD, clone E50-2440, Cat# 562680), Alexa Fluor 700-anti-mouse MHCII (eBioscience, clone M5/114.15.2, Cat# 107621), APC-eFluor780-anti-mouse CD3e (eBioscience, clone 145-2C11, Cat# 47-0031-80), eFluor450-anti-mouse CD19 (eBioscience, clone eBio1D3 (1D3), Cat# 48-0193-80), BV510-anti-mouse CD11c (BioLegend, clone N418, Cat# 117338), BV605-anti-mouse CD115 (BioLegend, clone AFS98, Cat# 135517), BV650-anti-mouse/human CD11b (BioLegend, clone M1/70, Cat# 101239), BV711-anti-mouse Ly6G (BioLegend, clone 1A8, Cat# 127643), BV785-anti-mouse Ly6C (BioLegend, clone HK1.4, Cat# 128041), PE-Cy7-anti-mouse FceR1 (eBioscience, clone 44986, Cat# 25-5898-82), and BUV395-anti-mouse CD45 (BD, clone 30-F11, Cat# 565967).
EPDM elastomer case for cryopreservation
The EPDM case was constructed from Aeroflex tubes (Aeroflex Co., ltd., Thailand), with thickness options of 6 mm and 10 mm. The main body of the case was formed by cutting the tubes to heights of ~125 mm for 6 mm thickness and 135 mm for 10 mm thickness. The lid was crafted from the same material of matching thickness, shaped into circular pieces with a diameter of 16 mm to fit the inner diameter of the tube. Once the lid was placed into the bottom of the main body, it was securely taped in place. Following the insertion of the blood collection tube into the main body, the top was sealed using the lid.
Cryopreservation
The blood of the mice (2 ml) was collected in a 7 ml blood collection tube (NIPRO, cat#: OP-EK0205). Without removing the cap, an equal volume of cell cryoprotective agent (CP-1® High Grade, KYOKUTO PHARMACEUTICAL INDUSTRIAL CO., cat#: 27207) was injected into the blood collection tube. At this point, a blood transfer device (BD Biosciences, cat#: 364880) is attached to the syringe containing the 2 ml of cryoprotective agent. After injecting the cell cryoprotective agent into the blood collection tube, the blood transfer device was removed from the tube while pressing and holding the plunger down. The blood collection tube was kept horizontally, and the blood was mixed with the cell cryoprotective agent by shaking the tube at indicated times along its long axis two times per second. Immediately after mixing, the mixture was placed into a freezing case or enveloped with paper towels (Kimtowel: NIPPON PAPER CRECIA CO., LTD., JAPAN), and frozen in a −80 °C freezer. For the small-scale cryopreservation, 500 μl of the CP-1 solution (12% hydroxyethyl starch and 10% DMSO in saline) or 20% DMSO was added and mixed with 500 μl of murine blood in a 1.5 ml centrifuge tube. The tube was enveloped in paper towels and frozen in a −80 °C freezer. For paper towel wrapping, a blood collection tube was wrapped in two paper towels and became ~4 cm in diameter after wrapping.
Measurement of freezing rate
CP-1 (2 ml) and the same amount of saline solution (finally 50% CP-1 solution) were filled in a 7 ml blood collection tube at room temperature, and stored at a −80 °C freezer (Thermo Fisher Scientific Inc, MA) for around 4 h. The temperature of the solution in blood collection tubes was measured at 5-s intervals with Sheathed Thermocouples (CHINO CORPORATION, Tokyo, JAPAN) and a data logger (MadgeTech, Inc., NH), under 4 conditions: the tube was placed in an EPDM elastomer case (6 mm or 10 mm thickness: Aeroflex Co., ltd., Thailand), the tube wrapped in paper towels (Kimtowel: NIPPON PAPER CRECIA CO., LTD., JAPAN), and the tube without any case or wrap.
