Abstract
In EGFR-mutated lung cancer, the duration of response to tyrosine kinase inhibitors (TKIs) is limited by the development of acquired drug resistance. Despite the crucial role played by apoptosis-related genes in tumor cell survival, how their expression changes as resistance to EGFR-TKIs emerges remains unclear. Here, we conduct a comprehensive analysis of apoptosis-related genes, including BCL-2 and IAP family members, using single-cell RNA sequence (scRNA-seq) and spatial transcriptomics (ST). scRNA-seq of EGFR-mutated lung cancer cell lines captures changes in apoptosis-related gene expression following EGFR-TKI treatment, most notably BCL2L1 upregulation. scRNA-seq of EGFR-mutated lung cancer patient samples also reveals high BCL2L1 expression, specifically in tumor cells, while MCL1 expression is lower in tumors compared to non-tumor cells. ST analysis of specimens from transgenic mice with EGFR-driven lung cancer indicates spatial heterogeneity of tumors and corroborates scRNA-seq findings. Genetic ablation and pharmacological inhibition of BCL2L1/BCL-XL overcome or delay EGFR-TKI resistance. Overall, our findings indicate that BCL2L1/BCL-XL expression is important for tumor cell survival as EGFR-TKI resistance emerges.
Subject terms: Non-small-cell lung cancer, Transcriptomics
Introduction
Despite improvements in therapeutics and advancements in research, lung cancer remains a leading cause of cancer-related deaths [1]. The discovery of mutations in the epidermal growth factor receptor (EGFR) gene allowed for the development of tyrosine kinase inhibitors (TKIs) as first-line treatment for advanced/metastatic non-small-cell lung cancer harboring EGFR mutations. Treatment with EGFR-TKIs results in a rapid response and improves both progression-free and overall survival [2–4]. Despite these responses, acquired resistance to EGFR-TKIs invariably develops. Such limitations of EGFR-TKI monotherapy prompted us to investigate mechanisms underlying drug resistance and tolerance. It is reported that tumor cells transiently maintain a drug-tolerant state following treatment with targeted therapies, allowing tumor cell subpopulations to withstand and adapt to those drugs [5–7]. This subpopulation, called drug-tolerant persister (DTP) cells, exhibits a relatively slow cell cycle and survives during drug treatment. These characteristics are reversible once the drug is withdrawn. Currently, there is no standardized protocol to establish DTP pre-clinical models [8].
Evasion of apoptotic cell death is a well-known phenomenon in tumor cells, one that leads to drug resistance [7]. A common molecular mechanism used by tumor cells to achieve drug resistance is the overexpression of anti-apoptotic proteins and downregulation of pro-apoptotic proteins [9]. Therefore, we focused on apoptosis-related genes to define mechanisms of EGFR-TKI resistance. The B Cell Lymphoma 2 (BCL-2) family includes both pro-apoptotic (BAX, BAK) and anti-apoptotic (BCL-2, BCL-XL, BCL-w, MCL1) members. Their balanced activity largely governs cell fate decisions between cell survival and death [10]. The inhibitor of apoptosis protein (IAP) family, which consists of eight members (NAIP, BIRC2, BIRC3, XIAP, BIRC5, BIRC6, BIRC7, and BIRC8), is also highly expressed in a variety of human malignancies [11]. Recent studies suggest that anti-apoptotic molecules such as BCL-XL and MCL1 may function in DTP formation in EGFR-mutated lung cancer [12–16]. However, many previous studies relied primarily on cell line models, potentially overlooking the impact of tumor heterogeneity and the tumor microenvironment.
Currently, single-cell RNA sequence (scRNA-seq) technologies can assess gene expression at single-cell resolution and provide extensive insight into the cellular composition of tumors [17, 18]. Although lack of a spatial context of cells is a challenge for scRNA-seq analysis, recent advances in spatial transcriptomics (ST) have enabled elucidation of spatial heterogeneity and the tumor microenvironment [19], and are revolutionizing our understanding of cancer biology. However, changes in expression profiles of apoptosis-related genes occurring as resistance to EGFR-TKI emerges have not been evaluated using such multi-omic approaches.
In this study, we perform scRNA-seq to analyze gene expression profiles, including those of apoptosis-related genes, and their response to treatment in EGFR-mutated lung cancer using human clinical samples and cell lines and also perform ST analyses using transgenic mouse specimens. We find that BCL2L1, which encodes the anti-apoptotic protein BCL-XL, is highly expressed predominantly in tumor cells, where it likely plays a crucial role in tumor cell survival as drug tolerance and acquired resistance to EGFR-TKIs emerge. We also demonstrate the efficacy of targeting BCL-XL in combination with EGFR-TKI treatment to prevent resistance, not only in cell lines but in transgenic mice, which better reflect characteristics of tumors and the tumor microenvironment, as well as anti-tumor immune responses.
Results
BCL2L1 promotes survival of DTPs and drug-resistant cells after osimertinib treatment in cell line models
To investigate cellular responses underlying the acquisition of osimertinib resistance, we used scRNA-seq data from polyclonal osimertinib-resistant cells that we previously generated using H1975 or PC9-erlotinib-resistant (ER) lines [15]. Specifically, we analyzed the expression of apoptosis-related genes in parental lines, OR30 cells, which survive in the presence of 30 nM osimertinib, and OR2000 cells, which are resistant to 2000 nM osimertinib, as respective models of untreated, DTP, or tumor-recurrent states (see Fig. 1 for study workflow) [15]. We observed significantly higher BCL2L1 expression in OR30 and OR2000 cells than in parental cells, while MCL1 expression was comparable in parental cells and OR30 cells and decreased in OR2000 cells in both lines (Fig. 2A, B and Supplementary Fig. 1A, B). Expression of other apoptosis-related genes of the BCL-2 and IAP families was either undetectable in >75% of cells or showed no consistent trend in the cell lines used (Fig. 2A, B, Supplementary Fig. 2A–C and 3A–C). This analysis suggests that increased BCL2L1 expression is a predominant change seen as cells acquire EGFR-TKI resistance.
