Abstract
A low arousal threshold (LAT) is a pathophysiological trait of obstructive sleep apnea (OSA) that may be associated with brainstem ascending reticular activating system-cortical functional connectivity changes. We evaluated resting-state connectivity between the brainstem nuclei and 105 cortical/subcortical regions in OSA patients with or without a LAT and healthy controls. Twenty-five patients with moderate to severe OSA with an apnea–hypopnea index between 20 and 40/hr (15 with and 10 without a LAT) and 15 age- and sex-matched controls were evaluated. Participants underwent functional magnetic resonance imaging after overnight polysomnography. Three brainstem nuclei—the locus coeruleus (LC), laterodorsal tegmental nucleus (LDTg), and ventral tegmental area (VTA)—associated with OSA in our previous study were used as seeds. Functional connectivity values of the two brainstem nuclei (LC and LDTg) significantly differed among the three groups. The connectivity of the LC with the precuneus was stronger in OSA patients than in controls regardless of the concomitant LAT. The connectivity between the LDTg and the posterior cingulate cortex was also stronger in OSA patients regardless of the LAT. Moreover, OSA patients without a LAT showed stronger LDTg-posterior cingulate cortex connectivity than those with a LAT (post hoc p = 0.013), and this connectivity strength was negatively correlated with the minimum oxygen saturation in OSA patients (r = − 0.463, p = 0.023). The LAT in OSA patients was associated with altered LDTg-posterior cingulate cortex connectivity. This result may suggested that cholinergic activity may play a role in the LAT in OSA patients.
Subject terms: Sleep disorders, Neural circuits, Functional magnetic resonance imaging
Introduction
Obstructive sleep apnea (OSA) is a condition where the upper airway narrows or collapses during sleep. This can cause frequent arousals, sleep fragmentation, intermittent desaturation, and sympathetic activation1,2. Anatomically, most OSA patients have a narrowed upper airway that results in increased negative pressure during inspiration3. Moreover, nonanatomical factors, such as low arousal threshold (LAT), high loop gain, and poor upper airway muscle responsiveness, also contribute to the pathophysiology of OSA3,4. Arousal is associated with apnea termination and can play a protective role in OSA4. However, frequent arousal from sleep is also linked to sympathetic activation and leads to cardiometabolic complications or memory disturbance observed in OSA patients5,6.
The arousal threshold is a measure of the respiratory effort level that triggers arousal during sleep. Arousal responses differ between OSA patients, and 30–50% of them have a LAT7. Those with a LAT are prone to be easily aroused from sleep in response to relatively mild respiratory stimuli. The LAT can be identified noninvasively according to the following polysomnography results: (apnea–hypopnea index (AHI) < 30/hr) + (minimum oxygen saturation > 82.5%) + (fraction of hypopnea > 58.3%). Each criterion is scored as 1, and a score of more than 2 indicates a LAT in OSA patients8.
OSA patients with a LAT (OSA + LAT) are less obese, older, more likely to be female, and more likely to have rapid eye movement sleep-predominant OSA than those without a LAT (OSA-LAT)7,9,10. A LAT is associated with sleep discontinuity and poor compliance with PAP therapy9,11. Moreover, a LAT may be a therapeutic target in selected patients with OSA12. However, no studies have evaluated neural substrates or possible pathomechanisms associated with a LAT in OSA patients.
The ascending reticular activating system is a network of brainstem nuclei that is connected with cortical and subcortical regions and is involved in arousal and vigilance13. Patients with OSA may have a neural arousal-associated pattern generator that responds to an obstructive respiratory event14. We previously reported altered resting-state functional connectivity among three brainstem nuclei [the locus coeruleus (LC), laterodorsal tegmental nucleus (LDTg), and ventral tegmental area (VTA)] and cortical/subcortical regions in patients with moderate to severe OSA compared to that in controls15.
We hypothesized that the LAT in OSA patients may be related to the altered brainstem nuclei-cortical/subcortical functional connectivity. Our aim was to assess resting-state functional connectivity between the three preidentified brainstem nuclei and 105 cortical/subcortical regions in patients with moderate to severe OSA with or without a LAT compared to that in healthy controls.