Recovery of cryopreserved cells
Frozen samples were thawed quickly by warming them in a 37 °C water bath, with mixing every 30 s throughout the defrosting process. Two minutes after thawing began, half of the frozen sample started to defrost, at which point inversion mixing was repeated for 10 s. If the mixture was not sufficiently defrosted, an additional 5 s of mixing was provided to ensure thorough defrosting. Prior to complete defrosting, the blood sample was stored on ice. After defrosting, the blood sample was diluted with 40 mL of RPMI 1640 medium containing L-Glutamine and 5% FBS. Cell counts were performed after washing with a 2% FBS medium.
Measurement of cell viability
Following the washing procedure, the cell viability of the cryopreserved cells is determined using the Acridine Orange (AO)/Propidium lodide (PI) Stain method. This staining process results in live cells exhibiting green fluorescence due to AO, while dead cells fluoresce red with AO/PI. Subsequently, cell counts and the cell viability are calculated using the automated fluorescence cell counter (LUNATM, Cat#: L20001) and Cell counting slides (LUNATM, Cat#: L12001).
Flow cytometric analysis
PBMCs were suspended in FACS buffer (2% fetal bovine serum; FBS in phosphate-buffered saline; PBS) containing 1 nM EDTA, and stained with antibodies in FACS buffer. 4’,6-diamidino-2-phenylindole (DAPI) was added to exclude dead cells. Data were acquired by using FACSymphony™ S6 Cell Sorter (BD Biosciences) and analyzed with FlowJo10. PBMC data were served for machine learning. In the PBMC dataset, live CD45+ cell data were extracted using FlowJo V10.6.2 (Treestar) and the subpopulation generation method implemented in flowkit36. The fluorescence intensity value of each stained antibody is obtained as a feature value because the measured data are flow cytometry data. Therefore, the feature values were normalized, respectively, using the StandardScaler package implemented in sklearn. The dead cell marker and the features FSC-H/W and SSC-H/W were excluded from the obtained fluorescence intensities. Dimensional reduction was performed using UMAP27. Among the hyperparameters, n_neighbors (5, 25, 45, 65) and min_dist (0.1, 0.4, 0.7, 1.0) were tested under the 4 × 4 conditions and determined to be n_neighbors = 25 and min_dist = 0.4. K-means implemented in sklearn was used as the clustering method28, and the clustering was divided into ten populations based on the elbow method.
Statistical analysis
A combination of one-way ANOVA and Tukey’s test or two-tailed Student’s t-test was performed for statistical analysis. Sample size was not determined by statistical methods but was determined based on common practice to reach statistical significance.
Supplementary information
Acknowledgements
This work is supported by JAXA feasibility study (T.A.) and CREST from the Japan Science and Technology Agency (JPMJCR2011) (T.A.). Grants-in-Aid for Scientific Research from JSPS (17H04038, 17K08622, 20K07332, and 20H03441 to T.A. and N.A.).
Author contributions
Hiroto Ishii: Data curation, formal analysis, investigation, validation, writing; Rin Endo: Data curation, formal analysis, investigation, validation, writing—review and editing; Sanae Hamanaka: BMX investigation and development, supervision, writing; Nobuyuki Hidaka: BMX investigation and development; Maki Miyauchi: Investigation; Naho Hagiwara: Investigation; Takahisa Miyao: Supervision, investigation; Tohru Yamamori: Supervision, validation, project administration; Tatsuya Aiba: BMX investigation and development, supervision; Nobuko Akiyama: Supervision, investigation, writing—review and editing; Taishin Akiyama: Funding acquisition, investigation, project administration, supervision, validation, writing.
Data availability
Source data are provided in this paper and as Supplementary Data. Other source data such as FCS files and R scripts have been deposited to GitHub depository, https://github.com/HirotoI/cryopreserved_pbmc_FCM.git.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hiroto Ishii, Rin Endo, Sanae Hamanaka, Nobuyuki Hidaka.
