Abstract
The role of serotonin in human behaviour is informed by approaches which allow in vivo modification of synaptic serotonin. However, characterising the effects of increased serotonin signalling in human models of behaviour is challenging given the limitations of available experimental probes, notably selective serotonin reuptake inhibitors. Here we use a now-accessible approach to directly increase synaptic serotonin in humans (a selective serotonin releasing agent) and examine its influence on domains of behaviour historically considered core functions of serotonin. Computational techniques, including reinforcement learning and drift diffusion modelling, explain participant behaviour at baseline and after week-long intervention. Reinforcement learning models reveal that increasing synaptic serotonin reduces sensitivity for outcomes in aversive contexts. Furthermore, increasing synaptic serotonin enhances behavioural inhibition, and shifts bias towards impulse control during exposure to aversive emotional probes. These effects are seen in the context of overall improvements in memory for neutral verbal information. Our findings highlight the direct effects of increasing synaptic serotonin on human behaviour, underlining its role in guiding decision-making within aversive and more neutral contexts, and offering implications for longstanding theories of central serotonin function.
Subject terms: Human behaviour, Neurotransmitters, Cognitive control, Computational neuroscience, Learning and memory
Serotonin is involved in aversive processing, but how serotonin shapes behavior remains unclear. Here, the authors show that directly enhancing synaptic serotonin in humans reduces outcome sensitivity and increases behavioral inhibition in aversive contexts.
Introduction
Understanding the function of central serotonin (or 5-hydroxytryptamine, 5-HT) has been a focal goal of neuroscience research for nearly a century1, not least because of its central role in the effects of many psychiatric drugs, predominantly selective serotonin reuptake inhibitors (SSRIs) and street drugs (e.g., ±3,4-methylenedioxymethamphetamine [MDMA] and lysergic acid diethylamide)2,3. Serotonin is phylogenetically ancient, and its function translates across species to many lower- and higher-level behaviours; from feeding and sexual functioning to goal-directed, flexible cognition4–7. Amongst these, behavioural inhibition, memory, and aversive processing are historically considered the core, specialised functions of serotonin8–11. This is underpinned by converging preclinical and human work involving in vivo manipulation of synaptic 5-HT, predominantly with SSRIs or depletion of its amino acid precursor tryptophan (TRP)7,12, and observing behavioural change. In humans, however, marked differences in the direction of behavioural effects are observed across similar experimental approaches. For example, several studies report seemingly contradictory effects of SSRIs on tasks of aversive and reward processing (reinforcement learning); specifically, different reports show that SSRIs increase reward sensitivity13, increase loss sensitivity and decrease reward sensitivity14, and decrease sensitivity to both reinforcement valences15. Inconsistent behavioural effects of SSRIs are also observed across other domains, including behavioural inhibition and memory processing12,16–22; in some cases, these behavioural changes align with those seen after tryptophan depletion (e.g., reduced cognitive flexibility) despite the expectation that they would have opposing effects on net synaptic 5-HT16,23.
Determining a causal link between increased synaptic 5-HT and behaviour in humans via SSRIs is difficult due to the complex effects of SSRIs on 5-HT and colocalised neurotransmitter systems. For example, negative signalling feedback along the serotonergic pathway following autoreceptor activation early in treatment can limit cell firing, and therefore 5-HT release, in a regionally-specific manner24–26. Furthermore, deactivation of 5-HT transporters results in 5-HT uptake via dopamine transporters, leading to subsequent co-release of dopamine and 5-HT27. The effect of increased dopaminergic content and signalling is seen in acute and subchronic SSRI administration28–32, observable in striatal, prefrontal, and hippocampal structures implicated in reward processing, behavioural inhibition and memory functioning27,33–35.
Given the complex molecular and behavioural profile of SSRIs, alternative probes which increase synaptic 5-HT may help further clarify the role of 5-HT in human behaviour and cognition. One such alternative involves the use of a selective serotonin releasing agent (SSRA) (Fig. 1): unlike SSRIs which increase 5-HT levels indirectly through prolonging synaptic 5-HT, SSRAs stimulate direct exocytic release of 5-HT, without broad monoaminergic efflux (as seen in non-selective 5-HT releasers, such as MDMA)36,37. While SSRIs require ongoing neural firing for vesicular release of 5-HT into the synapse, the SSRA mechanism is not firing-dependent and thus not negated by dorsal raphe autoreceptor negative feedback which delays the therapeutic onset of action of SSRIs38–40.
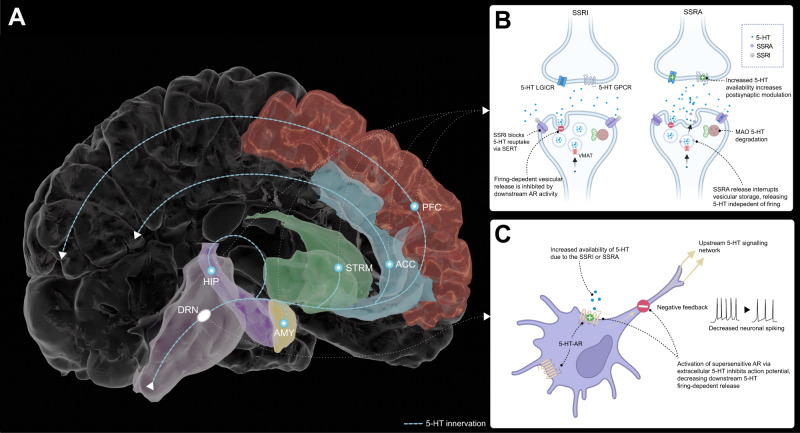
Fig. 1. Selective serotonin releasing agent is not negated by 5-HT1A supersensitivity, resulting in a rapid onset of pro-serotonergic activity.
A The majority of central serotonin (5-HT) innervation originates from the dorsal raphe nucleus (lilac), and is found within areas of the brain strongly implicated in mood regulation and cognitive function: amygdala (AMY; yellow), hippocampus (HIP; purple), striatal structures (STRM; green), anterior cingulate cortex (ACC; light blue) and the frontal lobe, including the prefrontal cortex (PFC; red). B Selective serotonin reuptake inhibitors (SSRI) and selective serotonin releasing agents (SSRA) both influence extracellular presynaptic serotonin concentrations, modulating downstream serotonergic activity, while the effects of SSRIs on synaptic 5-HT are delayed by autoreceptor hypersensitivity and may influence colocalised dopamine neurons. C 5-HT1a ARs are clustered in the dorsal raphe nucleus and are endogenously sensitive to extracellular serotonin, and upon activation produce a negative feedback loop which inhibits upstream firing-dependent serotonin release. The original atlas meshes in panel A are credited to A. M. Winkler (Brain For Blender), which have been modified for illustrative purposes, released under a Creative Commons Attribution ShareAlike 3.0 Unported license (https://creativecommons.org/licenses/by-sa/3.0/). Panels B and C were created with BioRender.com and released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en). AR autoreceptor; GPCR G protein-coupled receptor; LGICR Ligand-gated ion-channel receptors; MAO Monoamine oxidase, SERT serotonin transporter.
Until recently, it has been challenging to characterise the effects of SSRAs in humans because of the lack of available licensed pharmacological probes. However, in 2020, low dose fenfluramine (up to 26 mg daily; racemic mixture) was licensed for the treatment of Dravet epilepsy41. Preclinical work suggests low dose fenfluramine directly and rapidly increases synaptic 5-HT without modifying extracellular dopamine concentration in regions involved in mood regulation such as the striatum and hippocampus, in contrast to SSRIs40,42–53. Fenfluramine led to substantially greater extracellular 5-HT levels than the SSRI, fluoxetine, when administered at similar doses54. Further preclinical work suggests acute administration of fenfluramine increases synaptic 5-HT by 182-200% vs basal state53,55, while subchronic administration (4-5 days) retains increases in net 5-HT without influencing 5-HT terminal structural integrity53,56. In humans, acute and subchronic administration of d-fenfluramine decreases serotonergic radioligand binding of [18F]altanserin in a dose-dependent manner, suggestive of increased synaptic serotonin release in the brain57,58. With its recent relicensing for epilepsy syndromes41, fenfluramine provides an opportunity to probe the neurobehavioural effects of SSRAs in humans to answer outstanding questions about the role of synaptic 5-HT in human behaviour.
Here, we use this now-accessible approach to directly increase synaptic 5-HT in humans, examining its influence on domains of behaviour historically considered core functions of serotonin: aversive processing, behavioural inhibition, and memory. In line with our hypothesis that fenfluramine would result in a pattern of behaviour opposite to that seen with tryptophan depletion12,16,17,20,59–62, we show that increasing synaptic serotonin reduces reinforcement sensitivity for aversive outcomes; in addition, our findings indicate that increasing synaptic serotonin enhances behavioural inhibition and shifts bias towards impulse control during aversive interference. Finally, increasing synaptic serotonin increased memory for neutral verbal information.
Results
Does increased synaptic serotonin change reinforcement sensitivity for reward and loss?
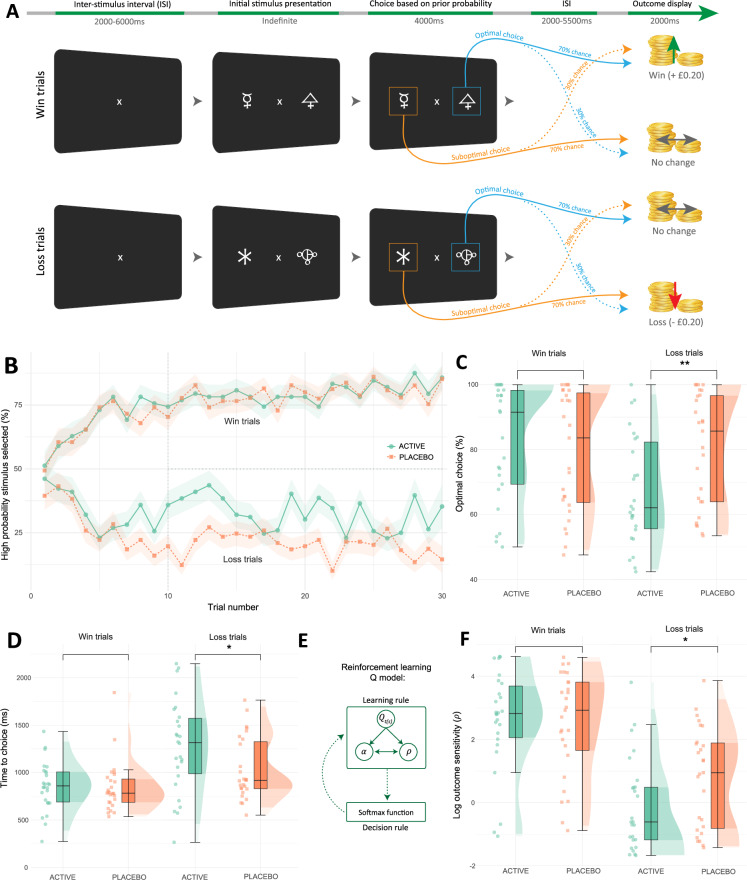
We investigated the effect of increased synaptic 5-HT (via fenfluramine) on reinforcement sensitivity for reward and loss outcomes during a probabilistic instrumental learning task described in Fig. 2A63,64. During this task, participants learned the probability of outcomes associated with symbols within pairs. Each pair represented a task condition: win trials (win money or no change) and loss trials (lose money or no change). Within a given task block, each trial type was associated with one pair of symbols and appeared 30 times per block (across three blocks). Optimal choices were made when selecting symbols which had a greater probability (70%) of leading to a favourable outcome (i.e., win in win trials and no change in loss trials). A computational reinforcement learning model was fit to participant choice behaviour to formalise the predicted change in optimal choices between allocation groups. Model parameters for each trial type were derived, providing a distinct explanation of learning and decision-making behaviour throughout the task: learning rate (), explaining the rate at which outcomes modify expectations, which was estimated separately for win and loss trials; and outcome sensitivity (), explaining the effective magnitude of experienced outcomes. Further information about the model, including parameter recovery and simulation procedures, are detailed within the Supplementary Methods.
Fig. 2. Task procedure, computational modelling, and analyses of the probabilistic instrumental learning task.