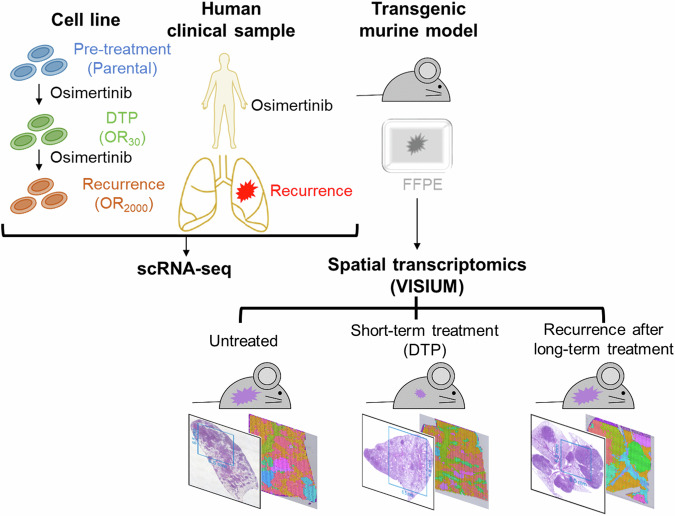
Fig. 1. Overview of study.
To assess apoptosis-related gene expression in tumors following EGFR-TKI treatment, we established cell line models (top, left) representing untreated, drug-tolerant persister (DTP), and acquired resistance states using EGFR-mutated lung cancer lines and subjected them to single-cell RNA sequencing (scRNA-seq). To validate those results and compare gene expression profiles between tumor and normal cells, we conducted scRNA-seq on samples collected from patients with EGFR-mutated lung cancer pre-treatment or after the development of EGFR-TKI resistance (top, middle). To confirm scRNA-seq findings and assess spatial heterogeneity, we prepared formalin-fixed paraffin-embedded (FFPE) lung tissue sections from transgenic mice (top, right) harboring EGFR-driven lung cancer representative of three distinct states: untreated, short-term drug treatment reflecting the DTP state, and tumor recurrence after long-term treatment. Spatial transcriptomics was subsequently conducted.
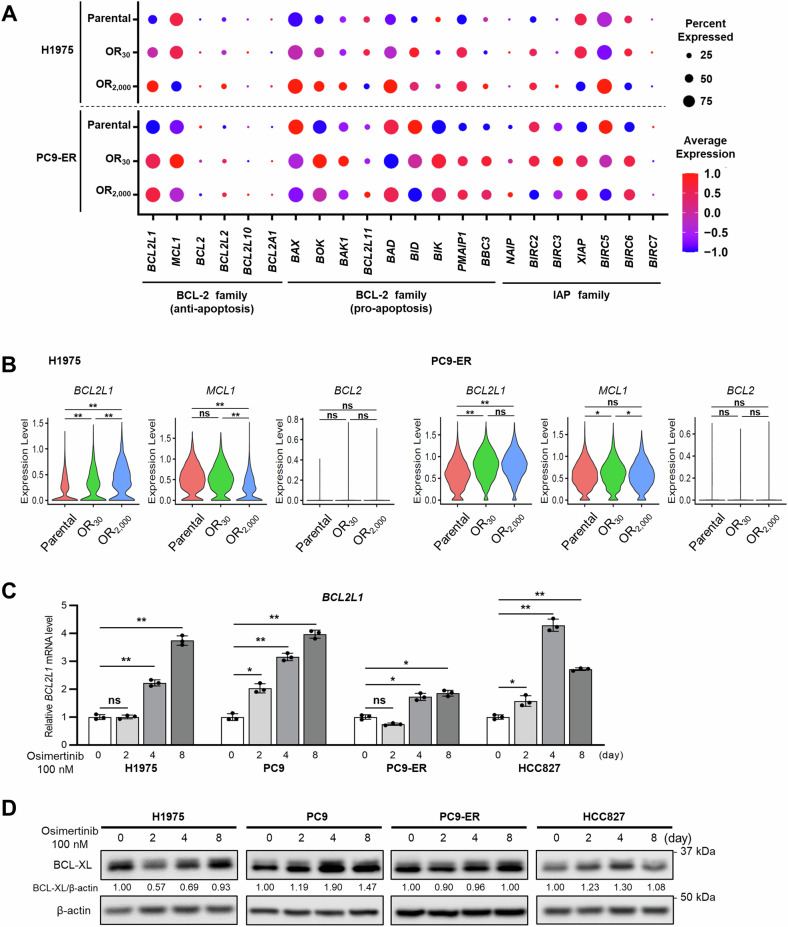
Fig. 2. Osimertinib induces BCL2L1 expression in EGFR-mutated lung cancer cell lines.
A Dotplots based on scRNA-seq showing apoptosis-related gene expression in H1975 (top) and PC9-ER (bottom) cells. B Violin plots based on scRNA-seq showing BCL2L1, MCL1, and BCL-2 expression in H1975 and PC9-ER cells. p-values were calculated using Kruskal–Wallis one-way ANOVA. C Relative BCL2L1 transcript levels in indicated EGFR-mutant cell lines based on real-time PCR. Each line was treated with 100 nM osimertinib and evaluated at 0, 2, 4, and 8 days. Data are means ± SD of triplicates from one experiment and are representative of three independent experiments. A Repeated measures one-way ANOVA with Dunnett’s test was used to compare treatment groups to a single control group. D Immunoblot analysis showing changes in BCL-XL expression in EGFR-mutant lung cancer lines treated with 100 nM osimertinib for 0, 2, 4, and 8 days. The BCL-XL/actin ratio was calculated using ImageJ software. All blots show representative images of three independent experiments. Asterisks indicate p-values as follows: *p < 0.05; **p < 0.005; ns, not significant. Data are presented as the mean ± SD.
To validate scRNA-seq results in the DTP state, we confirmed changes in mRNA and protein levels of anti-apoptotic genes after treatment with osimertinib using four EGFR-mutated cell lines. When cells were treated with 100 nM osimertinib, a concentration higher than IC50s [20], BCL2L1 mRNA levels significantly increased over time after osimertinib treatment in all lines tested (Fig. 2C). BCL-XL protein levels increased gradually after EGFR-TKI treatment or decreased 2 days after the start of EGFR-TKI treatment and then generally increased over 8 days of treatment (Fig. 2D). These results suggest that BCL-XL may be important for tumor cell survival during emergence of drug tolerance and development of acquired resistance to EGFR-TKIs.