Results
Patient characteristics
Twenty-five patients with moderate to severe OSA [apnea–hypopnea index (AHI) between 20/hr and 40/hr] and fifteen age- and sex-matched controls without OSA were evaluated. The mean participant age was 48 years old, and 34 (85.0%) were male, which was similar among the three groups. The Pittsburg Sleep Quality Index score was higher than that of the controls only in the OSA+LAT group (post hoc p = 0.005). The mean apnea, hypopnea, AHI, and minimum oxygen saturation in OSA patients were 8.6 ± 6.0/hr, 20.6 ± 5.9/hr, 29.2 ± 5.4/hr, and 81.0%, respectively. OSA + LAT patients showed a lower apnea index (post hoc p = 0.006) and higher minimum oxygen saturation than OSA-LAT patients (post hoc p = 0.001). No significant difference in sleep quality and daytime sleepiness was observed between the two groups (Table 1).
Table 1.
Patient characteristics.
| Control | OSA-LAT | OSA + LAT | p value | |
|---|---|---|---|---|
| n = 15 | n = 10 | n = 15 | ||
| Age | 46 ± 6 | 53 ± 10 | 46 ± 12 | 0.161 |
| Male (%) | 12 (80.0) | 8 (80.0) | 14 (93.3) | 0.52 |
| BMI | 25.5 ± 5.1 | 26.8 ± 4.6 | 25.4 ± 2.3 | 0.543 |
| Sleep Questionnaire | ||||
| BDI | 9.6 ± 5.7 | 10.5 ± 5.5 | 10.1 ± 5.4 | 0.846 |
| ESS | 6.4 ± 3.0 | 7.1 ± 3.7 | 7.1 ± 2.4 | 0.847 |
| PSQI | 5.6 ± 3.5 | 7.3 ± 3.1 | 10.4 ± 5.4** | 0.017 |
| PSG results | ||||
| TST (min) | 298.1 ± 90.4 | 280.7 ± 55.4 | 296.6 ± 36.5 | 0.59 |
| N1% | 17.5 ± 12.6 | 19.5 ± 8.9 | 24.3 ± 11.0* | 0.034 |
| N2% | 55.8 ± 11.2 | 47.4 ± 12.4 | 44.6 ± 13.8 | 0.047 |
| N3% | 10.8 ± 7.3 | 6.3 ± 11.4 | 7.3 ± 8.2 | 0.109 |
| REM% | 14.5 ± 7.6 | 13.8 ± 4.3 | 16.7 ± 6.5 | 0.498 |
| SE% | 83.0 ± 22.9 | 81.4 ± 10.8 | 86.4 ± 5.5 | 0.096 |
| SL (min) | 15.1 ± 24.4 | 10.1 ± 7.8 | 4.5 ± 3.1 | 0.192 |
| Arousal Index (/hr) | 15.1 ± 8.1 | 27.9 ± 9.8** | 30.2 ± 10.2** | 0.001 |
| Apnea Index (/hr) | 0.5 ± 1.3 | 12.6 ± 6.0** | 5.9 ± 4.4**## | < 0.001 |
| Hypopnea Index (/hr) | 2.7 ± 2.2 | 19.0 ± 6.8** | 21.7 ± 5.2** | < 0.001 |
| Hypopnea fraction (%) | 79.6 ± 33.4 | 59.7 ± 16.7 | 78.8 ± 15.2 | < 0.001 |
| AHI (/hr) | 3.2 ± 2.3 | 31.6 ± 6.3** | 27.6 ± 4.2** | 0.013 |
| minSat% | 90.9 ± 2.8 | 74.9 ± 7.2** | 85.1 ± 3.4**## | < 0.001 |
The data are expressed as the mean ± standard deviation or number (percent).
p value: for one-way ANOVA.
*and **: post hoc p value < 0.017 and < 0.01, respectively, compared with the controls, ##: post hoc p value < 0.01 compared to OSA-LAT patients.
Abbreviations: OSA, obstructive sleep apnea; LAT, low arousal threshold; BMI, body mass index; BDI, Beck Depression Inventory; ESS, Epworth Sleepiness Scale; PSQI, Pittsburgh Sleep Quality Index; TST, total sleep time; REM, rapid eye movement sleep; SE, sleep efficiency; SL, sleep latency; AHI, apnea–hypopnea index; minSat, minimum saturation.
