Abstract
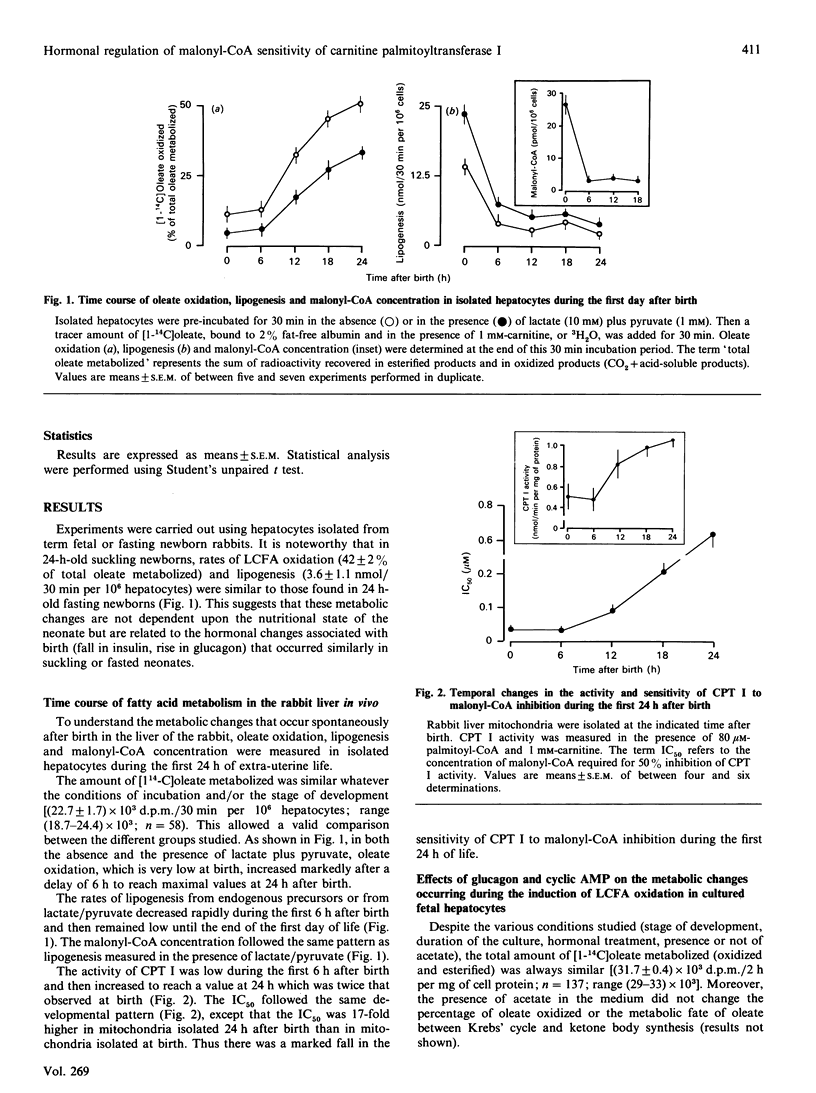
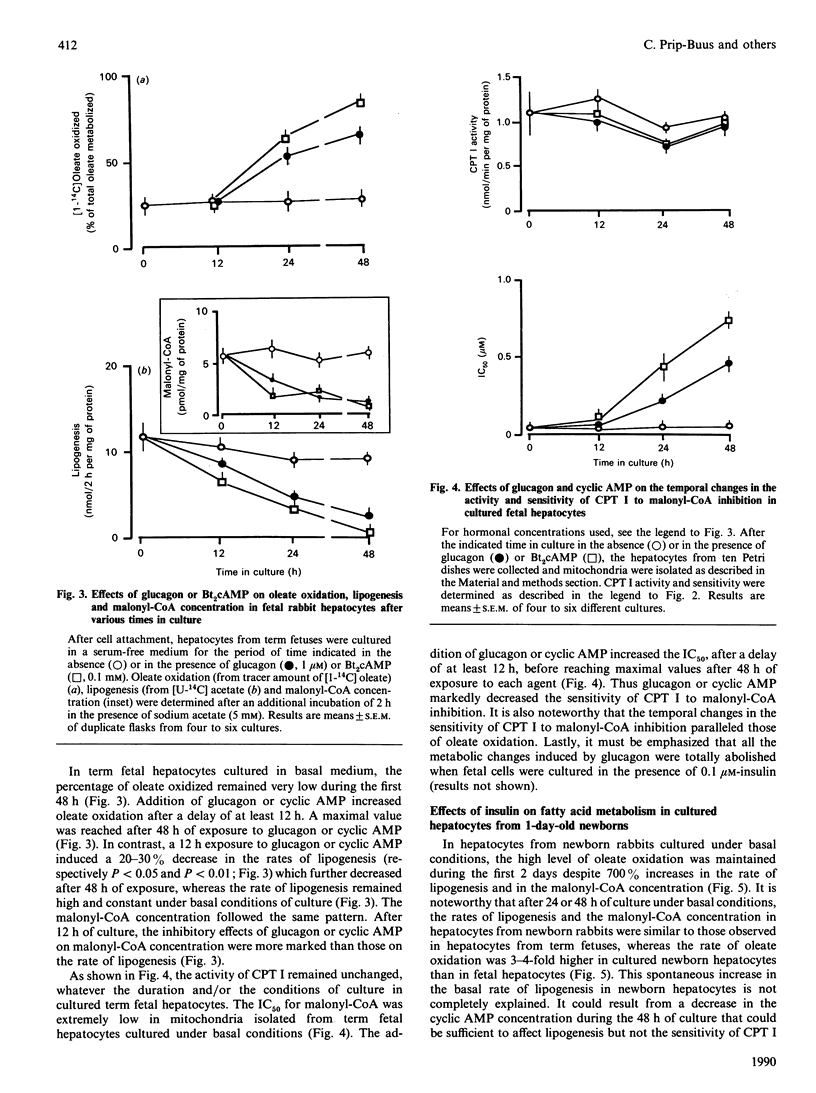
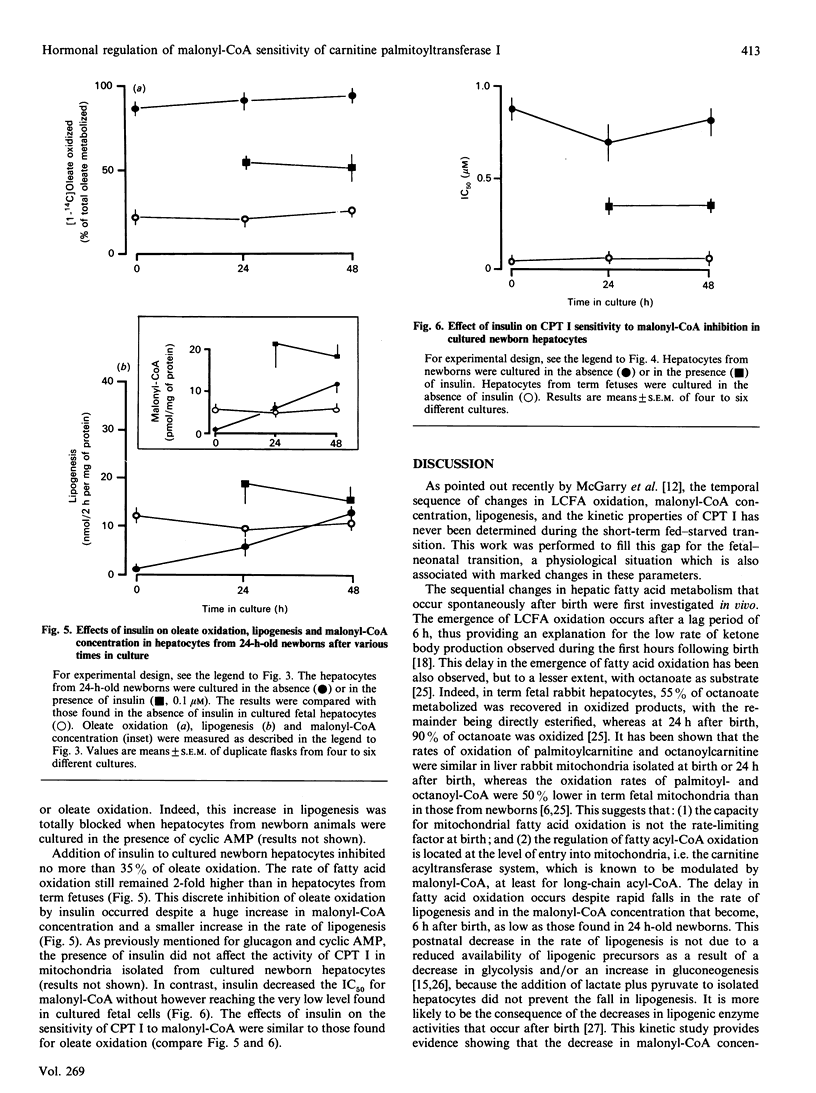
The temporal changes in oleate oxidation, lipogenesis, malonyl-CoA concentration and sensitivity of carnitine palmitoyltransferase I (CPT 1) to malonyl-CoA inhibition were studied in isolated rabbit hepatocytes and mitochondria as a function of time after birth of the animal or time in culture after exposure to glucagon, cyclic AMP or insulin. (1) Oleate oxidation was very low during the first 6 h after birth, whereas lipogenesis rate and malonyl-CoA concentration decreased rapidly during this period to reach levels as low as those found in 24-h-old newborns that show active oleate oxidation. (2) The changes in the activity of CPT I and the IC50 (concn. causing 50% inhibition) for malonyl-CoA paralleled those of oleate oxidation. (3) In cultured fetal hepatocytes, the addition of glucagon or cyclic AMP reproduced the changes that occur spontaneously after birth. A 12 h exposure to glucagon or cyclic AMP was sufficient to inhibit lipogenesis totally and to cause a decrease in malonyl-CoA concentration, but a 24 h exposure was required to induce oleate oxidation. (4) The induction of oleate oxidation by glucagon or cyclic AMP is triggered by the fall in the malonyl-CoA sensitivity of CPT I. (5) In cultured hepatocytes from 24 h-old newborns, the addition of insulin inhibits no more than 30% of the high oleate oxidation, whereas it stimulates lipogenesis and increases malonyl-CoA concentration by 4-fold more than in fetal cells (no oleate oxidation). This poor effect of insulin on oleate oxidation seems to be due to the inability of the hormone to increase the sensitivity of CPT I sufficiently. Altogether, these results suggest that the malonyl-CoA sensitivity of CPT I is the major site of regulation during the induction of fatty acid oxidation in the fetal rabbit liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boon M. R., Zammit V. A. Use of a selectively permeabilized isolated rat hepatocyte preparation to study changes in the properties of overt carnitine palmitoyltransferase activity in situ. Biochem J. 1988 Feb 1;249(3):645–652. doi: 10.1042/bj2490645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Silverstein L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner membrane properties and carnitine palmitoyltransferase A and