Supplementary information
The online version contains supplementary material available at 10.1038/s41526-024-00423-2.
References
- 1.Afshinnekoo, E. et al. Fundamental biological features of spaceflight: advancing the field to enable deep-space exploration. Cell183, 1162–1184 (2020). 10.1016/j.cell.2020.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demontis, G. C. et al. Human pathophysiological adaptations to the space environment. Front Physiol.8, 547 (2017). 10.3389/fphys.2017.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama, T. et al. How does spaceflight affect the acquired immune system? NPJ Microgravity6, 14 (2020). 10.1038/s41526-020-0104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crucian, B. et al. Terrestrial stress analogs for spaceflight associated immune system dysregulation. Brain Behav. Immun.39, 23–32 (2014). 10.1016/j.bbi.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 5.Mehta, S. K. et al. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity3, 11 (2017). 10.1038/s41526-017-0015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crucian, B. et al. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity1, 15013 (2015). 10.1038/npjmgrav.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stowe, R. P. et al. Leukocyte subsets and neutrophil function after short-term spaceflight. J. Leukoc. Biol.65, 179–186 (1999). 10.1002/jlb.65.2.179 [DOI] [PubMed] [Google Scholar]
- 8.Konstantinova, I. V. et al. Natural killer cells after ALTAIR mission. Acta Astronaut.36, 713–718 (1995). 10.1016/0094-5765(95)00161-1 [DOI] [PubMed] [Google Scholar]
- 9.Kaur, I., Simons, E. R., Castro, V. A., Mark Ott, C. & Pierson, D. L. Changes in neutrophil functions in astronauts. Brain Behav. Immun.18, 443–450 (2004). 10.1016/j.bbi.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 10.Crucian, B. E., Cubbage, M. L. & Sams, C. F. Altered cytokine production by specific human peripheral blood cell subsets immediately following space flight. J. Interferon Cytokine Res.20, 547–556 (2000). 10.1089/10799900050044741 [DOI] [PubMed] [Google Scholar]
- 11.Papalexi, E. & Satija, R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol.18, 35–45 (2018). 10.1038/nri.2017.76 [DOI] [PubMed] [Google Scholar]
- 12.Garrett-Bakelman, F. E. et al. The NASA Twins Study: a multidimensional analysis of a year-long human spaceflight. Science. 10.1126/science.aau8650 (2019). [DOI] [PMC free article] [PubMed]
- 13.Gertz, M. L. et al. Multi-omic, single-cell, and biochemical profiles of astronauts guide pharmacological strategies for returning to gravity. Cell Rep.33, 108429 (2020). 10.1016/j.celrep.2020.108429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overbey, E. G. et al. Challenges and considerations for single-cell and spatially resolved transcriptomics sample collection during spaceflight. Cell Rep. Methods2, 100325 (2022). 10.1016/j.crmeth.2022.100325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos, T. V., Mathew, A. J., Thompson, M. L. & Ehrhardt, R. O. Standardized cryopreservation of human primary cells. Curr. Protoc. Cell Biol.64, 1–8 (2014). 10.1002/0471143030.cba03is64 [DOI] [PubMed] [Google Scholar]
- 16.Pegg, D. E. Principles of cryopreservation. Methods Mol. Biol.368, 39–57 (2007). 10.1007/978-1-59745-362-2_3 [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama, W. M., Thompson, M. L. & Ehrhardt, R. O. Cryopreservation and thawing of cells. Curr. Protoc. Immunol. 10.1002/0471142735.ima03gs99 (2012). [DOI] [PubMed]