BCL2L1 is specifically highly expressed in tumors in clinical samples
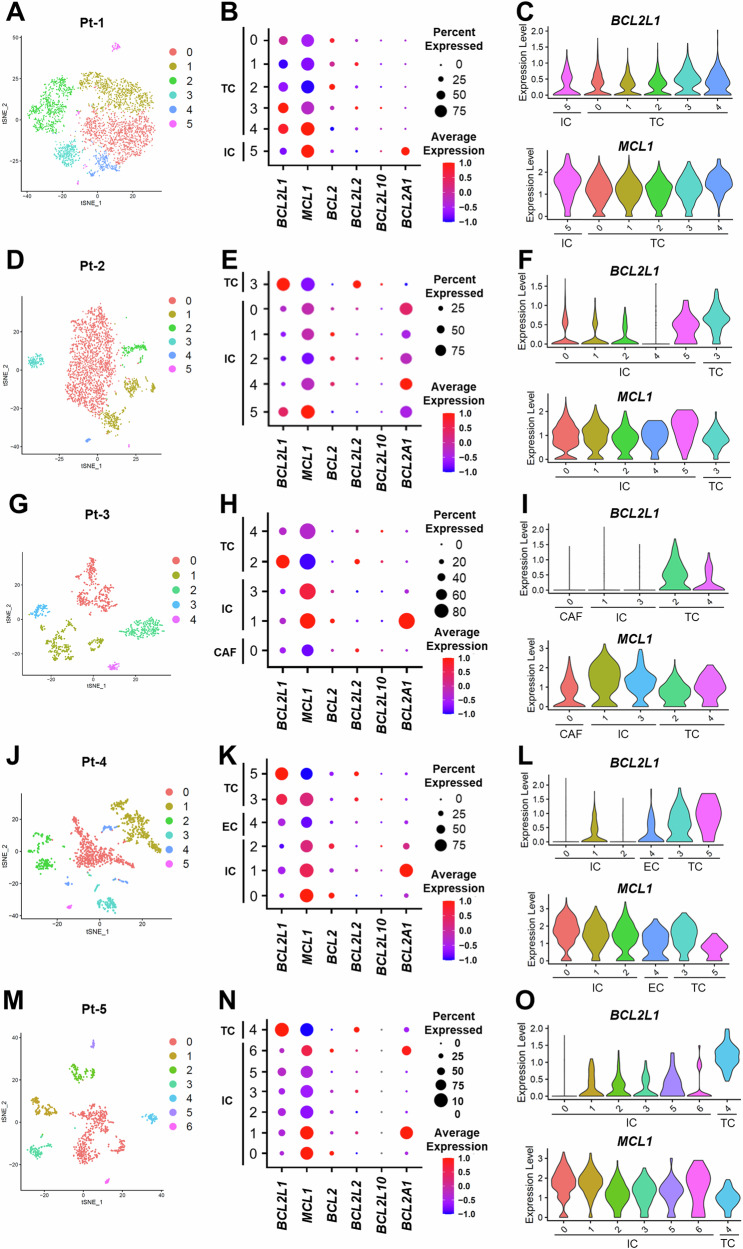
Next, we analyzed scRNA-seq data from patients with EGFR-mutated lung cancer before EGFR-TKI treatment and after the development of drug resistance to confirm results seen in cell lines and compare gene expression levels in tumor and non-tumor cells (Fig. 1). To do so, we analyzed scRNA-seq data from five clinical samples: two osimertinib failure cases (Pt-1 and Pt-4), two first- or second-generation EGFR-TKI failure cases (Pt-2 and Pt-3), and one untreated case (Pt-5). Characteristics of Pt-1, Pt-2, Pt-3, and Pt-4 have been previously reported [15], while characteristics of Pt-5 are shown in Supplementary Table 1. Clusters were annotated based on expression of representative marker genes, as previously reported [15]: those included EPCAM for epithelial cells, PTPRC for CD45-positive immune cells, VCAM1 for cancer-associated fibroblasts, and VWF for endothelial cells. We employed inferCNV to predict copy number variations to identify distinct copy number profiles in EPCAM-positive tumor cells compared to non-tumor cells (Supplementary Fig. 4A–C and 5A, B). In all cases, a high percentage of tumor cells expressed BCL2L1 and MCL1 as anti-apoptotic genes of the BCL-2 family (Fig. 3B, E, H, K, N). However, BCL2L1 was highly expressed specifically in tumor cells, while MCL1 was expressed at lower levels in tumors compared to non-tumor cells (Fig. 3A–O). Among pro-apoptotic BCL-2 family genes, BID expression was lower in tumor cells, and in the IAP family, XIAP expression tended to be higher in tumors relative to non-tumor cells (Supplementary Fig. 6). To validate our findings, we analyzed another scRNA-seq dataset (GSE146100) [21] from lung adenocarcinoma patients. We consistently found that BCL2L1 and XIAP were highly expressed specifically in tumor cells but not in non-tumor cells. Conversely, MCL1 and BID expression were lower in tumor cells compared to non-tumor cells. (Supplementary Fig. 7A–E).
Fig. 3. Apoptosis-related gene expression in clinical samples.
A, D, G, J, M t-SNE plots showing clusters based on scRNA-seq in indicated patients. B, E, H, K, N Dot plots showing differences in expression of anti-apoptotic genes in tumor versus non-tumor cells. C, F, I, L, O Violin plots showing BCL2L1 (upper) and MCL1 (lower) expression in indicated cell types, indicating that BCL2L1 is specifically and highly expressed in tumor cells. TC tumor cell, IC immune cell, EC endothelial cell, CAF cancer-associated fibroblast.