Differences in brainstem nuclei–cortex connectivity between controls and OSA patients
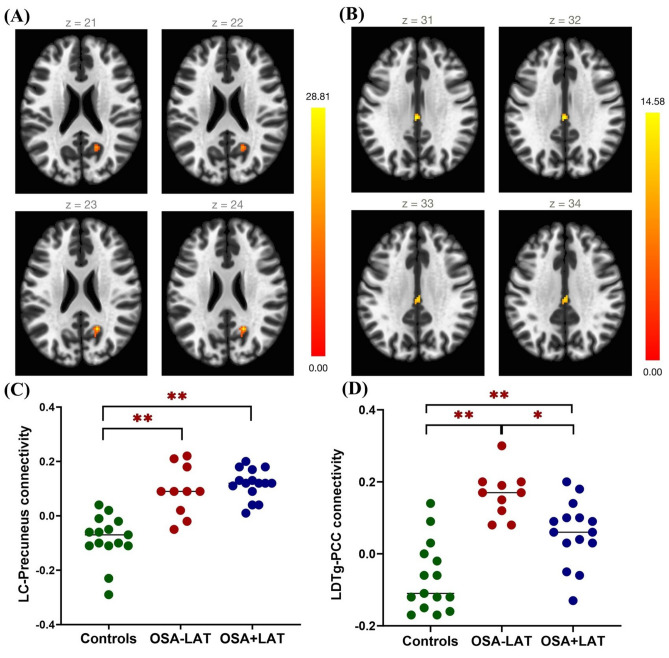
LC-precuneus and LDTg– posterior cingulate cortex functional connectivity differed among the three groups. Voxelwise functional connectivity t-maps of the LC and LDTg in the controls, OSA-LAT, and OSA + LAT patients are presented in Supplementary Fig. S1. Controls exhibited negative connectivity between the LC and the precuneus and between the LC and the posterior cingulate cortex. In contrast, OSA patients exhibited positive connectivity between the LC and the precuneus and the between the LC and the cingulate cortex regardless of the LAT. For LDTg, controls exhibited negative connectivity with the right lateral occipital cortex and cingulate cortex. In contrast, OSA-LAT patients showed positive LDTg connectivity with the cingulate cortex, and OSA + LAT patients showed positive LDTg connectivity with the precuneus regions.
Compared with the controls, OSA patients showed stronger functional connectivity between the LC and the precuneus regardless of the concomitant LAT (post hoc p < 0.001). The connectivity between the LDTg and the posterior cingulate cortex was also stronger in OSA patients regardless of the LAT (post hoc p < 0.001). Moreover, the LDTg-posterior cingulate cortex connectivity was stronger in the OSA - LAT group than in the OSA+LAT group (post hoc p = 0.013) (Table 2, Supplementary Table 1, and Fig. 1).
Table 2.
Between-group differences in functional connectivity (seed-to-voxel connectivity adjusted for age).
| Coordinates (MNI) | Peak region | Cluster size | Peak p (uncorr) | Size p (FDR) | ||
|---|---|---|---|---|---|---|
| X | y | z | ||||
| LC | ||||||
| + 18 | − 56 | + 24 | Precuneus cortex | 33 | < 0.000001 | 0.001163 |
| LDTg | ||||||
| − 02 | − 26 | + 32 | Posterior cingulate cortex | 33 | 0.000023 | 0.001381 |
Abbreviations: LC, locus coeruleus; LDTg, laterodorsal tegmental nucleus.
Figure 1.
Axial views of differences in voxelwise functional connectivity between the LC and the cortex or LDTg and the cortex when comparing patients with OSA-LAT vs. OSA + LAT vs. healthy controls. (A) Statistical maps of the regions where functional connectivity with the LC significantly differed among the three groups. (B) Statistical maps of the regions where connectivity with the LDTg significantly differed among the three groups (cluster-level p < 0.01, with false discovery rate correction p < 0.05). (C) The average functional connectivity value of the LC to the precuneus among controls, OSA-LAT, and OSA + LAT patients. (D) The average FC value of the LDTg to the posterior cingulate cortex among controls, OSA-LAT patients, and OSA + LAT patients. *p < 0.017, **p < 0.01, compared between two groups, post hoc Bonferroni correction. Abbreviations: OSA, obstructive sleep apnea; LAT, low arousal threshold; LC, locus coeruleus; LDTg, laterodorsal tegmental nucleus.