- 18.Rizzardi, L. F. et al. Evaluation of techniques for performing cellular isolation and preservation during microgravity conditions. NPJ Microgravity2, 16025 (2016). 10.1038/npjmgrav.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satpathy, S. et al. The Simple prEservatioN of Single cElls method for cryopreservation enables the generation of single-cell immune profiles from whole blood. Front Immunol.14, 1271800 (2023). 10.3389/fimmu.2023.1271800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl Acad. Sci. USA110, 2946–2951 (2013). 10.1073/pnas.1222738110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whaley, D. et al. Cryopreservation: an overview of principles and cell-specific considerations. Cell Transpl.30, 963689721999617 (2021). 10.1177/0963689721999617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stiff, P. J., Murgo, A. J., Zaroulis, C. G., DeRisi, M. F. & Clarkson, B. D. Unfractionated human marrow cell cryopreservation using dimethylsulfoxide and hydroxyethyl starch. Cryobiology20, 17–24 (1983). 10.1016/0011-2240(83)90054-8 [DOI] [PubMed] [Google Scholar]
- 23.Makino, S. et al. A simplified method for cryopreservation of peripheral blood stem cells at −80 degrees C without rate-controlled freezing. Bone Marrow Transpl.8, 239–244 (1991). [PubMed] [Google Scholar]
- 24.Katayama, Y. et al. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transpl.19, 283–287 (1997). 10.1038/sj.bmt.1700644 [DOI] [PubMed] [Google Scholar]
- 25.Arshad, N., Qasim, G. & Beagan, A. M. Investigations on the morphological, mechanical, ablative, physical, thermal, and electrical properties of EPDM-based composites for the exploration of enhanced thermal insulation potential. Polymers. 10.3390/polym14050863. (2022). [DOI] [PMC free article] [PubMed]
- 26.Liu, Z., Gu, Y., Shin, A., Zhang, S. & Ginhoux, F. Analysis of myeloid cells in mouse tissues with flow cytometry. STAR Protoc.1, 100029 (2020). 10.1016/j.xpro.2020.100029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 10.1038/nbt.4314. (2018). [DOI] [PubMed]
- 28.Murphy, R. F. Automated identification of subpopulations in flow cytometric list mode data using cluster analysis. Cytometry6, 302–309 (1985). 10.1002/cyto.990060405 [DOI] [PubMed] [Google Scholar]
- 29.Lugner, M. et al. Comparison between data-driven clusters and models based on clinical features to predict outcomes in type 2 diabetes: nationwide observational study. Diabetologia64, 1973–1981 (2021). 10.1007/s00125-021-05485-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiossone, L. et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood113, 5488–5496 (2009). 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- 31.Narasimhan, P. B., Marcovecchio, P., Hamers, A. A. J. & Hedrick, C. C. Nonclassical monocytes in health and disease. Annu. Rev. Immunol.37, 439–456 (2019). 10.1146/annurev-immunol-042617-053119 [DOI] [PubMed] [Google Scholar]
- 32.Jungheinrich, C. & Neff, T. A. Pharmacokinetics of hydroxyethyl starch. Clin. Pharmacokinet.44, 681–699 (2005). 10.2165/00003088-200544070-00002 [DOI] [PubMed] [Google Scholar]
- 33.Sarkar, S., Kalia, V. & Montelaro, R. C. Caspase-mediated apoptosis and cell death of rhesus macaque CD4+ T-cells due to cryopreservation of peripheral blood mononuclear cells can be rescued by cytokine treatment after thawing. Cryobiology47, 44–58 (2003). 10.1016/S0011-2240(03)00068-3 [DOI] [PubMed] [Google Scholar]
- 34.Owen, R. E. et al. Loss of T cell responses following long-term cryopreservation. J. Immunol. Methods326, 93–115 (2007). 10.1016/j.jim.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol.172, 2731–2738 (2004). 10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- 36.White, S. et al. FlowKit: a python toolkit for integrated manual and automated cytometry analysis workflows. Front Immunol.12, 768541 (2021). 10.3389/fimmu.2021.768541 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided in this paper and as Supplementary Data. Other source data such as FCS files and R scripts have been deposited to GitHub depository, https://github.com/HirotoI/cryopreserved_pbmc_FCM.git.