Identification of inter- and intra-tumor heterogeneity using spatial transcriptomics
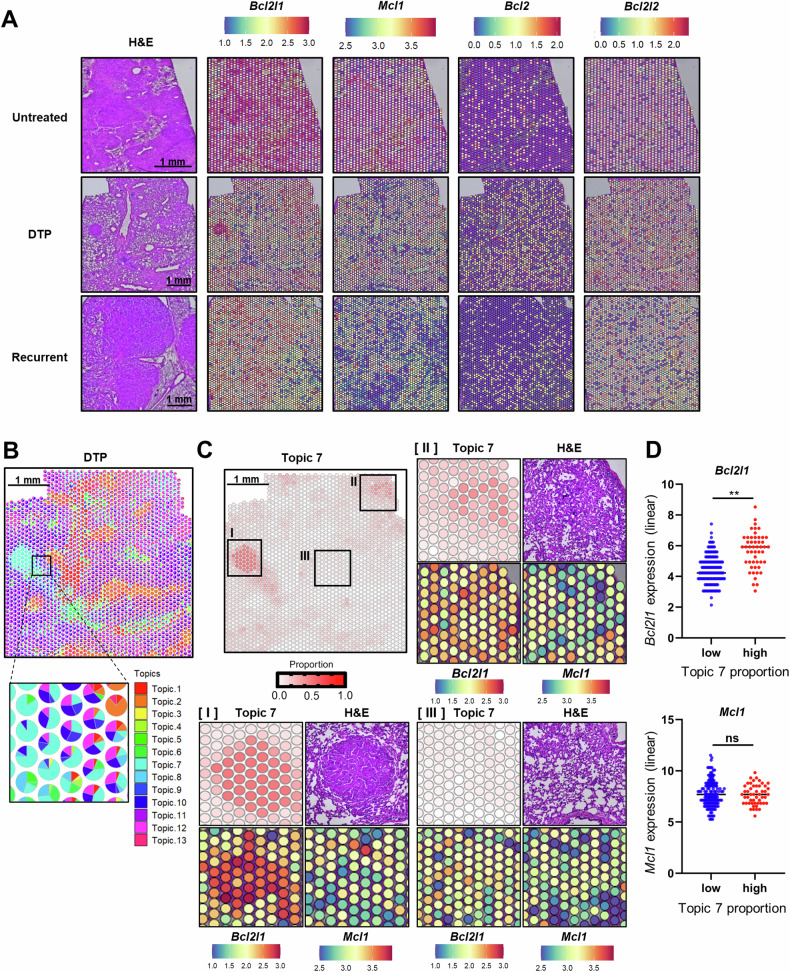
Next, to confirm scRNA-seq findings and assess potential inter- and intra-tumor heterogeneity of anti-apoptotic gene expression, we visualized gene expression spatially using Visium analysis of sections from lung-specific EGFR-L858R-T790M (EGFRTL/CCSP-rtTA) transgenic mice to compare samples that were (1) untreated, (2) treated short-term to represent the DTP state, and (3) treated long-term to represent the recurrent state. Although we observed intra-tumor heterogeneity, Bcl2l1 expression in tumor lesions was higher than in non-tumor lesions in all states (Fig. 4A), whereas Mcl1 expression was less tumor-specific and relatively lower in recurrent lesions. Furthermore, Bcl2 and Bcl2l2 expression in tumor cells were relatively low and not increased by EGFR-TKI treatment (Fig. 4A). These results overall are consistent with scRNA-seq findings.
Fig. 4. Visualization of inter- and intra-tumor heterogeneity using spatial transcriptomics.
A H&E staining and Bcl2l1, Mcl1, Bcl2, and Bcl2l2 expression in three lung-specific EGFR-L858R-T790M (EGFRTL/CCSP-rtTA) transgenic mouse samples representing untreated (top), DTP (middle), and tumor-recurrent (bottom) states. B Deconvolved cell-type proportions for the DTP dataset, represented as pie charts for each pixel. C Pixel proportions showing the distribution of topic 7. Three areas with high (I), medium (II), and low (III) proportions of topic 7 are extracted. Topic 7 proportion, H&E staining, and Bcl2l1 and Mcl1 expression levels are visualized in each area. D Spots were divided into low (≥25%, <50%) and high (≥50%) groups based on the size of the Topic 7 proportion. Bcl2l1 and Mcl1 expression levels were compared between these two groups. p-values were calculated using the unpaired two-tailed Welch’s t-test. Asterisks indicate p-values as follows: **p < 0.005. ns not significant, H&E hematoxylin and eosin, DTP drug-tolerant persister.
Each Visium spot contains multiple cells (spot diameter = 55 µm). Therefore, we used a reference-free deconvolution approach (STdeconvolution) [22] to identify cell types as “Topics” from spot gene expression profiles and assessed their relative proportions in that population. In the DTP state, we identified 13 different cell types (“Topics”) and visualized their relative proportions within a single spot (Fig. 4B, Supplementary Figs. 8 and 9A). The deconvolved transcriptional profile of each “Topic” is shown in Supplementary Table 2 and Supplementary Fig. 9B. Topic 7 was annotated as tumor cells based on that profile, and Topic 7 distribution was consistent with residual tumor lesions, based on hematoxylin and eosin staining (Fig. 4C). We then visualized Bcl2l1 and Mcl1 levels based on high (Fig. 4C I), moderate (Fig. 4C II), and low (Fig. 4C III) Topic 7 proportions. Interestingly, Bcl2l1 expression levels correlated positively with the size of the Topic 7 proportion, while Mcl1 expression levels did not. Indeed, Bcl2l1 expression was significantly higher in spots with high (≥50%) Topic 7 proportions than in those with low (≥25%, <50%) Topic 7 proportions. In contrast, no significant difference in Mcl1 expression was detected between the two groups (Fig. 4D). Taken together, Bcl2l1 is more highly expressed in residual tumor cells in comparison to neighboring stromal and immune cells.
BCL-XL loss in lung cancer cells blocks the emergence of drug-tolerant cells and suppresses osimertinib resistance in vitro and in vivo
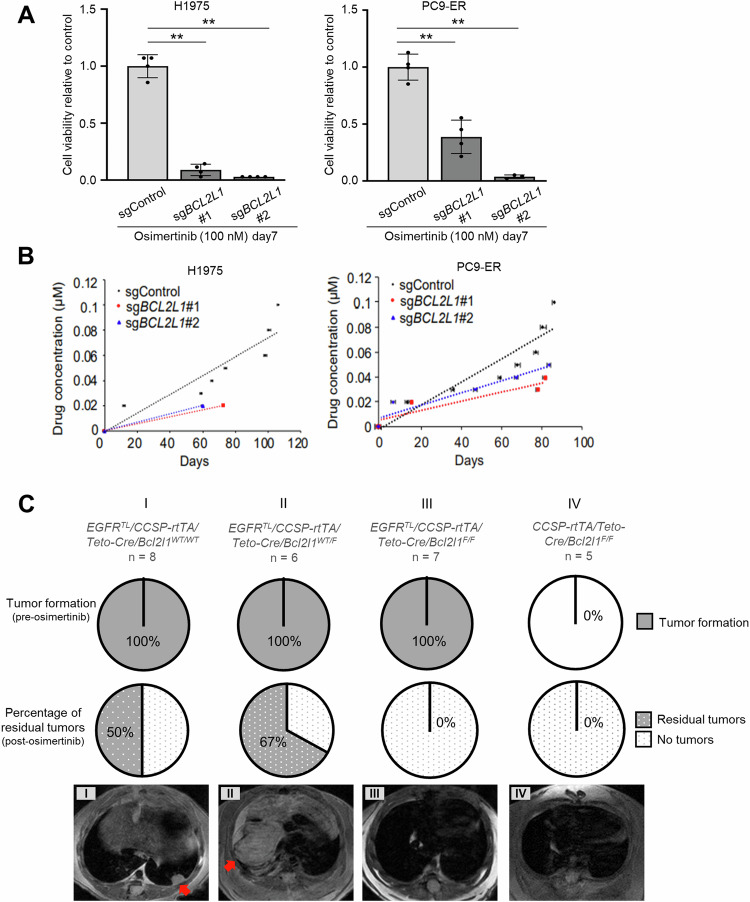
Our findings suggest that tumor-specific BCL2L1 expression likely plays an important role in tumor cell survival, especially in DTP and recurrent states. Thus, we asked whether genetic BCL2L1 deletion would prevent the emergence of EGFR-TKI-resistant tumors. To do so, we first generated BCL2L1 knockout (KO) H1975 and PC9-ER cells using CRISPR–CAS9 (Supplementary Fig. 10A) and treated control and BCL2L1 KO cells for 7 days with osimertinib. After counting surviving cells, we observed significantly fewer cells in BCL2L1 KO compared to control cells (Fig. 5A). To determine whether BCL2L1 deletion delayed the emergence of resistant clones, we treated control and BCL2L1 KO cells with increasing concentrations of osimertinib. Control H1975 and PC9-ER cells showed resistance to 100 nM osimertinib in ~100 and 80 days, respectively. However, BCL2L1 KO H1975 and PC9-ER cells failed to grow in the medium with 20 nM and 30–40 nM osimertinib, respectively (Fig. 5B).