A The task consists of choosing a symbol within a pair, with two interleaved pairs per task block. Each pair represents a high probability of winning (win trials) or high probability of loss (loss trials). Win trials (30 per block) result in winning or no change, while loss trials (30 per block) result in loss or no change. Within pairs, symbols had fixed reciprocal probabilities (70%, 30%), with outcomes displayed at trial end. Participants were instructed to make choices for most likely maximal monetary gain (awarded at study completion). B Group learning rates across trial types (blocks averaged). High probability stimulus selected (Y axis): mean percentage of high probability win or loss choices. The shaded area around lines represents standard error (SE). C Decreased optimal choices in the fenfluramine (active) group during loss trials via estimated margin means (EMM) (reward trials EMM( ± SE) = 0.68 ± 3.18, p = 0.8307; loss trials EMM = −8.62 ± 3.18, p = 0.0078, Cohen’s d = −0.75 [95% CI −1.30, −0.19]). D Increased response time (ms; milliseconds) in the fenfluramine group during loss trials (reward trials EMM = 13.9 ± 95.6, p = 0.8845; loss trials EMM = 246.0 ± 95.6, p = 0.0115, d = 0.71 [0.15, 1.26]). E The Q computational model contains two primary parts: a learning rule and decision rule. The learning rule describes trial-by-trial updates of value expectation (), and choice probability is determined via the decision rule. Model parameters alter distinct aspects of the decision-making process: outcome sensitivity () and learning rate () (see Supplementary Methods for details)64. F Decreased outcome sensitivity () in the fenfluramine group during loss trials (reward trials EMM = 0.10 ± 0.43, p = 0.8203; loss trials EMM = −0.90 ± 0.43, p = 0.0392, d = −0.57 [−1.11, −0.03]). B–D, F include N = 53 individuals; boxplots represent interquartile range (IQR); central line depicts the median. Whiskers represent ±1.5 IQR, encompassing most data points; half-violin plots depict the data distribution. ** p ≤ 0.01, * p ≤ 0.05 indicate group differences by two-tailed EMM tests (Bonferroni-Holm corrected). ISI Inter-stimulus interval.
In line with our hypothesis, fenfluramine allocation reduced the number of optimal choices during loss trials (ANCOVA group x task condition: F[1,50] = 5.14, p = 0.03, ηp2 = 0.07 [95% CI 0.00, 0.24]; reward condition EMM = 0.68 ± 3.18, p = 0.83; loss condition EMM ± SE = −8.62 ± 3.18, p < 0.01, Cohen’s d = −0.75 [95% CI −1.30, −0.19]) (Fig. 2B-C). Consistent with this, learning models fit to the data revealed increased synaptic 5-HT reduced outcome sensitivity for loss trials (ANCOVA group x task condition: F[1,50] = 5.73, p = 0.02, ηp2 = 0.10 [0.00, 0.28]; reward condition EMM = 0.10 ± 0.43, p = 0.82; loss condition EMM = −0.90 ± 0.43, p = 0.04, d = −0.57 [−1.11, −0.03]) (Fig. 2D). In contrast, modelled learning rate for both conditions did not vary across groups in a statistically significant manner (ANCOVA group x task condition: F[1,50] = 1.22, p = 0.27; main effect of group: F[1,50] = 0.92, p = 0.34) (Supplementary Fig. 10). Similarly, fenfluramine allocation increased time to choice selection during loss conditions (ANCOVA group x task condition: F[1,50] = 5.52, p = 0.02, ηp2 = 0.11 [0.00, 0.29]; reward condition EMM = 13.9 ± 95.6, p = 0.89; loss condition EMM = 246.0 ± 95.6, p = 0.01, d = 0.71 [0.15, 1.26]) (Fig. 2F), which would also be consistent with a relative reduction in loss sensitivity in this group.
Overall, these findings demonstrate that net increases in synaptic 5-HT (via serotonin releasing agent fenfluramine) decrease reinforcement sensitivity to loss outcomes, opposite to the effect of 5-HT depletion (TRP) where loss sensitivity increases61,62. While alternative computational accounts for the observed behaviour could include increased value decay or choice stochasticity, there are no reports of 5-HT manipulation influencing these components of behaviour64.
Does elevated 5-HT modulate behavioural inhibition, choice impulsivity, and vulnerability to aversive emotional interference?
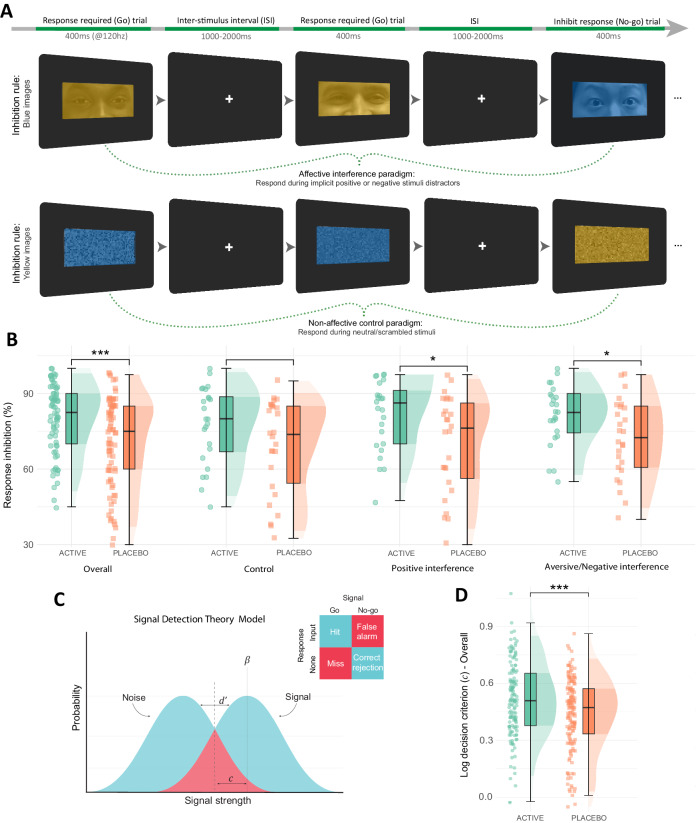
Next, we assessed the impact of increased synaptic 5-HT on response inhibition (an index of behavioural inhibition), choice impulsivity, and interference during the Affective Interference Go/No-Go task. In this task, participants respond (go) or withhold responses (no-go) according to rules which change over time (e.g., instructions: do not press the button if you see a blue/yellow image) while being exposed to emotional distractors (fearful or happy faces, or control images) (Fig. 3A). Fenfluramine allocation increased response inhibition, measured by mean percentage of accurately withheld responses to no-go trials (ANCOVA main effect of group: F[1,47] = 11.26, p < 0.01, ηp2 = 0.15 [0.00, 0.37]; all conditions EMM = 9.69 ± 2.63, p < 0.001, d = 0.60 [0.27, 0.93]) (Fig. 3B). Further, there was no statistically significant group effect for go trial accuracy (ANCOVA main effect of group: F[1,47] = 0.83, p = 0.37) (Supplementary Fig. 14B).
Fig. 3. Task procedure and accuracy analyses for the Affective Interference Go/No-Go task.
A An example of trial flow across two blocks from the affective interference go/no-go task, with one block depicting affective interference (above) and the other showing the non-affective (scrambled) control paradigm (below)143. The sequence of trials is left to right. The first two trials in each example illustrate go trials where participants respond with a key input (80% of trials); the third trial in the sequence illustrates a no-go trial where participants must withhold responses (20% of trials). There were six task blocks (two per task condition), with 80 trials within each block. Partial faces displayed in the top row are from the RADIATE stimulus set144,145. Models in each image consented to the photography and release of their photos for research purposes. B Higher response inhibition (mean %) performance was observed in the fenfluramine (active) group compared with the placebo group across all conditions via estimated marginal means tests (EMM) (overall EMM = 9.69 ± 2.63, p = 0.0003, d = 0.60 [0.27, 0.93]; control EMM = 8.58 ± 4.56, p = 0.0616; positive interference EMM = 11.25 ± 4.56, p = 0.0147, d = 0.69 [0.13, 1.26]; aversive interference EMM = 9.25 ± 4.56, p = 0.0442, d = 0.58 [0.01, 1.14]). C Application of signal detection theory indices to the go/no-go task, where correct and incorrect go/no-go responses are described on a sensory continuum of noise and signal (see further details in Supplementary Methods). D Fenfluramine allocation resulted in higher values for signal detection theory criterion index (indicative of more conservative/cautious decision-making) across all task conditions (EMM = 0.08 ± 0.02, p = 0.0007, d = 0.39 [0.16, 0.62]). B, D Include data for N = 50 individuals; boxplots represent the interquartile range (IQR), while the central line depicts the median. The whiskers extend to approximately ± 1.5 times the IQR, encompassing the bulk of the data points; half-violin plots depict the data distribution; ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 indicate group differences by two-tailed EMM tests (Bonferroni-Holm corrected). ISI Inter-stimulus interval.
Signal detection theory analyses was undertaken to determine if group differences in response inhibition were driven by perceptual decision-making (Fig. 3C). Fenfluramine allocation resulted in more cautious decision-making throughout (log criterion ; ANCOVA main effect of group: F[1,47] = 13.54, p < 0.001, ηp2 = 0.19 [0.02, 0.39]; all conditions EMM = 0.08 ± 0.02, p < 0.001, d = 0.39 [0.16, 0.62]) (Fig. 3D), but there was no statistically significant group effect on signal discriminability (see Supplementary Note 4).
There was a positive relationship between go trial response times and both response inhibition (r = 0.61, p < 0.001, two-tailed) and cautious decision-making (log decision criterion []; r = 0.77, p < 0.001, two-tailed), as shown in Supplementary Fig. 15. These findings represent an inherent speed-accuracy trade-off within the limited response window for the task (400 ms), where a favourable optimisation for task strategy is slowing response times to ensure greater response inhibition accuracy. As a result, faster response time for go trials may be considered an index of choice impulsivity. Indeed, it is argued that the tendency to react in a premature manner without adequate signal processing constitutes the fundamental aspects of impulsive behaviour65,66.
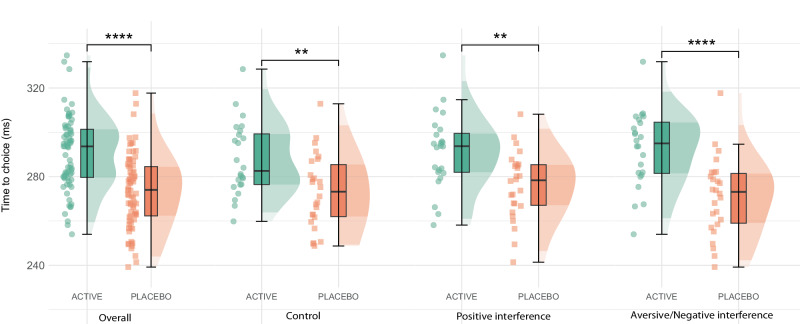
Increasing synaptic 5-HT (via fenfluramine) resulted in reductions in choice impulsivity, indicated by increased time to choice for go trials, across all task conditions (ANCOVA main effect of group: F[1,47] = 22.00, p < 0.001; ηp2 = 0.27 [0.07, 0.46]) (Fig. 4). Moreover, there was an interaction between group and task interference (happy, fearful or control distractors) on choice impulsivity (ANCOVA group x task condition: F[2,95] = 3.22, p < 0.05, ηp2 = 0.08 [0.00, 0.20]). Specifically, choice impulsivity was most reduced when aversive emotional distractors were present in the fenfluramine group (EMM = 21.3 ± 4.71, p < 0.0001, d = 1.28 [0.70, 1.86]) compared with both control (EMM = 14.6 ± 4.71, p < 0.01, d = 0.88 [0.31, 1.44]) and positive emotional distractors (EMM = 15.4 ± 4.71, p < 0.01, d = 0.93 [0.36, 1.50]). In a separate analysis of the fenfluramine group, there was a main effect for valence on choice impulsivity when isolating aversive vs control conditions (ANCOVA main effect: F[1,22] = 4.87, p = 0.04, ηp2 < 0.01 [0.00, 0.19]); however, there was no statistically significant main effect of valence in the placebo group (see Supplementary Table 8).
Fig. 4. Time to choice analysis for the Affective Go/No-Go Task.
General decreases in choice impulsivity (or, choice time for correct go trials) (ms; milliseconds) were observed in the fenfluramine (active) group via estimated marginal means tests (EMM) (overall EMM = 17.2 ± 2.72, p = 3.733661e-09, d = 1.03 [0.68, 1.37]; control EMM = 14.6 ± 4.71, p = 0.0024, d = 0.88 [0.31, 1.44]; positive interference EMM = 15.4 ± 4.71, p = 0.0013, d = 0.93 [0.36, 1.50]; aversive interference EMM = 21.3 ± 4.71, p = 1.31162e-5, d = 1.28 [0.70, 1.86]); this effect was most pronounced during aversive interference. Figure includes data for N = 50 individuals; boxplots represent the interquartile range (IQR), while the central line depicts the median. The whiskers extend to approximately ± 1.5 times the IQR, encompassing the bulk of the data points; half-violin plots depict the data distribution; **** p ≤ 0.0001, ** p ≤ 0.01 indicate group differences by two-tailed EMM tests (Bonferroni-Holm corrected).