Relationship with sleep parameters
We next evaluated the correlation between the LDTg–posterior cingulate cortex connectivity and parameters determining LAT (values of the AHI, hypopnea fraction, and minimum oxygen saturation) in OSA patients. No significant correlation was found after Bonferroni correction. We only observed a trend towards negative correlations between functional connectivity strength between the LDTg and the posterior cingulate cortex and minimum oxygen saturation after controlling for age as a covariate (r = − 0.463, p = 0.023, Fig. 2). The correlation between the connectivity strength and hypopnea fraction (r = − 0.237, p = 0.266), or AHI (r = 0.312, p = 0.137) were non-significant. Moreover, experimental correlation analysis of clinical and polysomnography parameters that differed between groups also showed no significant results.
Figure 2.
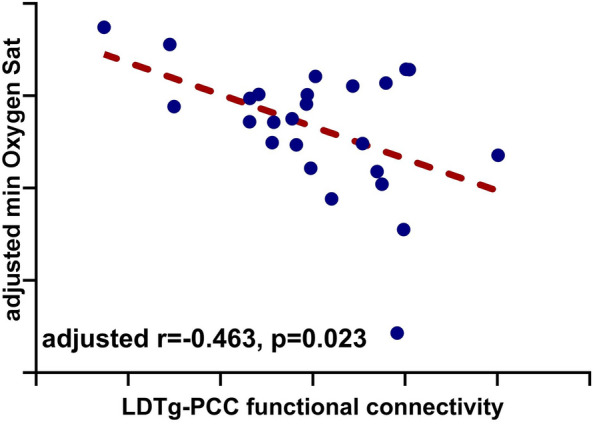
Correlation of connectivity strength between the LDTg-posterior cingulate cortex and minimum oxygen saturation. Partial Pearson correlation coefficient values showed negative correlations of LDTg-posterior cingulate cortex connectivity strength with minimum oxygen saturation. Abbretion: LDTg, Laterodorsal tegmental nucleus; FC, functional connectivity.
Discussion
We compared the resting-state functional connectivity of three ascending reticular activating system nuclei associated with OSA (the LC, LDTg, and VTA) and cortical/subcortical areas between OSA patients with or without a LAT and controls without OSA. As in our previous study15, LC and LDTg-cortical functional connectivity was enhanced in OSA patients. LC-precuneus cortex and LDTg–PCC connectivity was stronger in OSA patients than in controls regardless of the LAT. The LDTg-PCC connectivity was stronger in the OSA-LAT group than in the OSA + LAT group and tended to be negatively correlated with minimum saturation in OSA patients.
Patients with OSA + LAT usually awake more frequently during sleep, which may lead to lower sleep quality16. Accordingly, we found that only OSA + LAT, not OSA-LAT showed higher PSQI score thant the controls. However, because no significant difference in sleep questionnaire score was found between the OSA-LAT and OSA + LAT, and our experimental correlation analysis showed no significant association, effect of sleep-related symptom on functional connectivity was considered to be limited.
LDTg-posterior cingulate cortex connectivity differed according to the LAT in OSA patients. Pertinently, cholinergic neurons from the LDTg are part of the ascending reticular activating system, which is associated with arousal, and our results suggested that cholinergic activity may contribute to the concomitant LAT in OSA patients. The posterior cingulate cortex is also known as a crucial structure associated with arousal, and its functional connectivity changes according to arousal or awareness status17. Posterior cingulate cortex activation may be affected by cholinergic function because enhancing cholinergic activity through acetylcholine esterase inhibitors increases cerebral blood flow to the posterior cingulate cortex18.