Fig. 5. BCL-XL deletion in lung cancer blocks the emergence of drug-tolerant cells and counters osimertinib resistance in vitro and in vivo.
A The number of cells remaining on day 7 after treatment with 100 nM osimertinib in H1975 (left) and PC9-ER (right) cells transduced with indicated sgRNAs. Data are the mean ± SD of triplicates from one experiment and are representative of three independent experiments. p-values were calculated using one-way ANOVA followed by the Tukey–Kramer multiple-comparison test. Asterisks indicate p-values as follows: **p < 0.005. B Comparison of the time course of acquired osimertinib resistance in H1975 (left) and PC9-ER (right) cells transduced with indicated sgRNAs. Cells were chronically exposed to gradually increasing osimertinib doses. C (top) The proportion of indicated transgenic mice exhibiting tumor formation after doxycycline administration; (middle) the percentage of mice exhibiting residual tumors after completion of osimertinib treatment; and (bottom) representative MRI images after completion of osimertinib treatment. Red arrows indicate lung tumors.
To confirm these findings in vivo, we crossed EGFRTL/CCSP-rtTA mice [23] with conditional Bcl2l1 knockout mice [24] that had been crossed with tetracycline-inducible Cre-expressing mice (TetO-Cre/Bcl2l1WT/F or TetO-Cre/Bcl2l1F/F). In resultant EGFRTL/CCSP-rtTA/TetO-Cre/Bcl2l1WT/WT or WT/F or F/F mice, Cre recombination and induction of EGFR-L858R-T790M occur selectively in pulmonary epithelial cells upon doxycycline treatment (Supplementary Fig. 10B). After doxycycline treatment, we observed tumor formation in all murine lungs, regardless of Bcl2l1 genotype, suggesting that Bcl2l1 KO has no effect on tumor formation (Fig. 5C). After induction of tumor formation by doxycycline treatment, we treated mice with osimertinib using a modified intermittent dosing protocol defined in the Methods section [25]. MRI analysis showed that half of the EGFRTL/CCSP-rtTA/TetO-Cre/Bcl2l1WT/WT mice exhibited resistant tumors, and 4 of 6 mice that carried EGFRTL/CCSP-rtTA/TetO-Cre/Bcl2l1WT/F exhibited resistant tumors after 4 cycles of treatment (Fig. 5C I and II). In contrast, no EGFRTL/CCSP-rtTA/TetO-Cre/Bcl2l1F/F mice exhibited resistant tumors (Fig. 5C III). These observations suggest that BCL2L1/BCL-XL expression enables tumor cells to resist eradication by EGFR-TKIs.
Combining osimertinib with BCL-XL inhibitors suppresses osimertinib resistance
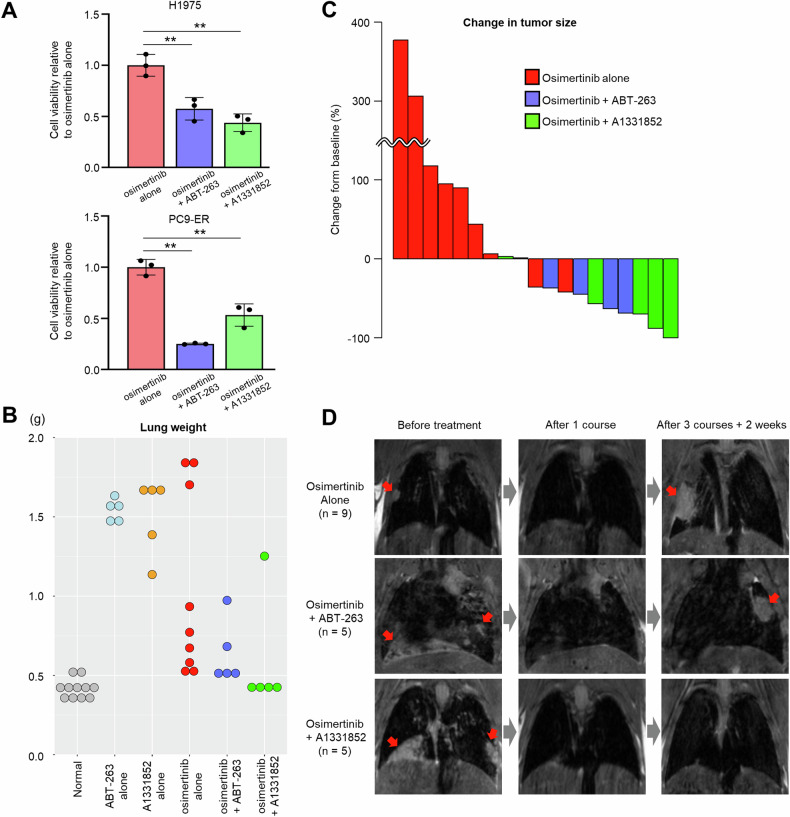
To determine whether pharmacological BCL-XL inhibition combined with osimertinib treatment blocks drug resistance in EGFR-mutated lung cancer, we treated H1975 or PC9-ER cells 7 days with either osimertinib alone or with a combination of osimertinib plus a BCL-XL inhibitor (either ABT-263 or A1331852). Quantification of surviving cells revealed a significant reduction in cell viability following combination therapy with either inhibitor compared to osimertinib alone (Fig. 6A). To determine whether BCL-XL inhibitor treatment delayed the emergence of resistant clones, we treated H1975 cells with a combination of osimertinib plus either of the 2 BCL-XL inhibitors using increasing osimertinib concentrations. Cells treated with osimertinib plus either of the 2 BCL-XL inhibitors required a longer time period to develop resistance to osimertinib compared to cells treated with osimertinib alone (Supplementary Fig. 11).