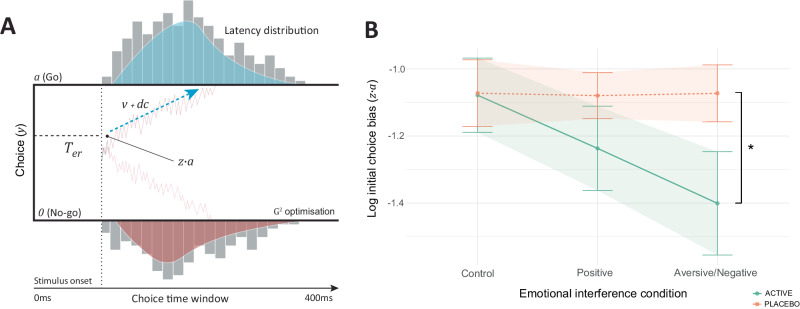
Computational drift diffusion modelling (Fig. 5A) was undertaken to investigate evidence accumulation patterns throughout the Affective Interference Go/No-Go task (for further model details, including recovery and simulation procedures, see Supplementary Methods). Fenfluramine allocation shifted initial choice bias () toward impulse control (no-go, lower boundary) during aversive interference only (ANCOVA group x task condition: F[2,95] = 3.45, p = 0.03, ηp2 = 0.06 [0.00, 0.17]; control condition EMM = −0.01 ± 0.15, p = 0.96; positive interference EMM = −0.16 ± 0.15, p = 0.31; aversive interference EMM = −0.33 ± 0.15, p = 0.03, d = −0.60 [−1.17, −0.04]) (Fig. 5B). There was no statistically significant group effect across other model parameters, including boundary separation (a) and drift rate (v) (see Supplementary Note 4). As 75% of task trials fit to the DDM were go trials, and there was no statistically significant group effect on accuracy for these trials, group differences in model parameters may not occur when accuracy is similar despite differences in choice time67.
Fig. 5. Computational drift diffusion modelling and choice bias during affective interference.
A A drift diffusion model (DDM) was fit to participant behaviour during the Affective Go/No-Go Task. The DDM provides distinct explanations for evidence accumulation before reaching a decision (i.e., Go or No-Go) during the task, explaining distinct parts of the decision-making process which contribute to task behaviour. The DDM describes decision-making using five parameters: 1) Boundary separation (), which describes the required quantity of evidence for making a decision. 2) Non-decision time () is the period between stimulus onset and the start of the evidence accumulation, where foremost sensory and perceptual processes occur, notably emotional facial expression encoding146. 3) Initial choice bias () represents bias toward one of the choice boundaries ( [Go] and 0 [No-go]) at the start of evidence accumulation. 4) Drift rate () describes the rate of evidence accumulation before arriving at a choice boundary. 5) Drift criterion () is a constant applied to the mean drift rate which is evidence independent. The model was fit to behaviour using the Gsquare (G2) approach which uses maximum likelihood estimation, where choice time distributions were divided into five quantiles: 10th, 30th, 50th, 70th and 90th147,148. Model-fitted synthetic data and observed task data were closely matched, and all model parameters were recoverable (for further details, please see Supplementary Methods). B During interference with aversive emotional information (fearful faces), fenfluramine (active) allocation resulted in an initial choice bias () toward the impulse control (no-go) choice boundary via estimated marginal means tests (EMM) (control condition EMM = −0.01 ± 0.15, p = 0.9692; positive interference EMM = −0.16 ± 0.15, p = 0.3093; aversive interference EMM = −0.33 ± 0.15, p = 0.0352, d = −0.60 [−1.17, −0.04]) (N = 50). This suggests group differences in go trial choice time observed during the task, specifically during aversive interference, were driven by a bias toward the no-go boundary in the fenfluramine group. B Includes data for N = 50 individuals; lines and plot points depict mean value, with error bars and shaded areas around each line depicting standard mean error; * p ≤ 0.05 indicates group difference by two-tailed EMM tests (Bonferroni-Holm corrected).
Taken together, these findings suggest that increasing synaptic 5-HT enhances behavioural inhibition across emotional and more neutral contexts, an effect which was driven by more cautious decision-making. Moreover, increased 5-HT levels appear to shift bias towards impulse control during aversive affective interference at the start of evidence accumulation, consequentially lowering choice impulsivity.
Assessing the influence of increased synaptic serotonin on memory processing
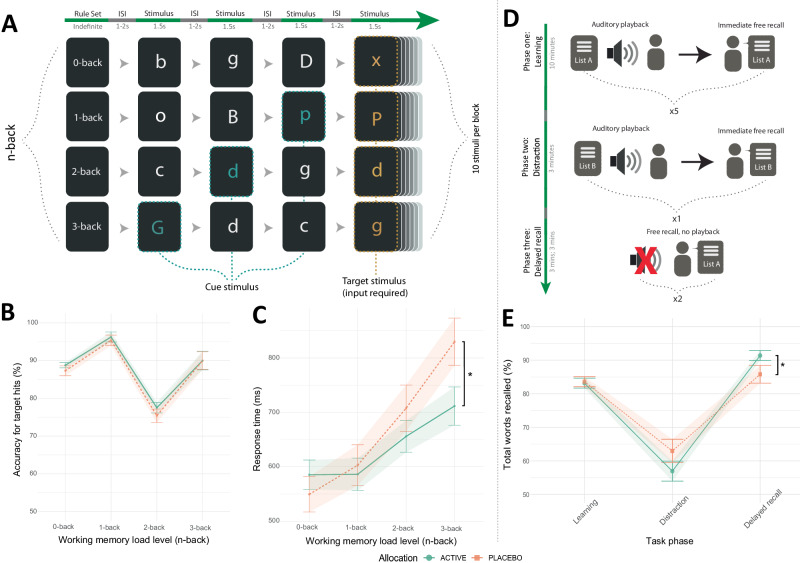
Finally, we assessed the influence of fenfluramine administration on memory function. During a task of complex verbal working memory processing (Verbal n-back; Fig. 6A), participants were required to recall if a target letter occurred within a pre-specified sequential pattern (i.e., 0-, 1-, 2-, or 3-back letters ago). There was no statistically significant group effect for total number of correctly recalled targets (ANCOVA group analysis: F[1,47] = 0.70, p = 0.41) (Fig. 6B). However, during the highest task difficulty (3-back), fenfluramine allocation resulted in faster recall for correct targets (ANCOVA group x task condition: F[3,143] = 3.66, p = 0.01, ηp2 = 0.05 [0.00, 0.13]; 0-back EMM: 35.9 ± 50.20, p = 0.48; 1-back EMM: −16.7 ± 50.20, p = 0.74; 2-back EMM: −51.8 ± 50.20, p = 0.30; 3-back EMM: −118.20 ± 50.20, p = 0.02, d = −0.67 [−1.23, −0.10]) (Fig. 6C).
Fig. 6. Effects of the serotonin releasing agent fenfluramine across tasks of memory function (n-Back and Auditory Verbal Learning Task).
A Verbal n-back task example task flow for all four task conditions (top to bottom: 0-back, 1-back, 2-back and 3-back). The sequence of trials is left to right. Before each block of 10 stimuli, participants were given a rule for targets (e.g., instructions: press spacebar if you see the same letter that appeared two letters ago [2-back]). Each condition was repeated four times (16 blocks total). B No statistically significant difference in target accuracy was observed across groups (Supplementary Note 6). C Reduced response time (ms; milliseconds) for correct choices in the fenfluramine (active) group at highest n-back memory load via estimated marginal means tests (EMM) (0-back EMM: 35.9 ± 50.20, p = 0.4760; 1-back EMM: −16.7 ± 50.20, p = 0.7395; 2-back EMM: −51.8 ± 50.20, p = 0.3035; 3-back EMM: −118.20 ± 50.20, p = 0.0196, d = −0.67 [−1.23, −0.10]. D Auditory Verbal Learning Task flow across three task phases: phase one (learning/encoding), phase two (distraction), and phase three (delayed recall). During phase one, participants listened to a recording of 15 verbal items (List A) at a slowed pace (1 s interval between words), followed by an immediate free recall of list items. After this occurred five times, phase two (distraction) required learning a novel list of items for immediate recall (List B). Phase three (delayed recall) required free recall (without list playback) of items from List A immediately after phase two and then fifteen minutes later. E The fenfluramine group showed increased accuracy during the delayed recall phase of the Auditory Verbal Learning Task relative to placebo (learning EMM = −0.07 ± 0.14, p = 0.6378; distraction recall EMM = −0.90 ± 0.70, p = 0.1961); delayed recall EMM = 0.84 ± 0.35, p = 0.0165, d = 0.34 [0.06, 0.61]). B, C contain data for N = 50 individuals, while (E) contains data for N = 51 individuals; lines and plot points depict mean value, with error bars and shaded areas around each line depicting standard mean error; *p ≤ 0.05 indicate group differences by two-tailed EMM tests (Bonferroni-Holm corrected). ISI Inter-stimulus interval.
During a task of long-term memory encoding and retrieval (Auditory Verbal Learning Task; Fig. 6D), participants were required to learn a list of 15 verbal items and correctly recall these items during learning (immediate recall) and after a short period (delayed recall). Fenfluramine allocation resulted in higher total accuracy during delayed recall, while there was no statistically significant group effect for learning trials (ANCOVA group x task condition: F[2,1474] = 6.23, p = 0.01, ηp2 < 0.01 [0.00, 0.02]; delayed recall EMM = 0.84 ± 0.35, p = 0.02, d = 0.34 [0.06, 0.61]; learning EMM = −0.07 ± 0.14, p = 0.63; distraction recall EMM = −0.90 ± 0.70, p = 0.20) (Fig. 6E). There was no statistically significant group effect for word repetitions or intrusions (Supplementary Fig. 19).
There was no statistically significant group effect for performance on tasks of visuo-spatial working memory (Oxford Memory Task) and implicit visual learning (Contextual Cueing Task) (see Supplementary Note 7−8, Supplementary Table 4 and Supplementary Figs. 22, 24).
Taken as a whole, these findings suggest increasing synaptic 5-HT enhances memory processing for verbal information.
Relationship of elevated 5-HT with self-report questionnaire measures, gender-related covariance, and cortisol concentration
There was no statistically significant group effect on self-report ratings of subjective cognition, side effects, motivation, and affect (Tables 1–2; Supplementary Table 3). At study completion, the placebo group (70%) were better than chance at correctly guessing their allocation compared with the fenfluramine group (50%), however this was not a statistically significant difference (χ² = 3.92, p = 0.27). Gender did not covary with the effects of fenfluramine administration on task behaviour reported in previous sections (Supplementary Table 5). There was no statistically significant difference between groups in salivary cortisol during the initial dosing period, suggesting a lack of acute modulation of hypothalamic-pituitary-adrenal (HPA) axis function from fenfluramine.
Table 1.
Subjective outcome measures of cognition, affect, and mood across allocation groups – post-intervention descriptive statistics and inferential analysis
| Fenfluramine (n = 26) M (S.D.) |
Placebo (n = 27) M (S.D.) |
Inferential analysis a | ||
|---|---|---|---|---|
| F-statistic [df] | p | |||
| Cognition | ||||
| Perceived Deficits Questionnaire | ||||
| Baseline | 10.27 (11.80) | 15.12 (13.60) | -- | -- |
| Follow-up | 13.46 (13.49) | 13.35 (12.99) | 0.01 [1,37] | 0.781 |
| Affect | ||||
| Positive and Negative Affect Schedule | ||||
| Negative items, baseline | 10.86 (1.28) | 12.00 (1.81) | -- | -- |
| Negative items, follow-up | 10.86 (1.24) | 11.61 (1.97) | 5.00 [1,38] | 0.0313 b |
| Positive items, baseline | 30.09 (6.37) | 28.17 (7.76) | -- | -- |
| Positive items, follow-up | 27.62 (6.74) | 26.22 (7.90) | 0.277 [1,38] | 0.602 |
| Visual Analogue Scale | ||||
| Negative items, baseline | 148.92 (87.76) | 171.55 (68.73) | -- | -- |
| Negative items, follow-up | 152.12 (86.90) | 155.62 (79.94) | 0.29 [1,46] | 0.593 |
| Positive items, baseline | 736.13 (110.33) | 664.38 (83.23) | -- | -- |
| Positive items, follow-up | 699.62 (101.57) | 710.15 (99.07) | 0.00 [1,44] | 0.984 |
| Mood | ||||
| Beck Depression Inventory | ||||
| Baseline | 2.92 (3.14) | 5.00 (4.44) | -- | -- |
| Follow-up | 3.08 (2.99) | 2.60 (3.24) | 0.05 [1,43] | 0.826 |
| Spielberger Trait Anxiety Subscale | ||||
| Baseline | 23.32 (5.51) | 23.09 (3.99) | -- | -- |
| Follow-up | 23.00 (5.64) | 22.38 (5.03) | 0.07 [1,36] | 0.790 |
| Spielberger State Anxiety Subscale | ||||
| Baseline | 1.52 (2.00) | 2.26 (2.47) | -- | -- |
| Follow-up | 1.48 (1.83) | 2.33 (3.00) | 3.03 [1,36] | 0.090 |
aInferential analysis via baseline-adjusted ANCOVA modelling across allocation groups (active vs placebo).
bPost-hoc EMM analysis (two-tailed) revealed no significant score difference on negative PANAS items across allocation groups (EMM = −0.75 ± 0.50, 95% CI [−1.76, 0.26], p = 0.141), while at baseline placebo scored significantly higher on negative PANAS items than fenfluramine (EMM = −1.14 ± 0.46, 95% CI [−2.06, −0.21], p = 0.02, Hedges’ g = −0.70). Between visits, the mean score for this item did not change in fenfluramine group while the placebo group reduced by a mean difference of 0.39.