Among the factors determining LAT, minimum oxygen saturation showed the strongest association with the LDTg-posterior cingulate cortex connectivity strength. OSA-LAT patients are often exposed to a higher hypoxic burden than OSA + LAT patients because arousal may not occur even in situations of severe hypoxia. In healthy volunteers, sustained hypoxia delays the time to arousal and increases the respiratory arousal threshold19. Hypoxic burden may affect LDTg-cortical connectivity. Hypoxia decreases the number of cholinergic neurons20 and acetylcholine synthesis in rat brains21. Exposure to hypoxia also reduces cerebral blood flow to the posterior cingulate cortex22. Increased FC between LDTg and posterior cingulate cortex in OSA may be a compensatory mechanism for hypoxic burden in patients with OSA, which can be more severe in OSA-LAT patients than in those with OSA + LAT.
Our results may imply that cholinergic activity is associated with OSA and the LAT. This suggested that modulating cholinergic function may be a therapeutic option that can improve OSA severity or the arousal threshold. Several studies have reported the effect of the acetylcholine esterase inhibitor donepezil on OSA severity. A placebo-controlled randomized trial showed that 10 mg donepezil in Alzheimer's disease patients with OSA improved AHI values and oxygen saturation23. Another study with nondemented participants reported improvement in AHI values, desaturation index values, and minimum oxygen saturation after one month of donepezil use24. However, the effect was not consistent. One randomized double-blind crossover study did not find a significant reduction in the AHI, minimum oxygen saturation or arousal threshold after donepezil administration for a single night25. Another randomized study also did not find changes in AHI values or minimum oxygen saturation after patients using donepezil for 1 month26.
Several limitations should be considered when interpreting the results of our study. This was a single-center study with a limited number of participants. To minimize the effect of OSA severity on brainstem-cortical FC, we only evaluated patients with an AHI between 20 and 40/hr. Moreover, the demographics of our OSA + LAT patients differed from those in prior studies. Therefore, caution is warranted when generalizing our results to other populations. Age was included as a covariate, but OSA disease duration couldn’t be due to practical limitations. The LAT was not measured directly but was estimated using the polysomnography parameter, which is known to have high sensitivity and specificity8. Moreover, the association between the LAT and LDTg- posterior cingulate cortex functional connectivity does not imply a causal relationship between the two. Further pharmacological or neuromodulation studies will be needed to establish a causal relationship between the connectivity and LAT.
Conclusions
This study suggested that cholinergic LDTg– posterior cingulate cortex functional connectivity is associated with the LAT in patients with moderate to severe OSA. Among the factors determining LAT, minimum oxygen saturation exhibited the strongest correlation with LDTg- posterior cingulate cortex connectivity. Future studies with larger numbers of patients are needed to elucidate the clinical implications of LDTg- posterior cingulate cortex connectivity for determining individualized treatment options in OSA patients.
Methods
Participants
We recruited 25 moderate to severe OSA patients with AHI between 20 and 40/hr (10 without LAT and 15 with LAT) and 15 controls without OSA. We previously recruited 50 moderate to severe OSA patients and 20 controls without OSA to report that higher AHI values were associated with increased ARAS-cortical FC15. Because one of the criteria for identifying LAT is AHI value < 30/hr8, we included only those with AHI between 20 and 40/hr to minimize the effect of the AHI on the ARAS-cortical FC. Twenty of the patients from our previous study who met the criteria (7 without LAT and 13 with LAT) and 5 additional newly recruited patients (3 without LAT and 2 with LAT) were included in this study. Fifteen age and gender-matched controls were selected from 20 healthy volunteers from our previous study15. For age matching, controls within ± 5 years of age difference were selected. Individuals with structural abnormalities, other sleep or neurological diseases (e.g., stroke or neurodegenerative disease) were excluded.
The participants first completed sleep questionnaire and then underwent overnight-PSG in accordance with their habitual sleep time. The resting-state fMRI was completed early in the morning, 2–3 h after wake-up time while awake.
This study was approved by the Institutional Review Board of Kyung Hee University Hospital in Gangdong (IRB no: 2020-12-010), and written informed consent was obtained from all participants. This study was performed in accordance to the Declaration of Helsinki.
Clinical assessment
Subjective sleep symptoms were evaluated by sleep questionnaires prior to PSG. The questionnaire included the Beck Depression Inventory (BDI)-II27, Epworth Sleepiness Scale (ESS)28, and Pittsburgh Sleep Quality Index (PSQI)29, which evaluate depression symptoms, daytime sleepiness, and sleep quality, respectively.