Fig. 6. Combining osimertinib with pharmacological BCL-XL inhibition suppresses osimertinib resistance.
A The number of cells remaining after 7 days of treatment with 100 nM osimertinib plus either 1 µM ABT-263 or 1 µM A1331852 in H1975 (top) and PC9-ER (bottom) cells. Data are the mean ± SD of triplicates from one experiment and are representative of three independent experiments. p-values were calculated using one-way ANOVA followed by the Tukey–Kramer multiple-comparison test. Asterisks indicate p-values as follows: **p < 0.005. B Lung weight at the end of the experiment. Normal lungs were derived from CCSP-rtTA transgenic mice (no EGFRTL expression). C Waterfall plot showing changes in tumor size from baseline (before treatment) to the experimental endpoint. The bars shown represent a single mouse. D Representative MRI images of mouse lung before and after drug treatment. Red arrows indicate lung tumors. Drug administration protocols are provided in the Methods section.
To confirm these findings in vivo, we treated EGFRTL/CCSP-rtTA mice with osimertinib plus either ABT-263 or A1331852, and also with each of the 3 drugs as single agents, and assessed lung weight at the endpoint. The maximum weight of normal lungs obtained from CCSP-rtTA transgenic mice (no EGFRTL expression) was <0.53 g. Notably, we observed lung weights <0.53 g in 2 of 9 (22%) osimertinib-only mice, in 3 of 5 (60%) osimertinib + ABT-263 mice, and in 4 of 5 (80%) osimertinib + A1331852 mice (Fig. 6B). We observed that in groups treated with either ABT-263 or A1331852 alone, all lungs weighed >1.0 g and exhibited massive tumors (Fig. 6B). When comparing tumor sizes post-treatment to their pre-treatment size, only 2 of 9 mice (22%) in the osimertinib-only group showed tumor reduction. Moreover, in groups treated with osimertinib combined with either ABT-263 or A1331852, 4 of 5 mice in each group (80%) exhibited tumor reduction (Fig. 6C). Interestingly, tumor size reduction was greater in the osimertinib + A1331852 combination group. Representative MRI images of this analysis are shown in Fig. 6D.
Discussion
In this study, we used comprehensive scRNA-seq and ST analysis to identify changes in the expression of apoptosis-related genes after EGFR-TKI treatment and to assess tumor heterogeneity in EGFR-mutated lung cancer. We demonstrated that BCL2L1 is specifically expressed in tumor cells and plays an important role in tumor survival, especially in DTPs and in cells that have acquired resistance to osimertinib treatment.
Apoptosis-related genes reportedly function in tumorigenesis and tumor growth, as well as in drug resistance in some solid tumors [26, 27]. Recently, various DTP cell markers have been reported to function in or mediate EGFR-TKI resistance in EGFR-mutated lung cancer, and these could, therefore, serve as novel therapeutic targets [15, 28, 29]. Apoptosis-related genes are known to function in DTP cell formation by acting downstream of these DTP cell marker genes [8, 30]. Our study demonstrated that BCL-XL expression increases at both the mRNA and protein levels following EGFR-TKI treatment and showed that deletion or inhibition of BCL-XL overcame or delayed EGFR-TKI resistance. Interestingly, we observed tumor formation in BCL2L1 KO mouse lungs, suggesting that BCL2L1 plays a crucial role in drug resistance but has no impact on tumorigenesis in EGFR-mutated lung cancer. Others report that MCL1 activity is important for DTP cell emergence [14]. It is also reported that inhibition of both BCL-XL and MCL1 in cell culture models promotes extensive apoptosis in EGFR-mutated lung cancer cells that display an EMT phenotype [31]. However, MCL1 activity in tumor cells varies depending on transcriptional and cellular contexts. In EGFR-mutated and ALK-rearranged lung cancer cell lines, MCL1 translocates to the nucleus and binds to FBW7 after TKI treatment, which leads to the degradation of MCL1 [32, 33]. MCL1 is also expressed in normal immune cells and is involved in immune cell survival [34]. In the TCGA dataset of lung adenocarcinoma, expression of MCL1, BCL2, BCL2L2, and BCL2A1 was significantly higher in non-tumor relative to tumor cells (Supplementary Fig. 12). Moreover, our scRNA-seq analysis using human clinical specimens supported the idea that MCL1 is more highly expressed in non-tumor than in tumor cells, and our ST data also suggests that Mcl1 expression is not specific to tumors. Interestingly, in a clinical trial against relapsed or refractory hematological malignancies, some MCL1 inhibitors were reported to promote on-target/off-tumor toxicity, and in that trial, administration of the MCL1 inhibitor AMG-397 was suspended due to cardiac side effects [35]. In addition, in a phase I study of the MCL1 inhibitor AMG-176 against relapsed or refractory multiple myeloma, treatment-emergent adverse events of grade 3 or higher occurred in 62% of patients, the most common being neutropenia, anemia, and hypertension [36]. The maximum tolerable AMG-176 dose was not reached, and this trial is also on voluntary hold. Therefore, MCL1 inhibitors are not well-tolerated and are associated with side effects potentially due to effects on normal cells. On the other hand, phase I clinical trials of osimertinib combined with ABT-263 (Navitoclax) for patients with EGFR-mutated lung cancer after EGFR-TKI failure have been conducted, and dosing of osimertinib 80 mg daily and navitoclax 150 mg daily was well tolerated [37]. Our study showing that BCL2L1 expression is higher in tumor cells than in non-tumor cells supports the outcome of this clinical trial. However, combining ABT-263 with osimertinib has not shown striking positive effects for patients with EGFR-mutated lung cancer after EGFR-TKI failure. ABT-263 inhibits not only BCL-XL but also BCL-2 and BCL-w. Our scRNA-seq and ST analyses revealed that BCL2 (BCL-2) and BCL2L2 (BCL-w) are expressed at low levels in tumor cells and have low tumor specificity (Figs. 3B, E, H, K, N and 4A). Consequently, there may be little benefit to suppressing BCL-2 and BCL-w in this context. Combining A1331852 with osimertinib showed the highest rate of tumor reduction in our in vivo experiments, and selective BCL-XL inhibitors like A1331852 may have more tumor-specific effects, potentially reducing toxicity.