B. Effect of diabetes and starvation. Biochem J. 1985 Dec 1;232(2):445–450. doi: 10.1042/bj2320445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Gamble M. S. Regulation of carnitine palmitoyltransferase by insulin results in decreased activity and decreased apparent Ki values for malonyl-CoA. J Biol Chem. 1987 Feb 15;262(5):2050–2055. [PubMed] [Google Scholar]
- Cook G. A., King M. T., Veech R. L. Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. J Biol Chem. 1978 Apr 25;253(8):2529–2531. [PubMed] [Google Scholar]
- Cook G. A., Nielsen R. C., Hawkins R. A., Mehlman M. A., Lakshmanan M. R., Veech R. L. Effect of glucagon on hepatic malonyl coenzyme A concentration and on lipid synthesis. J Biol Chem. 1977 Jun 25;252(12):4421–4424. [PubMed] [Google Scholar]
- Decaux J. F., Ferré P., Robin D., Robin P., Girard J. Decreased hepatic fatty acid oxidation at weaning in the rat is not linked to a variation of malonyl-CoA concentration. J Biol Chem. 1988 Mar 5;263(7):3284–3289. [PubMed] [Google Scholar]
- Duee P. H., Pegorier J. P., el Manoubi L., Herbin C., Kohl C., Girard J. Hepatic triglyceride hydrolysis and development of ketogenesis in rabbits. Am J Physiol. 1985 Nov;249(5 Pt 1):E478–E484. doi: 10.1152/ajpendo.1985.249.5.E478. [DOI] [PubMed] [Google Scholar]
- Duée P. H., Pégorier J. P., el Manoubi L., Ferré P., Bois-Joyeux B., Girard J. Development of gluconeogenesis from different substrates in newborn rabbit hepatocytes. J Dev Physiol. 1986 Oct;8(5):387–394. [PubMed] [Google Scholar]
- El Manoubi L., Callikan S., Duee P. H., Ferre P., Girard J. Development of gluconeogenesis in isolated hepatocytes from the rabbit. Am J Physiol. 1983 Jan;244(1):E24–E30. doi: 10.1152/ajpendo.1983.244.1.E24. [DOI] [PubMed] [Google Scholar]
- Ferré P., Satabin P., Decaux J. F., Escriva F., Girard J. Development and regulation of ketogenesis in hepatocytes isolated from newborn rats. Biochem J. 1983 Sep 15;214(3):937–942. doi: 10.1042/bj2140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J., Duée P. H., Ferré P., Pégorier J. P., Escriva F., Decaux J. F. Fatty acid oxidation and ketogenesis during development. Reprod Nutr Dev. 1985;25(1B):303–319. doi: 10.1051/rnd:19850221. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán M., Geelen M. J. Short-term regulation of carnitine palmitoyltransferase activity in isolated rat hepatocytes. Biochem Biophys Res Commun. 