Polysomnography
Overnight PSG was performed using Grass-Telefactor twin version 2.6 (West Warwick, RI, USA) and scored manually according to the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events, version 2.630. Arousal was defined as abrupt frequency shift on electroencephalography channel for at least ≥ 3 s. Obstructive apnea was indicated as a decrease in airflow amplitude of ≥ 90% for at least ≥ 10 s along with evident respiratory effort. Hypopnea was indicated as a decrease in airflow amplitude of ≥ 30% relative to baseline for at least ≥ 10 s, accompanied by either ≥ 3% oxygen desaturation or an arousal. The AHI was calculated as the sum of apnea and hypopnea events per hour during sleep.
Image acquisition and preprocessing
MRI was performed the morning after polysomnography using a 3.0 Tesla MRI system with a 32-channel encoding head coil (Ingenia, Philips Medical System, Best, the Netherlands) and acquired and analyzed as in our previous study15,31. The following parameters were used to acquire resting-state fMRI data: repetition time (TR) = 2000 ms; echo time (TE) = 35 ms; field of view (FOV) = 220 × 220 mm; flip angle (FA) = 90°; acquisition voxel size = 3.3 × 3.3 × 3.3; reconstructed voxel size = 1.7 × 1.7 × 3.3 mm3; echo-planar imaging (EPI) factor = 33; and number of slices = 34. Structural three-dimensional (3D) T1-weighted (T1W) images were acquired with the following parameters: TR = 8.1 ms, TE = 3.7 ms, FA = 8°, FOV = 236 × 236 mm2, and voxel size = 1 × 1 × 1 mm3.
Statistical Parametric Mapping (SPM) version 12 software (Wellcome Department of Cognitive Neurology, London, UK) and the default preprocessing pipeline in the CONN-fMRI FC toolbox (https://www.nitrc.org/projects/conn) version 21a32 were used to process the images after discarding the first five scans as was done in our previous study15,31.
We applied default preprocessing pipeline in the CONN toolbox for preprocessing. Briefly, this included realigning the images to the first volume, unwarping, and adjusting for slice acquisition timing (interleaved bottom-up order). Next, the functional images were co-registered with the participants' structural data and spatially normalized to a standard MNI template space. To improve signal-to-noise ratio, we applied a spatial smoothing with 2-mm full-width half-maximum (FWHM) isotropic Gaussian kernel. Additionally, a component-based noise correction technique (CompCor) was employed to regress out and correct for head motion, physiological noise, and nuisance signals, including six motion parameters, signals from white matter, and cerebrospinal fluid (CSF) voxels. Finally, a band-pass filter (0.009–0.08 Hz) was applied and the signal was linearly detrended.
There was no significant difference in six motion realignment parameters (controls: 0.125 ± 0.057 vs. OSA-LAT: 0.145 ± 0.047 vs. OSA + LAT: 0.136 + 0.035, p = 0.577) or global signal change and framewise displacement (controls: 0.803 ± 0.048 vs. OSA-LAT: 0.779 ± 0.051 vs. OSA + LAT: 0.789 + 0.042, p = 0.433).
Region of interest seed region
We used three brainstem nuclei (LC, LDTg, VTA) identified in our previous study as region of interests. The brainstem nuclei were derived from the Harvard Ascending Arousal Network Atlas provided by the Martinos Center for Biomedical Imaging (Charleston, Massachusetts, USA, https://www.nmr.mgh.harvard.edu/resources/aan-atlas)33.
Seed-to-voxel functional connectivity analysis
Seed-to-voxel analysis was performed to measure functional connectivity between each brainstem nucleus and 105 cortical/subcortical regions of the Harvard–Oxford atlas embedded in the CONN toolbox34. Partial Pearson’s correlation analyses were performed to determine the temporal correlations between the blood oxygen level-dependent (BOLD) signals of each ARAS nucleus and other voxels in the whole brain. Group differences in ARAS-cortical FC were evaluated using generalized linear model (GLM) one-way analysis of variance (ANOVA) adjusting for age. To identify significant clusters, an uncorrected peak value threshold of a p value < 0.001 and a familywise error (FWE)-corrected threshold of p < 0.05 were applied.