A limitation of our study is the sample size in ST analysis; however, our ST results consistently supported findings obtained from experiments using cell lines and human clinical specimens. Studies of genetic profiles in DTP cells surviving therapeutic assault have thus far relied on cell line-based experiments to define molecular mechanisms underlying DTP cell formation, as ethical constraints preclude the collection of human clinical specimens for this purpose. However, cell line-based experiments do not reflect tumor complexity or diversity or the effects of the tumor microenvironment on DTP cells. We enabled the establishment of a DTP model using EGFR-driven lung cancer transgenic mice. This approach allowed for a comprehensive analysis of the dynamics of apoptosis-related genes, including spatial information, during the process of acquiring resistance to EGFR-TKIs. However, further studies are required to understand the mechanism by which DTP cells acquire full drug resistance to EGFR-TKIs and to determine whether our results can be applied to the emergence of resistance to other targeted therapies. Another limitation is that each Visium spot covers multiple cells in our analysis. Therefore, gene expression levels in each spot do not accurately reflect the expression of individual cells, making it challenging to analyze detailed interactions between DTP cells and TME. However, our analysis of ST data showed a positive correlation between tumor cell proportions and Bcl2l1 expression levels (Fig. 4D), indicating that DTP cells exhibit high Bcl2l1 expression regardless of co-localized non-tumor cells.
In summary, our multifaceted approach, incorporating new technologies such as scRNA-seq and ST as well as conventional cell line systems and animal models, highlights the importance of BCL2L1 expression in tumor cell survival as drug resistance emerges and cells acquire resistance to EGFR-TKI.
Methods
Cell lines
NCI-H1975 (H1975) cells and HCC827 cells were purchased from ATCC. PC9 cells were kindly provided by Dr. Pasi Jänne. All cell lines were confirmed by short-tandem repeat DNA profiling and routinely tested for mycoplasma using the Mycoalert Mycoplasma Detection Kit (Lonza). PC9-ER cells were established as described [38]. All lines were maintained in RPMI1640 (Corning) plus heat-inactivated 10% FBS (Corning) and 1% penicillin–streptomycin (Corning). Cells were cultured in 5% CO2 at 37 °C.
Generation of stable cell lines harboring sgRNA
pSpCas9-2A-Puro (px459) v2.0 plasmid was obtained from Addgene (Cambridge, MA, USA). DNA Oligos were synthesized and ligated into px459 according to the manufacturer’s protocol. H1975 and PC9-ER lines were transfected with sgRNA plasmids, and stable lines were selected in puromycin to generate BCL2L1-knockout (KO) cells. KO efficiency was determined by western blot analysis. Oligo sequences are shown in Supplementary Table 3.
Western blotting
Cell cytoplasmic extracts were prepared as previously described [15]. SDS-PAGE was performed, and proteins were transferred to PVDF membranes (Millipore Sigma), which were blocked in 5% nonfat milk and PBST and incubated overnight at 4 °C with primary antibodies diluted 1:1000 in 5% BSA. Primary antibodies included those to BCL-XL (Cell Signaling Technology, 2764S) and β-actin (Cell Signaling Technology, 4970S). After incubation with HRP-conjugated secondary antibodies diluted 1:5000 in 5% nonfat milk and PBST, we detected signals with ECL Prime Western Blotting detection reagent (ThermoFisher) using an Amersham imager 600 (GE Healthcare). Expression levels were quantified using Image-J software.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from cells using an RNA Isolation Kit (ZYMO, Direct-zol RNA Miniprep Plus) and converted to cDNA via RT-PCR using the High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). Real-time PCR was performed in triplicate using iTaq Universal SYBR Green Supermix (Bio-Rad). Primers are shown in Supplementary Table 4. mRNA abundance was normalized to that of GAPDH.
Clinical specimens
All patients provided written informed consent before sampling, in accordance with the Declaration of Helsinki. This study was performed in a blinded manner and approved by the National Cancer Center Ethics Committee.
Single-cell RNA-seq analysis
scRNA-seq data acquisition and analysis workflow were previously described [15]. Briefly, scRNA-seq analysis was performed using R (v4.2.1). Using Seurat ver4.3.0 [39], low-quality reads and PCR “sister” duplicates were removed. To filter out low-quality cell data, the following threshold was determined for each sample. For cell lines, (i) >7500 UMI per cell, (ii) >1000 genes per cell, and (iii) <10% mitochondrial gene expression; for Pt-1 and -3, (i) >5000 UMI per cell, (ii) >1000 genes per cell, and (iii) <20% mitochondrial gene expression; for Pt-2, -4 and -5, (i) >5000 UMI per cell, (ii) >1000 genes per cell, and (iii) <10% mitochondrial gene expression. Stats of the scRNA-seq analysis are shown in Supplementary Table 1.
Transgenic murine models
All animal studies were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center. EGFR-L858R-T790M (EGFRTL)/CCSP-rtTA bi-transgenic mice and teto-Cre transgenic mice were previously described [23, 40]. Bcl2l1 floxed mice were provided by Dr. Lothar Hennighausen at the National Institute of Diabetes and Digestive and Kidney Diseases. Samples were collected until the sample size was sufficient to give comparison and reliable estimates. Once male and female juvenile mice reached three to five weeks of age, they were treated with doxycycline for eight to ten weeks to induce EGFRTL expression and excise Bcl2l1. Immediately after doxycycline treatment, mice underwent magnetic resonance imaging (MRI). For in vivo studies, we modified a protocol previously published to establish an erlotinib-resistant mouse model [25]. In brief, osimertinib at 5 mg kg−1 per day, ABT-263 (Chemgood, C-1009) at 100 mg kg−1 per day, and A1331852 (MedChemExpress, HY-19741) at 25 mg kg−1 per day were administered by oral gavage 6 days per week with drug-on/drug-off cycles described below, while maintaining mice on a doxycycline-containing diet: (i) mice for ST and for the pharmacological BCL-XL inhibition study, 2 weeks on/2 weeks off for 2 courses, then 4 weeks on; (ii) Bcl2l1 knockout evaluation study, 4 weeks on/2 weeks off for 4 courses, then 2 weeks on. Animals were randomly grouped for the pharmacological BCL-XL inhibition study. No randomization was required in the other study using mouse models since no treatment conditions were compared. The investigators were blinded to group allocation during data analysis. Tumor growth was monitored by MRI. The sum of maximum tumor diameters was calculated using Sante DICOM Viewer Lite 3.2.4. Response criteria were defined as follows: partial response (PR), at least a 30% decrease in the sum of diameters of target lesions, taking baseline sum diameters as a reference; progressive disease (PD), at least a 20% increase in the sum of diameters of target lesions, taking the smallest sum in the study as a reference; and stable disease (SD), neither sufficient shrinkage to qualify for a PR nor sufficient increase in tumor size to qualify for PD. Mouse lungs were dissected and subjected to histological analysis. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin in the Histology Core Facility at Beth Israel Deaconess Medical Center.