Table 2.
Mixed-effects linear modelling of longitudinal (daily) Visual Analogue Scale ratings and Side Effects Profile data
| Model estimate a | 95% CI | t-value | p | |
|---|---|---|---|---|
| Visual Analogue Scale | ||||
| Positive items | −17.63 | −67.80, 32.55 | −0.69 | 0.494 |
| Negative items | 28.20 | −8.32, 64.32 | 1.35 | 0.178 |
| Side Effects Profile | ||||
| Appetite, decreased | −0.07 | −0.35, 0.21 | −0.50 | 0.622 |
| Appetite, increased | 0.11 | −0.02, 0.24 | 1.74 | 0.089 |
| Drowsiness/Fatigue | −0.02 | −0.23, 0.26 | −0.13 | 0.901 |
| Insomnia | 0.01 | −0.06, 0.23 | 1.18 | 0.242 |
| Sexual side effects | 0.01 | −0.12, 0.14 | 0.14 | 0.888 |
| Sweating | 0.04 | −0.01, 0.09 | 1.48 | 0.147 |
| Tremors | 0.01 | −0.03, 0.06 | 0.57 | 0.571 |
| Agitation | 0.01 | −0.11, 0.11 | 0.05 | 0.959 |
| Anxiety | 0.07 | −0.04, 0.18 | 1.27 | 0.211 |
| Diarrhoea | −0.11 | −0.26, 0.04 | −1.41 | 0.166 |
| Dry Mouth | −0.02 | −0.24, 0.21 | −0.15 | 0.879 |
| Indigestion | 0.05 | −0.01, 0.12 | 1.63 | 0.110 |
| Nausea | −0.05 | −0.20, 0.10 | −0.64 | 0.528 |
| Upset stomach | −0.05 | −0.21, 0.12 | −0.54 | 0.595 |
aMain effect of group (active vs placebo) via time-adjusted mixed-effects linear models with restricted maximum likelihood estimation.
Discussion
The present findings demonstrate the direct effects of increased synaptic serotonin on human behaviour, underlining its role in guiding decision-making across aversive and more neutral contexts (i.e., where valence is not explicitly manipulated). Specifically, we observed reduced sensitivity for outcomes in aversive contexts; enhanced behavioural inhibition and increased bias favouring impulse control during aversive interference; and enhanced memory function for verbally encoded information. These findings offer broad implications for longstanding theories of how central 5-HT influences human behaviour and contributes to psychiatric aetiology.
Throughout instrumental learning, increased synaptic 5-HT (via fenfluramine) reduced sensitivity to aversive outcomes. This effect is opposite to that described following central depletion of serotonin with tryptophan-depletion, where enhanced negative prediction errors during probabilistic instrumental learning and bias toward aversive stimuli during Pavlovian conditioning have been observed8,60,62,68. Further, in a Pavlovian-to-instrumental transfer paradigm, independent depletion of 5-HT and dopamine respectively enhanced aversive and decreased rewarding Pavlovian-to-instrumental transfer69. As SSRAs and TRP result in opposite effects on net synaptic 5-HT, the opposite behavioural pattern observed here is consistent with a key role for serotonin in modulating loss sensitivity12,38.
There was no statistically significant difference in reward processing between the SSRA and placebo groups. Despite the shared purpose of increasing synaptic 5-HT, SSRI administration has been associated with decreased sensitivity for rewarding outcomes in some studies14,15,70. Reduced reward sensitivity has been attributed to unwanted SSRI treatment effects, notably emotional blunting and reduced efficacy in targeting anhedonia71. Importantly, in preclinical work, SSRI administration results in indirect modulation of dopaminergic signalling pathways involved in reward processing28–31. In similar work, however, the SSRA used here (low dose fenfluramine, racemic mixture) retains selectivity for 5-HT45,55,72,73 and is inactive at dopaminergic synapses56,74, while having a binding affinity for 5-HT transporters which is <0.5% of that typically seen in SSRIs such as citalopram75 (see the Supplementary Discussion for further details on the past uses of the experimental probe). Thus, these results highlight potentially specific effects of serotonin on loss processing, whereas contradictory effects of SSRIs previously reported may relate to effects beyond the serotonin system. It would be worthwhile to investigate if the effect of the SSRA on aversive processing could prove advantageous for the treatment of depression without exacerbating features of anhedonia.
In the preclinical literature, pharmacological (fenfluramine) and optogenetic stimulation of serotonergic neurons in the dorsal raphe nucleus (DRN) results in no change in reward learning in animal models; however, stimulation of non-serotonergic DRN neurons via amphetamine and optogenetics results in increases in reward choice preference76. Moreover, increased firing of amygdala 5-HT projecting neurons is observed during aversive but not reward prediction errors, an effect which appears to be modulated by a functionally discrete DRN to basal amygdala 5-HT pathway77,78. Further optogenetic labelling work suggests that increased DRN 5-HT firing promotes aspects of reward processing79–81, potentially facilitated by co-release of glutamate and subsequent activation of mesoaccumbens dopamine neurons82. Given the methodological disparity between this literature and the present work, drawing direct parallels is challenging, particularly as associations between neuronal firing patterns and synaptic serotonin are region-specific83–87.
Interpreting the ecological meaning of performance during reinforcement learning is challenging. A reduction in sensitivity to loss outcomes may be adaptive or detrimental depending on real-world context. Despite reduced optimal choices for loss trials in the SSRA group, there was no difference in total money earned across groups (see Supplementary Note 3). Moreover, reduced loss learning cannot be attributed to undesired effects such as impaired cognition; indeed, concurrent improvements in learning and memory tasks were observed in the SSRA group.
Increasing synaptic 5-HT (via fenfluramine) enhanced behavioural inhibition, an effect driven by more cautious decision-making. Impairment of 5-HT function decreasing behavioural inhibition is well-observed in animals, and to a lesser extent in humans9,17. However, the opposite approach of increasing synaptic 5-HT with SSRIs yields a comparably less clear picture cross-species. In humans, SSRI challenge results in improvement or no change in action cancellation ability (stop signal)16,88, while action restraint ability (go/no-go) remains unchanged or impaired17–19,89. Frontal functional activity increases during action restraint following SSRI challenge, however this is not linked to a corresponding change in ability18,89. Likewise, SSRIs yield no clear effect on behavioural inhibition in animals17,90. The seemingly irreconcilable effects of SSRIs on behavioural inhibition may be attributed to the vulnerability of the agent to experimental noise; notably, its acute-to-chronic mechanistic shift and off-target dopaminergic effects. Nevertheless, the present study demonstrates objective improvements in action restraint by increasing synaptic 5-HT. Given disorders of behavioural control and impulsivity (e.g., ADHD) are associated with 5-HT dysregulation90, exploring potential clinical applications of SSRAs within these populations may prove beneficial.
During behavioural inhibition, increased synaptic 5-HT resulted in a bias for impulse control during aversive interference, alongside a corresponding drop in choice impulsivity. This indicates an optimisation in task strategy during aversive interference, which is congruent with increased 5-HT reducing sensitivity to aversive outcomes. These findings align with the longstanding conceptualisation of 5-HT as an inhibitor which becomes active in aversive contexts91,92. Indeed, in individuals with depression and tryptophan-depleted healthy adults, choice impulsivity increases for explicit negative emotional targets in a go/no-go paradigm93–96. However, the effects of increased 5-HT on behavioural inhibition reported here were not experimentally confined to aversive contexts; notably, we observed a decrease in choice impulsivity during a control condition without affective interference. Potentially then, 5-HT performs an active role of limiting impulsive action across emotional and neutral contexts, but this is amplified in aversive contexts.
Increasing synaptic 5-HT also enhanced retrieval and speed of processing during memory tasks involving verbal information. Observable changes in verbal but not visuospatial learning is reliably observed following TRP depletion12. The effects of tryptophan depletion on complex verbal working memory are less clear, owing to insufficient studies in this area. SSRI challenge, however, leads to highly variable effects on memory function; while improvements have been observed, typically null findings are reported21,22,97. Unlike fenfluramine, the threshold of synaptic 5-HT required for observable change may not be achieved during the brief SSRI regimen of most studies ( ≤ 7 days), where the problem of autoreceptor supersensitivity persists39,98. Importantly, 5-HT receptor subtypes strongly associated with memory functioning (i.e., 5-HT3,4,6 receptors) have significantly lower binding affinities for endogenous 5-HT relative to other 5-HT receptors (e.g., 5-HT1A,B,D,E,F; 5-HT2A-C)99–102. Thus, crossing a putative 5-HT concentration threshold may be required to observe change in memory function, potentially explaining our findings.
As with most psychoactive substances, including SSRIs103–107, the SSRA fenfluramine may produce ancillary off-target pharmacological effects alongside its primary mechanism. While retaining neurotransmitter selectivity for the serotonergic system at low doses, fenfluramine has modest affinity for the 5-HTR subtypes and sigma-1 (σ1) receptors. In the case of 5-HTR, these effects appear to be specific to 5-HT2A/B/CR108, while there is inconclusive evidence of the involvement of other receptors, such as 5-HT4109; however, the binding affinity of fenfluramine for 5-HT2A/B/CR is at most <1% of that of competitive endogenous 5-HT100,108,110. Indeed, given the high concentration of 5-HT following exocytic release and the finite availability of 5-HT receptors38,55,111, the resulting ancillary effects of 5-HTR agonism/antagonism may be negligible. Moreover, fenfluramine appears to be both a positive allosteric modulator and antagonist of σ1R112,113; while endogenous neurosteroid sigma-1 agonists may be potentiated through positive allosteric modulation, leading to improvements in cognitive ability113–115, it is unclear to what extent this effect may be offset by the sigma-1 antagonist properties of fenfluramine in the healthy brain112,115–117.
Pharmacodynamic data on norfenfluramine, the neuroactive metabolite of fenfluramine, is limited; in one study, d-norfenfluramine administration in mice resulted in small increases (relative to 5-HT) of synaptic noradrenaline118. In two further studies, however, noradrenaline levels were unaltered by d-norfenfluramine and dl-norfenfluramine administration119,120. Moreover, dl-fenfluramine administration in humans produces plasma concentrations of dl-fenfluramine and dl-norfenfluramine at a 1:3 ratio, respectively, with only a fraction of that being d-norfenfluramine118,121. Nevertheless, contrasting the neurobehavioural profile of SSRA fenfluramine with S-enantiomers (selective to 5-HT) of SRAs such as 4-methyl-N-methylcathinone39, once clinically available, could provide further insight. Beyond this, while there is human evidence of in vivo serotonergic modulation by d-fenfluramine with [18F]altanserin PET57, further human PET/SPECT investigations of low dose fenfluramine may offer additional insights into its neurochemical profile. Finally, when interpreting the present findings in the context of past studies of TRP, worth noting is the disputed contribution of non-serotonergic modulation via the kynurenine pathway from TRP107,122.
Methodological differences across studies complicate the interpretation of findings, past and present, within a broader literature context. While earlier we mentioned key differences in response inhibition paradigms (i.e., Stop Signal vs Go/No-Go tasks), paradigms which capture reinforcement learning in humans also splinter in a manner which hinders direct comparison. Notably, reinforcement learning paradigms with or without components such as reversal or model-free/model-based learning involve different computational models and neural pathways64,92,123–127; consequentially, model-free learning implemented in the present work is challenging to compare with TRP/SSRI work involving model-based and reversal learning128–130. For example, previous work suggests 5-HT depletion (via pharmacological lesioning) modulates reinforcement sensitivity to misleading punishments and rewards during reversal learning131. It is important, therefore, to interpret the present findings within the context of its methodological fit to past literature.