Statistical analysis
All data are presented as the mean ± standard deviation. One-way ANOVA and post hoc Bonferroni correction were used to evaluate differences in demographics and clinical measures among the three groups: OSA + LAT, OSA-LAT, and controls. A chi-square test was used to analyze the categorical data. Among all OSA patients, Pearson partial correlation coefficients were calculated to assess the relationships between the aberrant brainstem-cortical functional connectivity and clinical variables determining LAT (AHI, Hypopnea fraction, and spO2 nadir) after adjusting for age. The significance level was set to 0.05, and the level of significance was p ≤ 0.05/3 (0.017) for the post hoc analysis. All statistical comparisons were conducted using SPSS (Version 22.0, Chicago, IL, USA).
Supplementary Information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) (No. NRF-2020R1C1C1013160).
Author contributions
J.-I.B.: conceptualization, formal analysis, funding acquisition, roles/writing—original draft; G.-H.J.: data curation, investigation, writing—review & editing; C.-W.R.: data curation, supervision; S.P.: data curation, investigation; K.H.L.: data curation, investigation, S.O.H.: data curation, investigation, K.-Y.J.: supervision, writing—review & editing, validation; W.C.S.: conceptualization, supervision, writing—review & editing.
Data availability
Data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ki-Young Jung and Won Chul Shin.
Contributor Information
Ki-Young Jung, Email: jungky@snu.ac.kr.
Won Chul Shin, Email: shin1chul@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68394-8.
References
- 1.Eckert, D. J. & Younes, M. K. Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol.116(3), 302–313 (2014). 10.1152/japplphysiol.00649.2013 [DOI] [PubMed] [Google Scholar]
- 2.Jordan, A. S., McSharry, D. G. & Malhotra, A. Adult obstructive sleep apnoea. Lancet383(9918), 736–747 (2014). 10.1016/S0140-6736(13)60734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNicholas, W. T. & Pevernagie, D. Obstructive sleep apnea: Transition from pathophysiology to an integrative disease model. J. Sleep Res.31(4), e13616 (2022). 10.1111/jsr.13616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert, D. J. & Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc.5(2), 144–153 (2008). 10.1513/pats.200707-114MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra, A. & Jordan, A. The importance of arousal in obstructive sleep apnea—Updates from the American Thoracic Society 2016. J. Thorac. Dis.8(Suppl 7), S542 (2016). 10.21037/jtd.2016.06.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djonlagic, I. et al. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS ONE7(3), e34106 (2012). 10.1371/journal.pone.0034106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hang, L.-W., Huang, C.-S. & Cheng, W.-J. Clinical characteristics of Asian patients with sleep apnea with low arousal threshold and sleep structure change with continuous positive airway pressure. Sleep Breath.25, 1309–1317 (2021). 10.1007/s11325-020-02235-6 [DOI] [PubMed] [Google Scholar]
- 8.Edwards, B. A. et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med.190(11), 1293–1300 (2014). 10.1164/rccm.201404-0718OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinchuk, A. et al. Prevalence, associated clinical features, and impact on continuous positive airway pressure use of a low respiratory arousal threshold among male United States Veterans With Obstructive Sleep Apnea. J. Clin. Sleep Med.14(5), 809–817 (2018). 10.5664/jcsm.7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino, T. et al. Estimated respiratory arousal threshold in patients with rapid eye movement obstructive sleep apnea. Sleep Breath.26(1), 347–353 (2022). 10.1007/s11325-021-02399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu, H. et al. Low arousal threshold is associated with unfavorable shift of PAP compliance over time in patients with OSA. Sleep Breath.25, 887–895 (2021). 10.1007/s11325-020-02197-9 [DOI] [PubMed] [Google Scholar]