1988 Mar 15;151(2):781–787. doi: 10.1016/s0006-291x(88)80349-8. [DOI] [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Herbin C., Duée P. H., Pégorier J. P., Bladé C., Kohl C., Girard J. Premature appearance of gluconeogenesis and fatty acid oxidation in the liver of the postterm rabbit fetus. Pediatr Res. 1988 Feb;23(2):224–228. doi: 10.1203/00006450-198802000-00019. [DOI] [PubMed] [Google Scholar]
- Herbin C., Pegorier J. P., Duee P. H., Kohl C., Girard J. Regulation of fatty acid oxidation in isolated hepatocytes and liver mitochondria from newborn rabbits. Eur J Biochem. 1987 May 15;165(1):201–207. doi: 10.1111/j.1432-1033.1987.tb11212.x. [DOI] [PubMed] [Google Scholar]
- Iliffe J., Knight B. L., Myant N. B. Fatty acid synthesis in the brown fat and liver of foetal and newborn rabbits. Biochem J. 1973 May;134(1):341–343. doi: 10.1042/bj1340341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund H., Woldegiorgis G. Carnitine palmitoyltransferase: separation of enzyme activity and malonyl-CoA binding in rat liver mitochondria. Biochim Biophys Acta. 1986 Sep 12;878(2):243–249. doi: 10.1016/0005-2760(86)90152-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Goodman J., Houk J. M., Anderson S. Studies on the biochemistry of mitochondria and cell morphology in the neonatal swine hepatocyte. J Cell Biol. 1972 May;53(2):335–347. doi: 10.1083/jcb.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñas M., Benito M. Regulation of carnitine palmitoyltransferase activity in the liver and brown adipose tissue in the newborn rat: effect of starvation and hypothermia. Biochem Biophys Res Commun. 1986 Mar 13;135(2):589–596. doi: 10.1016/0006-291x(86)90034-3. [DOI] [PubMed] [Google Scholar]
- Pirola B. A., Borthwick I. A., Srivastava G., May B. K., Elliott W. H. Effect of lead ions on chick-embryo liver mitochondrial delta-aminolaevulinate synthase. Biochem J. 1984 Sep 15;222(3):627–630. doi: 10.1042/bj2220627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pégorier J. P., Duée P. H., Clouet P., Kohl C., Herbin C., Girard J. Octanoate metabolism in isolated hepatocytes and mitochondria from fetal, newborn and adult rabbit. Evidence for a high capacity for octanoate esterification in term fetal liver. Eur J Biochem. 1989 Oct 1;184(3):681–686. doi: 10.1111/j.1432-1033.1989.tb15067.x. [DOI] [PubMed] [Google Scholar]