In summary, we demonstrate direct effects of increased synaptic serotonin on human behaviour, underlining its role in guiding decision-making within aversive and neutral contexts. In aversive contexts, increased synaptic serotonin appears to reduce sensitivity for loss outcomes, and promotes a bias toward impulse control during behavioural inhibition. In neutral contexts, increased synaptic serotonin appears to enhance behavioural inhibition by promoting cautious decisions, as well as enhancing memory recall for verbal information.
Not only do the present findings offer implications for longstanding theories of central serotonin, but they also demonstrate the promise of the SSRA as an experimental probe, furthering the scope of fundamental work which aims to characterise the involvement of serotonin in human behaviour, and its contribution to psychiatric aetiology in clinical samples.
Given the prominence of impaired cognition and aversive/negative emotional biases as transdiagnostic targets within psychiatry (e.g., unipolar and bipolar depression; schizophrenia)21,71,132–135, investigating the therapeutic potential of the SSRA in clinical populations may be worthwhile. Such investigations may allow greater targeting of specific neurocognitive mechanisms across disorders in the absence of widespread, and often unwanted, effects including emotional blunting.
Methods
Participants and design
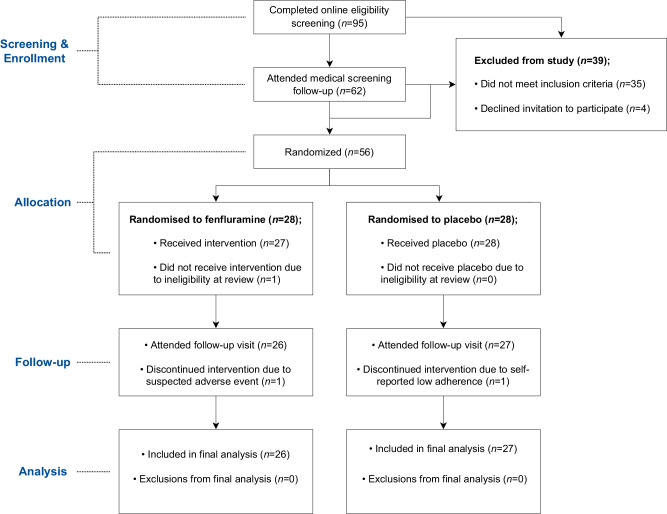
Fifty-six participants (28:28, SSRA:placebo; mean age = 20.2) were randomised to take part in the study. Recruitment occurred between June 2021 and June 2022. Potential participants were screened to exclude those who had recently used recreational drugs (3-month wash-out, except MDMA which had a wash out period of ≥ 1 year), who were pregnant, trying to become pregnant, or who were currently breastfeeding. All participants had a BMI between 18–30 and were fluent speakers of English. For full exclusion and inclusion criteria, please see Supplementary Methods. For full details of the recruitment process, see the study CONSORT flow diagram (Fig. 7). The final sample consisted of 53 young, non-clinical participants (mean age = 20.15; 32 female) which were allocated to administration of serotonin releasing agent fenfluramine (n = 26) or placebo (n = 27) for subchronic administration (8 ± 1 days). All participants in the final sample attended sessions before treatment and at follow-up. Participants were reimbursed 175 GBP upon completion of the study. Participants were requested to self-report on gender, and the potential covariance of gender with treatment effects was investigated post-hoc. The detailed results of these analyses are provided in the Supplementary Tables 5–7.
Fig. 7. CONSORT Diagram of participant flow throughout the study.
The flowchart shows the study recruitment process, which consisted of two screening visits (online and in-person) and two study visits (an initial dose visit and follow-up visit occurring 8 ± 1 days later). Eligible participants were invited to participate in the study and, upon acceptance, were randomised to receive either fenfluramine or placebo.
Eligible participants were randomised to administration of SSRA fenfluramine hydrochloride (15 mg b.i.d.; racemic mixture) or placebo for subchronic administration (8 ± 1 days), in a double-blind design. Both fenfluramine and the placebo were administered orally in a flavoured aqueous solution, with the placebo lacking an active pharmaceutical ingredient. Randomisation was performed by the Clinical Pharmacy Support Unit, Oxford Health NHS Foundation Trust (Oxfordshire, United Kingdom) using a stratified block randomisation algorithm, with stratification for gender and task stimulus version (for further details on task stimulus versions, see Supplementary Methods).
The study was approved by the University of Oxford Central University Research Ethics Committee (MSD-IDREC reference R69642/RE004) and pre-registered on the National Institute of Health Clinical Trials Database (NCT05026398). All primary outcomes within the pre-registered are reported within the current paper. Prior to study participation, participants provided informed consent. All study visits were conducted at the Department of Psychiatry, University of Oxford.
Procedure
Participants undertook two screening visits to assess study eligibility. In the first session, medical history and current medication use was assessed and the Structured Clinical Interview for DSM-V was conducted to screen for current or past psychiatric illness. In the second session, cardiovascular health (blood pressure; electrocardiography), renal and liver health (liver function, urea, and electrolyte blood tests) were assessed, and drug and pregnancy urine tests were performed. Eligible participants attended two study visits, baseline and post-intervention occurring 7, 8 or 9 days later. This study period was scheduled to avoid the premenstrual week for female participants. At baseline, participants completed a battery of cognitive and emotional computer tasks and questionnaires. Participants were then given their first dose of the SSRA or placebo, and monitored for three hours during which regular blood pressure and observational checks were made. Measurements of early changes in salivary cortisol concentration were taken to investigate potential modulation of HPA axis function that has been previously observed in studies of acute high dose fenfluramine136. Saliva samples were collected immediately before initial dose, one hour post-dose, and three hours post-dose. These samples were immunoassayed for cortisol levels over linear calibration curves (for further details, see Supplementary Methods). After the initial dose visit, participants were asked to independently take the fenfluramine or placebo daily, in addition to completing daily questionnaires (see Questionnaires measures section). At the post-intervention visit, participants completed the same task and questionnaire battery as at baseline and were then requested to estimate their allocation prior to debriefing.
Questionnaire measures
At each study visit, participants completed self-report questionnaires measuring affect, mood, anxiety, subjective cognitive functioning, and side-effects; the Spielberger State-Trait Anxiety Inventory [STAI-T], Beck Depression Inventory II [BDI], Positive and Negative Affect Schedule [PANAS], Visual Analogue Scale [VAS], Perceived Deficit Questionnaire – Depression [PDQ-D], and side effects profile questionnaire. Participants completed the VAS and side effects questionnaire once per day from the baseline visit until the follow-up visit.
Cognitive and Emotional Task Battery
Participants undertook an extensive cognitive and emotional task battery at both the initial dose visit (baseline) and follow-up visit. Participants undertook the following tasks in order: 1) Auditory Verbal Learning Task (AVLT) (Fig. 6D) – a measure of declarative memory encoding and retrieval where accuracy of recall was the measured outcome. Behavioural data for the task consisted of N = 51 individuals (mean age = 20.15; 31 female). 2) Affective Interference Go/No-Go Task (Fig. 3A) – a measure of behavioural inhibition under affective interference (positive [happy faces], aversive/negative [fearful faces], and neutral distractors) where accuracy of inhibited response to no-go trials (response inhibition), accuracy, and response time for go trials (an index of impulsivity137 were the non-model outcome measures. The block design of the task allows for analysis of set-shifting effects (executive shifting for task condition rule changes) on accuracy and response time. Participant task data was fit to a computational drift diffusion model (see Supplementary Methods for further details) which provided the following model parameters: boundary separation, initial choice bias, non-decision time, drift rate, and drift criterion. Behavioural and computational data for the task consisted of N = 50 individuals (mean age = 20.22; 32 female). 3) Verbal n-back task (Fig. 6A) – a measure of complex verbal working memory where accuracy and response time to target letters (i.e., matching a letter which appeared n-back [0, 1, 2, or 3] trials ago) were the outcome measures. Behavioural data for the n-back consisted of N = 50 individuals (mean age = 20.1; 31 female). 4) Probabilistic instrumental learning task ([Fig. 2A]; adapted from63) – a measure of reward and loss sensitivity during instrumental learning, which produced non-model outcome measures which were fit to computational reinforcement learning models. Non-model outcomes were optimal choice outcome (i.e., selecting the stimulus with a higher probability of a favourable outcome under each task condition: wins during win trials and no change during loss trials) and response time. The main computational model parameters were outcome sensitivity and learning rate, where learning rate was estimated separately for both win and loss trials (see Supplementary Methods for further details). Behavioural and computational data for the task consisted of N = 53 individuals (mean age = 20.15; 32 female). 5) Oxford Memory Task – a measure of visuospatial working memory which included localisation speed and stimulus selection accuracy outcomes. Behavioural data for the task consisted of N = 51 individuals (mean age = 20.16; 31 female). 6) Contextual cueing task – a measure of implicit learning and visual search ability where the outcome measure was accuracy and response times under novel/implicit cueing conditions. Behavioural data for the task consisted of N = 53 individuals (mean age = 20.15; 32 female). Full details of tasks included in this battery are included in the Supplementary Methods.
Statistical analysis
Data pre-processing and statistical analyses were carried out using R Software (version 4.3.1), and computational modelling was undertaken using MATLAB (R2022a), Docker (version 4.22.0), and Python (version 3.8.8); required software dependencies and packages are listed within the Supplementary Methods. Homogeneity in demographic variables across allocation groups was assessed using chi-squared independence tests (categorical, binary variables) and two-tailed independent t-tests (continuous, discrete variables). The effect of the SSRA on outcomes across the task battery and questionnaire ratings was analysed using between-groups (SSRA vs. placebo) mixed effects ANCOVA models (two-tailed) on post-intervention data, with baseline visit performance serving as a regressor and participant as a random effect for nested multilevel data structures (e.g., multiple task conditions). The approach of using baseline score as a regressor in this manner was selected as it allows isolation of the group effect from potential sources of bias (e.g., learning and practice effects)138,139. In comparison with other baseline-adjustment techniques, such as change score between before and after intervention, baseline-adjusted ANCOVA yields greater statistical efficiency and precision (irrespective of baseline balance/imbalance)140,141. Planned comparisons were carried out on outcome measures collected at follow-up using two-tailed estimated marginal means tests, where estimates are reported alongside standard mean error. Family-wise error for estimated marginal means tests was adjusted using the Bonferroni-Holm procedure. Models were assessed through histogram examination of standardised residuals to determine normality of distribution (non-normal computational values were log-transformed), and homogeneity of regression slopes and covariate independence were tested by modelling covariate interactions across factors (covariate × group × task condition). Two-tailed effect sizes metrics are reported for significant ANCOVA (partial eta squared, ηp2) and EMM (Cohen’s d, d) models alongside corresponding 95% confidence intervals (for effect size calculations, see Supplementary Methods). Post-hoc correlational analyses on the Affective Interference Go/No-Go task were performed using two-tailed Pearson’s product-moment correlation analysis. Baseline-adjusted ANCOVA models were generated post-hoc to investigate the potential covariance of gender with group effects across study outcomes, which are reported in Supplementary Tables 5–7. Group allocation guesses were analysed using chi-squared independence tests. In addition to ANCOVA analysis of questionnaire data at follow-up, daily questionnaire data (VAS and side effects profile) was joined longitudinally with initial dose and follow-up visit data and analysed using linear mixed effects models with restricted maximum likelihood estimation where time served as a regressor. Salivary cortisol was analysed across three timepoints (before dose, 1- and 3-hr post-dose) using linear mixed effects modelling with time as a regressor. Baseline task battery, cortisol and self-report questionnaire data are included in Supplementary Figs. 5, 7–9, 11–13, 16, 18, 20–23, 25. All inferential analyses were carried out at the 0.05 alpha level. A priori sample size calculation (Power [1 - β error probability]: 80%) determined that a sample of N = 52 (26 per group) was required to undertake two-tailed between-groups analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This study was funded by a grant from Zogenix International Ltd. (awarded to C.H.), prior to merge with UCB Pharma, and supported by the NIHR Oxford Health Biomedical Research Centre and the NIHR Oxford Cognitive Health Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We thank Dr Sandra Tamm, Dr Angharad de Cates, Dr Sara Costi, and Dr Alexander Smith for their assistance with medical screening and diagnostics procedures. We thank Prof Valerie Voon for suggestions for data analysis. We thank Dr Margarita Chibalina for their assistance with biological sample handling and processing. We thank Dr Jan Willem de Gee for publishing openly available computational modelling scripts. We thank Tara Pusinelli for assistance with data collection and entry. We thank Sorcha Hamilton for help with verifying code reproducibility.