- 12.Eckert, D. J. et al. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep37(4), 811–819 (2014). 10.5665/sleep.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlin, N. L. Brain circuitry mediating arousal from obstructive sleep apnea. Curr. Opin. Neurobiol.23(5), 774–779 (2013). 10.1016/j.conb.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahr, K. et al. Intensity of respiratory cortical arousals is a distinct pathophysiologic feature and is associated with disease severity in obstructive sleep apnea patients. Brain Sci.11(3), 282 (2021). 10.3390/brainsci11030282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byun, J.-I. et al. Altered functional connectivity of the ascending reticular activating system in obstructive sleep apnea. Sci. Rep.13(1), 8731 (2023). 10.1038/s41598-023-35535-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, W. H. et al. Association of low arousal threshold obstructive sleep apnea manifestations with body fat and water distribution. Life (Basel)13(5), 1218 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leech, R. & Sharp, D. J. The role of the posterior cingulate cortex in cognition and disease. Brain137(1), 12–32 (2014). 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iizuka, T. & Kameyama, M. Cholinergic enhancement increases regional cerebral blood flow to the posterior cingulate cortex in mild Alzheimer’s disease. Geriat. Gerontol. Int.17(6), 951–958 (2017). 10.1111/ggi.12818 [DOI] [PubMed] [Google Scholar]
- 19.Hlavac, M. C. et al. Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep29(5), 624–631 (2006). [PubMed] [Google Scholar]
- 20.Tanaka, H. et al. Effects of neonatal hypoxia on brainstem cholinergic neurons-pedunculopontine nucleus and laterodorsal tegmental nucleus. Brain Dev.17(4), 264–270 (1995). 10.1016/0387-7604(95)00043-B [DOI] [PubMed] [Google Scholar]
- 21.Gibson, G. E. & Duffy, T. E. Impaired synthesis of acetylcholine by mild hypoxic hypoxia or nitrous oxide. J. Neurochem.36(1), 28–33 (1981). 10.1111/j.1471-4159.1981.tb02373.x [DOI] [PubMed] [Google Scholar]
- 22.Rogan, M. et al. Hypoxia alters posterior cingulate cortex metabolism during a memory task: a 1H fMRS study. Neuroimage260, 119397 (2022). 10.1016/j.neuroimage.2022.119397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraes, W. et al. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study. Chest133(3), 677–683 (2008). 10.1378/chest.07-1446 [DOI] [PubMed] [Google Scholar]
- 24.Sukys-Claudino, L. et al. Beneficial effect of donepezil on obstructive sleep apnea: A double-blind, placebo-controlled clinical trial. Sleep Med.13(3), 290–296 (2012). 10.1016/j.sleep.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Li, Y. et al. The effect of donepezil on arousal threshold and apnea–hypopnea index. A randomized, double-blind, cross-over study. Ann. Am. Thorac. Soc.13(11), 2012–2018 (2016). 10.1513/AnnalsATS.201605-384OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunchaisri, N. & Chalermsuwiwattanakan, W. Efficacy of Donepezil in the treatment of obstructive sleep apnea: A placebo-controlled trial. J. Med. Assoc. Thai.99(Suppl 8), S31-s35 (2016). [PubMed] [Google Scholar]
- 27.Song, Y. M. et al. Reliability and validity of the Korean version of beck depression inventory-II via the internet: results from a university student sample. J. Korean Neuropsychiatr. Assoc.51(6), 402–408 (2012). 10.4306/jknpa.2012.51.6.402 [DOI] [Google Scholar]
- 28.Cho, Y. W. et al. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath.15(3), 377–384 (2011). 10.1007/s11325-010-0343-6 [DOI] [PubMed] [Google Scholar]
- 29.Sohn, S. I. et al. The reliability and validity of the Korean version of the Pittsburgh sleep quality index. Sleep Breath.16(3), 803–812 (2012). 10.1007/s11325-011-0579-9 [DOI] [PubMed] [Google Scholar]
- 30.American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events (American Academy of Sleep Medicine, 2015). [Google Scholar]
- 31.Byun, J. I. et al. Altered intrinsic brain functional network dynamics in moderate-to-severe obstructive sleep apnea. Sleep Med.101, 550–557 (2023). 10.1016/j.sleep.2022.12.003 [DOI] [PubMed] [Google Scholar]
- 32.Nieto-Castanon, A. & Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox (RRID:SCR_009550) Version 21 (Hilbert Press, 2021). [Google Scholar]
- 33.Edlow, B. L. et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol.71(6), 531–546 (2012). 10.1097/NEN.0b013e3182588293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect.2(3), 125–141 (2012). 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.