- Pégorier J. P., Duée P. H., Herbin C., Laulan P. Y., Bladé C., Peret J., Girard J. Fatty acid metabolism in hepatocytes isolated from rats adapted to high-fat diets containing long- or medium-chain triacylglycerols. Biochem J. 1988 Feb 1;249(3):801–806. doi: 10.1042/bj2490801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pégorier J. P., Garcia-Garcia M. V., Prip-Buus C., Duée P. H., Kohl C., Girard J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem J. 1989 Nov 15;264(1):93–100. doi: 10.1042/bj2640093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. A procedure for the rapid preparation of mitochondria from rat liver. Biochem J. 1982 Jun 15;204(3):731–735. doi: 10.1042/bj2040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Bird M. I., Carpenter C. A., Winter K. A., Wright J. J. Cycloheximide blocks changes in rat liver carnitine palmitoyltransferase 1 activity in starvation. Biochem J. 1984 Nov 15;224(1):201–206. doi: 10.1042/bj2240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Regulation of hepatic carnitine palmitoyltransferase activity during the foetal-neonatal transition. FEBS Lett. 1982 Dec 13;150(1):177–180. doi: 10.1016/0014-5793(82)81329-x. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. W., Harris R. A. Effect of starvation and diabetes on the sensitivity of carnitine palmitoyltransferase I to inhibition by 4-hydroxyphenylglyoxylate. Biochem J. 1987 Apr 15;243(2):405–412. doi: 10.1042/bj2430405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops J. K., Ross P., Arslanian M. J., Aune K. C., Wakil S. J., Oliver R. M. Physicochemical studies of the rat liver and adipose fatty acid synthetases. J Biol Chem. 1979 Aug 10;254(15):7418–7426. [PubMed] [Google Scholar]
- Zammit V. A. Carnitine acyltransferases in the physiological setting: the liver. Biochem Soc Trans. 1986 Aug;14(4):676–679. doi: 10.1042/bst0140676. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Altered release of carnitine palmitoyltransferase activity by digitonin from liver mitochondria of rats in different physiological states. Biochem J. 1985 Sep 1;230(2):389–394. doi: 10.1042/bj2300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Kolodziej M. P. Target size analysis by radiation inactivation of carnitine palmitoyltransferase activity and malonyl-CoA binding in outer membranes from rat liver mitochondria. Biochem J. 1989 Oct 1;263(1):89–95. doi: 10.1042/bj2630089. [DOI] [PMC free article] [PubMed] [Google Scholar]