Author contributions
M.C., S.M., C.H. and P.C. designed the study. M.C., H.T., H.I.C. and C.E.W. undertook data collection. M.C., H.T., H.I.C. and M.B. produced preprocessing task scripts. M.C. and C.E.W. undertook biological specimen processing. F.S. and M.B. provided computational modelling support. M.C. undertook all computational modelling and inferential analyses. H.T. and F.S. contributed equally to this work as second authors. M.C., S.M., C.H., P.C., and M.B. undertook data interpretation. M.C. drafted the article and produced illustrations. All authors contributed to revisions and approval of the final draft.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
Raw and modelled datasets generated for this study have been deposited on Zenodo142 and Github: https://github.com/mjcolwell/SSRA_human_behaviour_data_and_code.
Code availability
The code used to undertake data preprocessing, computational modelling, and inferential analyses in this study has been deposited on Zenodo142 and GitHub: https://github.com/mjcolwell/SSRA_human_behaviour_data_and_code.
Competing interests
C.H. has received consultancy fees from P1vital Ltd., Janssen Pharmaceuticals, UCB Pharma, Sage Therapeutics, Pfizer, Zogenix, Compass Pathways, and Lundbeck, and is a director of the company TnC Psychiatry and Neuroscience. S.M. has received consultancy fees from Zogenix, Sumitomo Dainippon Pharma, P1vital Ltd., UCB Pharma and Janssen Pharmaceuticals. C.H. and S.M. hold grant income from Zogenix, UCB Pharma, Syndesi and Janssen Pharmaceuticals. C.H. and P.C. hold grant income from a collaborative research project with Pfizer. M.B. has received travel expenses from Lundbeck for attending conferences and has acted as a consultant for J&J, Novartis, Boehringher and CHDR. He previously owned shares in P1vital Ltd. The other authors report no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Susannah E. Murphy, Catherine J. Harmer.
Contributor Information
Michael J. Colwell, Email: michael.colwell@psych.ox.ac.uk
Catherine J. Harmer, Email: catherine.harmer@psych.ox.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-50394-x.
References
- 1.Whitaker-Azmitia, P. M. The Discovery of Serotonin and its Role in Neuroscience. Neuropsychopharmacology21, 2–8 (1999). 10.1038/sj.npp.1395355 [DOI] [PubMed] [Google Scholar]
- 2.Cowen, P. J. & Browning, M. What has serotonin to do with depression? World Psychiatry14, 158 (2015). 10.1002/wps.20229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivier, B. Serotonin: A never-ending story. Eur. J. Pharm.753, 2–18 (2015). 10.1016/j.ejphar.2014.10.031 [DOI] [PubMed] [Google Scholar]
- 4.Voigt, J.-P. & Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res.277, 14–31 (2015). 10.1016/j.bbr.2014.08.065 [DOI] [PubMed] [Google Scholar]
- 5.Olivier, B., Van Oorschot, R. & Waldinger, M. D. Serotonin, serotonergic receptors, selective serotonin reuptake inhibitors and sexual behaviour. Int Clin. Psychopharmacol.13, S9–S14 (1998). 10.1097/00004850-199807006-00003 [DOI] [PubMed] [Google Scholar]
- 6.van der Plasse, G. et al. Medial prefrontal serotonin in the rat is involved in goal-directed behaviour when affect guides decision making. Psychopharmacol. (Berl.)195, 435–449 (2007). 10.1007/s00213-007-0917-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts, C., Sahakian, B. J. & Robbins, T. W. Psychological mechanisms and functions of 5-HT and SSRIs in potential therapeutic change: lessons from the serotonergic modulation of action selection, learning, affect, and social cognition. Neurosci. Biobehav Rev.119, 138–167 (2020). 10.1016/j.neubiorev.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Crockett, M. J., Clark, L., Apergis-Schoute, A. M., Morein-Zamir, S. & Robbins, T. W. Serotonin Modulates the Effects of Pavlovian Aversive Predictions on Response Vigor. Neuropsychopharmacology37, 2244–2252 (2012). 10.1038/npp.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soubrié, P. Reconciling the role of central serotonin neurons in human and animal behavior. Behav. Brain Sci.9, 319–335 (1986). 10.1017/S0140525X00022871 [DOI] [Google Scholar]
- 10.Deakin, J. F. W. Roles of serotonergic systems in escape, avoidance and other behaviours. Theory Psychopharmacol.2, 149–193 (1983). [Google Scholar]
- 11.McEntee, W. J. & Crook, T. H. Serotonin, memory, and the aging brain. Psychopharmacol. (Berl.)103, 143–149 (1991). 10.1007/BF02244194 [DOI] [PubMed] [Google Scholar]
- 12.Cowen, P. & Sherwood, A. C. The role of serotonin in cognitive function: evidence from recent studies and implications for understanding depression. J. Psychopharmacol.27, 575–583 (2013). 10.1177/0269881113482531 [DOI] [PubMed] [Google Scholar]
- 13.Scholl, J. et al. Beyond negative valence: 2-week administration of a serotonergic antidepressant enhances both reward and effort learning signals. PLoS Biol.15, e2000756 (2017). 10.1371/journal.pbio.2000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michely, J., Eldar, E., Erdman, A., Martin, I. M. & Dolan, R. J. Serotonin modulates asymmetric learning from reward and punishment in healthy human volunteers. Commun. Biol.5, 812 (2022). 10.1038/s42003-022-03690-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langley, C. et al. Chronic escitalopram in healthy volunteers has specific effects on reinforcement sensitivity: a double-blind, placebo-controlled semi-randomised study. Neuropsychopharmacology48, 664–670 (2023). 10.1038/s41386-022-01523-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skandali, N. et al. Dissociable effects of acute SSRI (escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology43, 2645–2651 (2018). 10.1038/s41386-018-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle, D. M., Bari, A. & Robbins, T. W. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacol. (Berl.)199, 439–456 (2008). 10.1007/s00213-008-1127-6 [DOI] [PubMed] [Google Scholar]
- 18.Macoveanu, J. et al. Serotonin 2 A Receptors, Citalopram and Tryptophan-Depletion: a Multimodal Imaging Study of their Interactions During Response Inhibition. Neuropsychopharmacology38, 996–1005 (2013). 10.1038/npp.2012.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guitart-Masip, M. et al. Differential, but not opponent, effects of l-DOPA and citalopram on action learning with reward and punishment. Psychopharmacol. (Berl.)231, 955–966 (2014). 10.1007/s00213-013-3313-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendelsohn, D., Riedel, W. J. & Sambeth, A. Effects of acute tryptophan depletion on memory, attention and executive functions: A systematic review. Neurosci. Biobehav Rev.33, 926–952 (2009). 10.1016/j.neubiorev.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 21.Colwell, M. J. et al. Pharmacological targeting of cognitive impairment in depression: recent developments and challenges in human clinical research. Transl. Psychiatry12, 484 (2022). 10.1038/s41398-022-02249-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassel, J.-C. CHAPTER 3.9 - Experimental Studies on the Role(s) of Serotonin in Learning and Memory Functions. in Handbook of Behavioral Neuroscience (eds Müller, C. P. & Jacobs, B. L.) vol. 21 429–447 (Elsevier, 2010).
- 23.Evers, E. A. T., Van der Veen, F. M., Fekkes, D. & Jolles, J. Serotonin and cognitive flexibility: neuroimaging studies into the effect of acute tryptophan depletion in healthy volunteers. Curr. Med Chem.14, 2989–2995 (2007). 10.2174/092986707782794032 [DOI] [PubMed] [Google Scholar]
- 24.Barton, C. L. & Hutson, P. H. Inhibition of hippocampal 5‐HT synthesis by fluoxetine and paroxetine: Evidence for the involvement of both 5‐HT1A and 5‐HT1B/D autoreceptors. Synapse31, 13–19 (1999). [DOI] [PubMed] [Google Scholar]
- 25.El Mansari, M., Sánchez, C., Chouvet, G., Renaud, B. & Haddjeri, N. Effects of Acute and Long-Term Administration of Escitalopram and Citalopram on Serotonin Neurotransmission: an In Vivo Electrophysiological Study in Rat Brain. Neuropsychopharmacology30, 1269–1277 (2005). 10.1038/sj.npp.1300686 [DOI] [PubMed] [Google Scholar]
- 26.de Groote, L., Olivier, B. & Westenberg, H. G. Extracellular serotonin in the prefrontal cortex is limited through terminal 5-HT1B autoreceptors: a microdialysis study in knockout mice. Psychopharmacol. (Berl.)162, 419–424 (2002). 10.1007/s00213-002-1117-z [DOI] [PubMed] [Google Scholar]
- 27.Zhou, F.-M. et al. Corelease of Dopamine and Serotonin from Striatal Dopamine Terminals. Neuron46, 65–74 (2005). 10.1016/j.neuron.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 28.Sekine, Y., Suzuki, K., Ramachandran, P. V., Blackburn, T. P. & Ashby, J. R. C. R. Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse61, 72–77 (2007). 10.1002/syn.20349 [DOI] [PubMed] [Google Scholar]
- 29.Yoshino, T., Nisijima, K., Katoh, S., Yui, K. & Nakamura, M. Tandospirone potentiates the fluoxetine-induced increases in extracellular dopamine via 5-HT1A receptors in the rat medial frontal cortex. Neurochem. Int.40, 355–360 (2002). 10.1016/S0197-0186(01)00079-1 [DOI] [PubMed] [Google Scholar]
- 30.Shuto, T. et al. Obligatory roles of dopamine D1 receptors in the dentate gyrus in antidepressant actions of a selective serotonin reuptake inhibitor, fluoxetine. Mol. Psychiatry25, 1229–1244 (2020). 10.1038/s41380-018-0316-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dremencov, E., el Mansari, M. & Blier, P. Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. J. Psychiatry Neurosci.34, 223–229 (2009). [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery, A. M. J., Rose, I. C. & Herberg, L. J. 5-HT1A agonists and dopamine: the effects of 8-OH-DPAT and buspirone on brain-stimulation reward. J. Neural Transm. / Gen. Sect. JNT83, 139–148 (1991). 10.1007/BF01244460 [DOI] [PubMed] [Google Scholar]
- 33.Pozzi, L., Invernizzi, R., Garavaglia, C. & Samanin, R. Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin: a comparison with citalopram. J. Neurochem.73, 1051–1057 (1999). 10.1046/j.1471-4159.1999.0731051.x [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi, K., Haneda, E., Higuchi, M., Suhara, T. & Suzuki, H. Chronic Fluoxetine Selectively Upregulates Dopamine D1-Like Receptors in the Hippocampus. Neuropsychopharmacology37, 1500–1508 (2012). 10.1038/npp.2011.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitaichi, Y. et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur. J. Pharm.647, 90–96 (2010). 10.1016/j.ejphar.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 36.Müller, F. et al. MDMA-induced changes in within-network connectivity contradict the specificity of these alterations for the effects of serotonergic hallucinogens. Neuropsychopharmacology46, 545–553 (2021). 10.1038/s41386-020-00906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustafa, N. S. et al. MDMA and the Brain: A Short Review on the Role of Neurotransmitters in Neurotoxicity. Basic Clin. Neurosci.11, 381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothman, R. B. & Baumann, M. H. Serotonin releasing agents: neurochemical, therapeutic and adverse effects. Pharm. Biochem Behav.71, 825–836 (2002). 10.1016/S0091-3057(01)00669-4 [DOI] [PubMed] [Google Scholar]
- 39.Mayer, F. P. et al. Serotonin-releasing agents with reduced off-target effects. Mol. Psychiatry10.1038/s41380-022-01843-w (2022). [DOI] [PMC free article] [PubMed]
- 40.Udo de Haes, J. I., Harada, N., Elsinga, P. H., Maguire, R. P. & Tsukada, H. Effect of fenfluramine‐induced increases in serotonin release on [18 F] MPPF binding: A continuous infusion PET study in conscious monkeys. Synapse59, 18–26 (2006). 10.1002/syn.20209 [DOI] [PubMed] [Google Scholar]
- 41.Knupp, K. G. et al. Efficacy and Safety of Fenfluramine for the Treatment of Seizures Associated With Lennox-Gastaut Syndrome: A Randomized Clinical Trial. JAMA Neurology79, 554–564 (2022). [DOI] [PMC free article] [PubMed]
- 42.Finnema, S. J., Varrone, A., Hwang, T.-J., Halldin, C. & Farde, L. Confirmation of fenfluramine effect on 5-HT1B receptor binding of [11 C] AZ10419369 using an equilibrium approach. J. Cereb. Blood Flow. Metab.32, 685–695 (2012). 10.1038/jcbfm.2011.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duval, F. et al. Serotonergic and noradrenergic function in depression: clinical correlates. Dialogues Clin. Neurosci.2, 299–308 (2022). [DOI] [PMC free article] [PubMed]
- 44.Marona-Lewicka, D. & Nichols, D. E. Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan. Eur. J. Pharm.258, 1–13 (1994). 10.1016/0014-2999(94)90051-5 [DOI] [PubMed] [Google Scholar]
- 45.Fattaccini, C. M., Gozlan, H. & Hamon, M. Differential effects of d-fenfluramine and p-chloroamphetamine on H7512-induced depletion of 5-hydroxytryptamine and dopamine in the rat brain. Neuropharmacology30, 15–23 (1991). 10.1016/0028-3908(91)90037-C [DOI] [PubMed] [Google Scholar]
- 46.Bonanno, G., Fassio, A., Severi, P., Ruelle, A. & Raiteri, M. Fenfluramine Releases Serotonin from Human Brain Nerve Endings by a Dual Mechanism. J. Neurochem63, 1163–1166 (1994). 10.1046/j.1471-4159.1994.63031163.x [DOI] [PubMed] [Google Scholar]
- 47.Baumann, M. H., Ayestas, M. A., Dersch, C. M. & Rothman, R. B. 1-(m-Chlorophenyl)piperazine (mCPP) Dissociates In Vivo Serotonin Release from Long-Term Serotonin Depletion in Rat Brain. Neuropsychopharmacology24, 492–501 (2001). 10.1016/S0893-133X(00)00221-9 [DOI] [PubMed] [Google Scholar]
- 48.Leonardi, E. T. K. & Azmitia, E. C. MDMA (Ecstasy) Inhibition of MAO Type A and Type B: Comparisons with Fenfluramine and Fluoxetine (Prozac). Neuropsychopharmacology10, 231–238 (1994). 10.1038/npp.1994.26 [DOI] [PubMed] [Google Scholar]
- 49.Campbell, S. & MacQueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci.29, 417–426 (2004). [PMC free article] [PubMed] [Google Scholar]
- 50.Lanzenberger, R. et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage63, 874–881 (2012). 10.1016/j.neuroimage.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 51.Jørgensen, L. M. et al. Cerebral 5-HT release correlates with [11 C] Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. J. Cereb. Blood Flow. Metab.37, 425–434 (2017). 10.1177/0271678X16629483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hume, S. et al. Effect of 5‐HT on binding of [11 C] WAY 100635 to 5‐HT1A receptors in rat brain, assessed using in vivo microdialysis and PET after fenfluramine. Synapse41, 150–159 (2001). 10.1002/syn.1069 [DOI] [PubMed] [Google Scholar]
- 53.Balcioglu, A. & Wurtman, R. J. Effects of fenfluramine and phentermine (fen–phen) on dopamine and serotonin release in rat striatum: in vivo microdialysis study in conscious animals. Brain Res813, 67–72 (1998). 10.1016/S0006-8993(98)01003-8 [DOI] [PubMed] [Google Scholar]
- 54.Sabol, K. E., Richards, J. B. & Seiden, L. S. Fluoxetine attenuates the DL-fenfluramine-induced increase in extracellular serotonin as measured by in vivo dialysis. Brain Res585, 421–424 (1992). 10.1016/0006-8993(92)91249-E [DOI] [PubMed] [Google Scholar]
- 55.Baumann, M. H. et al. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse36, 102–113 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Zaczek, R. et al. Effects of repeated fenfluramine administration on indices of monoamine function in rat brain: pharmacokinetic, dose response, regional specificity and time course data. J. Pharmacol. Exp. Therapeutics253, 104–112 (1990). [PubMed] [Google Scholar]
- 57.Quednow, B. B. et al. Assessment of serotonin release capacity in the human brain using dexfenfluramine challenge and [18 F]altanserin positron emission tomography. Neuroimage59, 3922–3932 (2012). 10.1016/j.neuroimage.2011.09.045 [DOI] [PubMed] [Google Scholar]
- 58.Ikoma, Y. et al. Measurement of changes in endogenous serotonin level by positron emission tomography with [18 F] altanserin. Ann. Nucl. Med35, 955–965 (2021). 10.1007/s12149-021-01633-4 [DOI] [PubMed] [Google Scholar]
- 59.Moore, P. et al. Clinical and Physiological Consequences of Rapid Tryptophan Depletion. Neuropsychopharmacology23, 601–622 (2000). 10.1016/S0893-133X(00)00161-5 [DOI] [PubMed] [Google Scholar]
- 60.Geurts, D. E. M., Huys, Q. J. M., den Ouden, H. E. M. & Cools, R. Serotonin and Aversive Pavlovian Control of Instrumental Behavior in Humans. J. Neurosci.33, 18932 (2013). 10.1523/JNEUROSCI.2749-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson, O. J., Cools, R. & Sahakian, B. J. Tryptophan depletion disinhibits punishment but not reward prediction: implications for resilience. Psychopharmacol. (Berl.)219, 599–605 (2012). 10.1007/s00213-011-2410-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cools, R., Robinson, O. J. & Sahakian, B. Acute Tryptophan Depletion in Healthy Volunteers Enhances Punishment Prediction but Does not Affect Reward Prediction. Neuropsychopharmacology33, 2291–2299 (2008). 10.1038/sj.npp.1301598 [DOI] [PubMed] [Google Scholar]
- 63.Pessiglione, M., Seymour, B., Flandin, G., Dolan, R. J. & Frith, C. D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature442, 1042–1045 (2006). 10.1038/nature05051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halahakoon, D. C. et al. Pramipexole Enhances Reward Learning by Preserving Value Estimates. Biol. Psychiatry10.1016/j.biopsych.2023.05.023 (2023). [DOI] [PubMed]
- 65.Dalley, J. W., Everitt, B. J. & Robbins, T. W. Impulsivity, compulsivity, and top-down cognitive control. Neuron69, 680–694 (2011). 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 66.Campanella, S. et al. Short-term impact of tDCS over the right inferior frontal cortex on impulsive responses in a go/no-go task. Clin. EEG Neurosci.49, 398–406 (2018). 10.1177/1550059418777404 [DOI] [PubMed] [Google Scholar]
- 67.Wiecki, T. V., Sofer, I. & Frank, M. J. HDDM: Hierarchical Bayesian estimation of the drift-diffusion model in Python. Front Neuroinform.14, 55610 (2013). [DOI] [PMC free article] [PubMed]
- 68.Hindi Attar, C., Finckh, B. & Büchel, C. The influence of serotonin on fear learning. PLOS ONE7, e42397 (2012). [DOI] [PMC free article] [PubMed]
- 69.Hebart, M. N. & Gläscher, J. Serotonin and dopamine differentially affect appetitive and aversive general Pavlovian-to-instrumental transfer. Psychopharmacol. (Berl.)232, 437–451 (2015). 10.1007/s00213-014-3682-3 [DOI] [PubMed] [Google Scholar]
- 70.McCabe, C., Mishor, Z., Cowen, P. J. & Harmer, C. J. Diminished Neural Processing of Aversive and Rewarding Stimuli During Selective Serotonin Reuptake Inhibitor Treatment. Biol. Psychiatry67, 439–445 (2010). 10.1016/j.biopsych.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vrieze, E. et al. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry73, 639–645 (2013). 10.1016/j.biopsych.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giambalvo, C. T. & Price, L. H. Effects of fenfluramine and antidepressants on protein kinase C activity in rat cortical synaptoneurosomes. Synapse50, 212–222 (2003). 10.1002/syn.10262 [DOI] [PubMed] [Google Scholar]
- 73.Raiteri, M., Bonanno, G. & Vallebuona, F. In vitro and in vivo effects of d-fenfluramine: no apparent relation between 5-hydroxytryptamine release and hypophagia. J. Pharmacol. Exp. Therapeutics273, 643 (1995). [PubMed] [Google Scholar]
- 74.Kannengiesser, M.-H., Hunt, P. F. & Raynaud, J.-P. Comparative actionof fenfluramine on the uptake and release of serotonin and dopamine. Eur. J. Pharm.35, 35–43 (1976). 10.1016/0014-2999(76)90298-3 [DOI] [PubMed] [Google Scholar]
- 75.Owens, M. J., Knight, D. L. & Nemeroff, C. B. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol. Psychiatry50, 345–350 (2001). 10.1016/S0006-3223(01)01145-3 [DOI] [PubMed] [Google Scholar]
- 76.McDevitt, R. A. et al. Serotonergic versus Nonserotonergic Dorsal Raphe Projection Neurons: Differential Participation in Reward Circuitry. Cell Rep.8, 1857–1869 (2014). 10.1016/j.celrep.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McHugh, S. B. et al. Aversive prediction error signals in the amygdala. J. Neurosci.34, 9024–9033 (2014). 10.1523/JNEUROSCI.4465-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sengupta, A. & Holmes, A. A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory. Neuron103, 489–505.e7 (2019). 10.1016/j.neuron.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen, J. Y., Amoroso, M. W. & Uchida, N. Serotonergic neurons signal reward and punishment on multiple timescales. Elife4, e06346 (2015). 10.7554/eLife.06346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhong, W., Li, Y., Feng, Q. & Luo, M. Learning and stress shape the reward response patterns of serotonin neurons. J. Neurosci.37, 8863–8875 (2017). 10.1523/JNEUROSCI.1181-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paquelet, G. E. et al. Single-cell activity and network properties of dorsal raphe nucleus serotonin neurons during emotionally salient behaviors. Neuron110, 2664–2679 (2022). 10.1016/j.neuron.2022.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang, H.-L. et al. Dorsal raphe dual serotonin-glutamate neurons drive reward by establishing excitatory synapses on VTA mesoaccumbens dopamine neurons. Cell Rep.26, 1128–1142 (2019). 10.1016/j.celrep.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Athilingam, J. C., Ben-Shalom, R., Keeshen, C. M., Sohal, V. S. & Bender, K. J. Serotonin enhances excitability and gamma frequency temporal integration in mouse prefrontal fast-spiking interneurons. Elife6, e31991 (2017). 10.7554/eLife.31991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pompeiano, M., Palacios, J. M. & Mengod, G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J. Neurosci.12, 440–453 (1992). 10.1523/JNEUROSCI.12-02-00440.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Almeida, J. & Mengod, G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5‐HT2A receptors in human and monkey prefrontal cortex. J. Neurochem103, 475–486 (2007). 10.1111/j.1471-4159.2007.04768.x [DOI] [PubMed] [Google Scholar]
- 86.Hentall, I. D., Kurle, P. J. & White, T. R. Correlations between serotonin level and single-cell firing in the rat’s nucleus raphe magnus. Neuroscience95, 1081–1088 (1999). 10.1016/S0306-4522(99)00516-3 [DOI] [PubMed] [Google Scholar]
- 87.Scuvée-Moreau, J. & Dresse, A. Influence of fenfluramine and norfenfluramine stereoisomers on the firing rate of central monoaminergic neurons in the rat. Eur. J. Pharm.179, 211–215 (1990). 10.1016/0014-2999(90)90421-2 [DOI] [PubMed] [Google Scholar]
- 88.Chamberlain, S. R. et al. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science (1979)311, 861–863 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Del-Ben, C. M. et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology30, 1724–1734 (2005). 10.1038/sj.npp.1300728 [DOI] [PubMed] [Google Scholar]
- 90.Winstanley, C. A. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br. J. Pharm.164, 1301–1321 (2011). 10.1111/j.1476-5381.2011.01323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dayan, P. & Huys, Q. J. M. Serotonin in affective control. Annu Rev. Neurosci.32, 95–126 (2009). 10.1146/annurev.neuro.051508.135607 [DOI] [PubMed] [Google Scholar]
- 92.Robinson, O. J. & Roiser, J. P. The role of serotonin in aversive inhibition: behavioural, cognitive and neural perspectives. Psychopathol. Rev.3, 29–40 (2016). 10.5127/pr.034013 [DOI] [Google Scholar]
- 93.Roiser, J. P. et al. The Effect of Acute Tryptophan Depletion on the Neural Correlates of Emotional Processing in Healthy Volunteers. Neuropsychopharmacology33, 1992–2006 (2008). 10.1038/sj.npp.1301581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy, F., Smith, K., Cowen, P., Robbins, T. & Sahakian, B. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacol. (Berl.)163, 42–53 (2002). 10.1007/s00213-002-1128-9 [DOI] [PubMed] [Google Scholar]
- 95.Erickson, K. et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am. J. Psychiatry162, 2171–2173 (2005). 10.1176/appi.ajp.162.11.2171 [DOI] [PubMed] [Google Scholar]
- 96.Crockett, M. J., Clark, L. & Robbins, T. W. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J. Neurosci.29, 11993–11999 (2009). 10.1523/JNEUROSCI.2513-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harmer, C. J., Bhagwagar, Z., Cowen, P. J. & Goodwin, G. M. Acute administration of citalopram facilitates memory consolidation in healthy volunteers. Psychopharmacol. (Berl.)163, 106–110 (2002). 10.1007/s00213-002-1151-x [DOI] [PubMed] [Google Scholar]
- 98.Cowen, P. J. Psychopharmacology of 5-HT1A receptors. Nucl. Med Biol.27, 437–439 (2000). 10.1016/S0969-8051(00)00108-6 [DOI] [PubMed] [Google Scholar]
- 99.Hartig, P. R. Molecular biology of 5-HT receptors. Trends Pharm. Sci.10, 64–69 (1989). 10.1016/0165-6147(89)90080-1 [DOI] [PubMed] [Google Scholar]
- 100.Paterson, L. M., Tyacke, R. J., Nutt, D. J. & Knudsen, G. M. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J. Cereb. Blood Flow. Metab.30, 1682–1706 (2010). 10.1038/jcbfm.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murphy, S. E., Wright, L. C., Browning, M., Cowen, P. J. & Harmer, C. J. A role for 5-HT4 receptors in human learning and memory. Psychol. Med50, 2722–2730 (2020). 10.1017/S0033291719002836 [DOI] [PubMed] [Google Scholar]
- 102.Smith, J. et al. Vortioxetine reduces BOLD signal during performance of the N-back working memory task: a randomised neuroimaging trial in remitted depressed patients and healthy controls. Mol. Psychiatry23, 1127–1133 (2018). 10.1038/mp.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dionisie, V., Filip, G. A., Manea, M. C., Manea, M. & Riga, S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: a review of human and rodent research studies. Inflammopharmacology29, 75–90 (2021). 10.1007/s10787-020-00777-5 [DOI] [PubMed] [Google Scholar]
- 104.Cruceanu, C., Lopez, J. P., Tsai, W.-T. & Turecki, G. Dysregulation of the glutamatergic receptors after antidepressant treatment in human neural progenitor cells. Mol. Psychiatry22, 1228–1229 (2017). 10.1038/mp.2016.138 [DOI] [PubMed] [Google Scholar]
- 105.Hashimoto, K. Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship. Cent. Nerv. Syst. Agents Medicinal Chem. (Former. Curr. Medicinal Chem.-Cent. Nerv. Syst. Agents)9, 197–204 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Damsa, C. et al. Dopamine-dependent’ side effects of selective serotonin reuptake inhibitors: a clinical review. J. Clin. Psychiatry65, 1064–1068 (2004). 10.4088/JCP.v65n0806 [DOI] [PubMed] [Google Scholar]
- 107.van Donkelaar, E. L. et al. Mechanism of acute tryptophan depletion: is it only serotonin? Mol. Psychiatry16, 695–713 (2011). 10.1038/mp.2011.9 [DOI] [PubMed] [Google Scholar]
- 108.Fitzgerald, L. W. et al. Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Mol. Pharm.57, 75–81 (2000). [PubMed] [Google Scholar]
- 109.Sourbron, J. & Lagae, L. Serotonin receptors in epilepsy: Novel treatment targets? Epilepsia Open7, 231 (2022). 10.1002/epi4.12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.di Giovanni, G., di Matteo, V. & Esposito, E. Serotonin-dopamine interaction: experimental evidence and therapeutic relevance. Prog. Brain Res.172, 122–123 (2008). [DOI] [PubMed]
- 111.Gerhardt, C. C. & van Heerikhuizen, H. Functional characteristics of heterologously expressed 5-HT receptors. Eur. J. Pharm.334, 1–23 (1997). 10.1016/S0014-2999(97)01115-1 [DOI] [PubMed] [Google Scholar]
- 112.Sourbron, J. et al. Serotonergic modulation as effective treatment for Dravet syndrome in a zebrafish mutant model. ACS Chem. Neurosci.7, 588–598 (2016). 10.1021/acschemneuro.5b00342 [DOI] [PubMed] [Google Scholar]
- 113.Martin, P. et al. Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav.105, 106989 (2020). 10.1016/j.yebeh.2020.106989 [DOI] [PubMed] [Google Scholar]
- 114.Hindmarch, I. & Hashimoto, K. Cognition and depression: the effects of fluvoxamine, a sigma‐1 receptor agonist, reconsidered. Hum. Psychopharmacol: Clin. Exp.25, 193–200 (2010). 10.1002/hup.1106 [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto, K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J. Pharm. Sci.127, 6–9 (2015). 10.1016/j.jphs.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 116.Hashimoto, K., Fujita, Y. & Iyo, M. Phencyclidine-Induced Cognitive Deficits in Mice are Improved by Subsequent Subchronic Administration of Fluvoxamine: Role of Sigma-1 Receptors. Neuropsychopharmacology32, 514–521 (2007). 10.1038/sj.npp.1301047 [DOI] [PubMed] [Google Scholar]
- 117.Guo, L. et al. SKF83959 Attenuates Memory Impairment and Depressive-like Behavior during the Latent Period of Epilepsy via Allosteric Activation of the Sigma-1 Receptor. ACS Chem. Neurosci.13, 3198–3209 (2022). 10.1021/acschemneuro.2c00629 [DOI] [PubMed] [Google Scholar]
- 118.Rothman, R. B., Clark, R. D., Partilla, J. S. & Baumann, M. H. (+)-Fenfluramine and its major metabolite,(+)-norfenfluramine, are potent substrates for norepinephrine transporters. J. Pharmacol. Exp. Ther.305, 1191–1199 (2003). 10.1124/jpet.103.049684 [DOI] [PubMed] [Google Scholar]
- 119.Invernizzi, R., Fracasso, C., Caccia, S., Garattini, S. & Samanin, R. Effects of intracerebroventricular administration of d-fenfluramine and d-norfenfluramine, as a single injection or 2-HR infusion, on serotonin in brain: Relationship to concentrations of drugs in brain. Neuropharmacology30, 119–123 (1991). 10.1016/0028-3908(91)90194-G [DOI] [PubMed] [Google Scholar]
- 120.Scheurink, A. J. W., Leuvenink, H. & Steffens, A. B. Metabolic and hormonal responses to hypothalamic administration of norfenfluramine in rats. Physiol. Behav.53, 889–898 (1993). 10.1016/0031-9384(93)90265-H [DOI] [PubMed] [Google Scholar]
- 121.Caccia, S., Conforti, I., Duchier, J. & Garattini, S. Pharmacokinetics of fenfluramine and norfenfluramine in volunteers given d- and dl-fenfluramine for 15 days. Eur. J. Clin. Pharm.29, 221–224 (1985). 10.1007/BF00547426 [DOI] [PubMed] [Google Scholar]
- 122.Crockett, M. J. et al. Converging evidence for central 5-HT effects in acute tryptophan depletion. Mol. Psychiatry17, 121–123 (2012). 10.1038/mp.2011.106 [DOI] [PubMed] [Google Scholar]
- 123.Skvortsova, V. et al. A Causal Role for the Pedunculopontine Nucleus in Human Instrumental Learning. Curr. Biol.31, 943–954.e5 (2021). 10.1016/j.cub.2020.11.042 [DOI] [PubMed] [Google Scholar]
- 124.Fellows, L. K. & Farah, M. J. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain126, 1830–1837 (2003). 10.1093/brain/awg180 [DOI] [PubMed] [Google Scholar]
- 125.Izquierdo, A., Brigman, J. L., Radke, A. K., Rudebeck, P. H. & Holmes, A. The neural basis of reversal learning: an updated perspective. Neuroscience345, 12–26 (2017). 10.1016/j.neuroscience.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Browning, M. & Lan, D. What can reinforcement learning models of dopamine and serotonin tell us about the action of antidepressants? Comput. Psychiatry6, 166 (2022). [DOI] [PMC free article] [PubMed]
- 127.Gläscher, J., Daw, N., Dayan, P. & O’Doherty, J. P. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron66, 585–595 (2010). 10.1016/j.neuron.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kanen, J. W. et al. Serotonin depletion impairs both Pavlovian and instrumental reversal learning in healthy humans. Mol. Psychiatry26, 7200–7210 (2021). 10.1038/s41380-021-01240-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Worbe, Y. et al. Valence-dependent influence of serotonin depletion on model-based choice strategy. Mol. Psychiatry21, 624–629 (2016). 10.1038/mp.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo, Q. et al. Comparable roles for serotonin in rats and humans for computations underlying flexible decision-making. Neuropsychopharmacology49, 600–608 (2024). 10.1038/s41386-023-01762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rygula, R. et al. Role of Central Serotonin in Anticipation of Rewarding and Punishing Outcomes: Effects of Selective Amygdala or Orbitofrontal 5-HT Depletion. Cereb. Cortex25, 3064–3076 (2015). 10.1093/cercor/bhu102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bilderbeck, A. C. et al. Associations between mood instability and emotional processing in a large cohort of bipolar patients. Psychol. Med46, 3151–3160 (2016). 10.1017/S003329171600180X [DOI] [PubMed] [Google Scholar]
- 133.Quarmley, M. et al. Reduced safety processing during aversive social conditioning in psychosis and clinical risk. Neuropsychopharmacology44, 2247–2253 (2019). 10.1038/s41386-019-0421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCutcheon, R. A., Keefe, R. S. E. & McGuire, P. K. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol. Psychiatry28, 1902–1918 (2023). 10.1038/s41380-023-01949-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Robinson, L. J. & Nicol Ferrier, I. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross‐sectional evidence. Bipolar Disord.8, 103–116 (2006). 10.1111/j.1399-5618.2006.00277.x [DOI] [PubMed] [Google Scholar]
- 136.Schürmeyer, T. H., Brademann, G. & von zur Mühlen, A. Effect of fenfluramine on episodic ACTH and cortisol secretion. Clin. Endocrinol. (Oxf.)45, 39–45 (1996). 10.1111/j.1365-2265.1996.tb02058.x [DOI] [PubMed] [Google Scholar]
- 137.Chowdhury, N. S., Livesey, E. J., Blaszczynski, A. & Harris, J. A. Pathological Gambling and Motor Impulsivity: A Systematic Review with Meta-Analysis. J. Gambl. Stud.33, 1213–1239 (2017). 10.1007/s10899-017-9683-5 [DOI] [PubMed] [Google Scholar]
- 138.Van Breukelen, G. J. P. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J. Clin. Epidemiol.59, 920–925 (2006). 10.1016/j.jclinepi.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 139.Wright, D. B. & Osborne, J. E. Dissociation, cognitive failures, and working memory. Am. J. Psychol.118, 103–114 (2005). 10.2307/30039045 [DOI] [PubMed] [Google Scholar]
- 140.Egbewale, B. E., Lewis, M. & Sim, J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Med. Res. Methodol.14, 1–12 (2014). 10.1186/1471-2288-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clifton, L. & Clifton, D. A. The correlation between baseline score and post-intervention score, and its implications for statistical analysis. Trials20, 1–6 (2019). 10.1186/s13063-018-3108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Colwell, M. J. SSRA and Human Behaviour Study - Associated Raw Data and Scripts. Zenodo10.5281/zenodo.12599247 (2024).
- 143.Colwell, M. J., Murphy, S. & Harmer, C. J. Emotional Go/No-Go Task (Oxford). 10.5281/ZENODO.6207865 (2022).
- 144.Conley, M. I. et al. The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Res. 270, 1059–1067 (2018). 10.1016/j.psychres.2018.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tottenham, N. et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 168, 242–249 (2009). 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halgren, E., Raij, T., Marinkovic, K., Jousmäki, V. & Hari, R. Cognitive response profile of the human fusiform face area as determined by MEG. Cereb. Cortex10, 69–81 (2000). 10.1093/cercor/10.1.69 [DOI] [PubMed] [Google Scholar]
- 147.Ratcliff, R., Huang-Pollock, C. & McKoon, G. Modeling individual differences in the go/no-go task with a diffusion model. Decision5, 42 (2018). 10.1037/dec0000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Gee, J. W. et al. Pupil-linked phasic arousal predicts a reduction of choice bias across species and decision domains. Elife9, e54014 (2020). 10.7554/eLife.54014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and modelled datasets generated for this study have been deposited on Zenodo142 and Github: https://github.com/mjcolwell/SSRA_human_behaviour_data_and_code.
The code used to undertake data preprocessing, computational modelling, and inferential analyses in this study has been deposited on Zenodo142 and GitHub: https://github.com/mjcolwell/SSRA_human_behaviour_data_and_code.