Library preparation and data pre-processing of Spatial Transcriptomics (ST)
ST experiments were performed using the 10× Visium Spatial Gene Expression kit. Protocols for Tissue Optimization and Library preparation were followed based on the manufacturer’s instructions. Library preparation data pre-processing and normalization were done in the Spatial Technology Unit at Beth Israel Deaconess Medical Center. In brief, a 5 μm-thick section was prepared from the FFPE blocks and processed using the Visium Spatial Gene Expression for FFPE Kit (10× Genomics) according to the manufacturer’s instructions. Sections were H&E stained and imaged, followed by probe hybridization and ligation. Libraries were sequenced to a depth of more than 25,000 read pairs per spot for Visium experiments. Reads were processed using Spaceranger v2.0.0 with mm10 (build 2020-A, 10× Genomics) and Visium Mouse Transcriptome Probe Set v1.0 as references. For Visium spatial transcriptomics data, tissue morphology was annotated at per spot level using the Loupe browser (v6.2.0, 10× Genomics) and STUtility v1.1.1 [41]. Spots underneath regions affected by processing artifacts were annotated as “exclude” and excluded from downstream analyses. The UMI counts were then normalized using the SCTransform (v0.3.5) function from the Seurat package with R (v4.2.1).
Cell-type deconvolution
To infer the spatial organization of certain cell types in Day 4 samples, we used a reference-free deconvolution approach using the STdeconvolve (ver 1.2.0) R package [22]. Briefly, to first select genes in the LDA model, genes detected in either <5% or in 100% of pixels were removed. We optimize the number of cell types K in the LDA model to minimize perplexity and the number of predicted cell-types with mean pixel proportion less than 5%, and then determined the number of cell types K in the LDA model to be 13.
Annotating deconvolved cell-types
Deconvolved cell types (topics) were annotated based on PanglaoDB [42], a database with single-cell datasets and lists of cell-type markers for various human and mouse tissues. Annotated cell types were subjected to functional enrichment analysis using g:profiler [43]. Each cell type was then manually annotated on the basis of top-enriched pathways and marker gene upregulation.
Statistics
Statistical analyses were performed with GraphPad Prism 9.2.0. Two-group means comparison was analyzed using the unpaired two-tailed Welch’s t test. To evaluate multigroup data sets, one-way ANOVA with a Tukey–Kramer multiple-comparison test was used for the parametric test, and the Kruskal–Wallis one-way ANOVA was used for the non-parametric comparison. Also, a Repeated Measures one-way ANOVA with a Dunnett’s test was used to compare a treatment group to a single control group. p-values < 0.05 were considered statistically significant. Data are presented as mean ± SD.
Supplementary information
Supplementary Figure 1-12 and Table 1, 3, 4
Acknowledgements
Bcl2l1 conditional knockout mice were kindly provided by Dr. Lothar Hennighausen at the National Institute of Diabetes and Digestive and Kidney Diseases. All ST experiments were performed at the Spatial Technologies Unit (STU), Beth Israel Deaconess Medical Center, Harvard Medical School Initiative for RNA Medicine (RRID:SCR_024905). Spatial tissue profiling data analyses were performed by support from the Spatial Technologies Unit personnel on the BIDMC Ithaca High Performance Computing cluster. We thank Drs. Nikolaos Kalavros, Ioannis S. Vlachos, and the personnel of the STU for assistance with ST data analysis. We thank Drs. Aaron Grant and Cody Callahan for MRI imaging analysis. We thank all members of the Kobayashi laboratory for helpful discussions. This work was supported by NIH grants R01CA240257 (S.S.K.) and R37CA218707 (D.B.C.), and LUNGevity/EGFR Resisters Research Award 1144389 (to S.S.K.).
Author contributions
M.I., M.F., and S.S.K. conceived the experiments. M.I. and Y.K. contributed to analysis of scRNA-seq data. M.I. conducted bioinformatics analysis of Visium data. M.I., M.F., and I.S.K. performed biochemical analyses. M.I., M.F., V.H., and I.S.K. performed in vivo experiments. H.U. acquired and processed clinical specimens. D.C. and S.S.K. provided funding. S.S.K. supervised the work. M.I., M.F., and S.S.K. wrote the paper. All authors reviewed the paper, discussed the results, and commented on the paper.
Data availability
Datasets generated or analyzed during the current study are available from the corresponding author on reasonable request. The public single-cell sequencing dataset applied in this study is on the GEO database with number GSE146100.
Code availability
The version and parameters for the R packages used in this study are available in the Material and Methods section. Any additional information required to reanalyze the data reported in this study is available from the corresponding author on reasonable request.
Competing interests
The authors declare the following financial interests/personal relationships which may be considered potential competing interests: H.U. reports grants or contracts from Takeda, Boehringer Ingelheim, and Taiho; personal fees (honoraria) from Novartis, Taiho, Chugai, MSD, AstraZeneca, Daiichisankyo. S.S.K. reports research support from Boehringer Ingelheim, MiRXES, Johnson&Johnson, and Taiho Therapeutics, as well as personal fees (honoraria) from AstraZeneca, Boehringer Ingelheim, Bristol Meyers Squibb, Chugai Pharmaceutical, and Takeda Pharmaceuticals, plus royalties from Life Technologies; all interests are outside the submitted work. D.C. reports receiving consulting fees and honoraria from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Blueprint Medicines, and Janssen; institutional research support from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Merck Sharp and Dohme, Merrimack Pharmaceuticals, Bristol Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, Tesaro, Taiho Pharmaceutical Company, and Daiichi Sankyo; and consulting fees from Teladoc and Grand Rounds by Included Health plus royalties from Life Technologies; all interests are outside the submitted work. The remaining authors declare no competing interests.
Footnotes
Edited by Maurizio Fanciulli
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Motohiro Izumi, Masanori Fujii.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-024-06940-y.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 2.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28:S24–31. 10.1038/onc.2009.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 4.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 5.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. 10.1016/j.cell.2010.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rusan M, Li K, Li Y, Christensen CL, Abraham BJ, Kwiatkowski N, et al. Suppression of adaptive responses to targeted cancer therapy by transcriptional repression. Cancer Discov. 2018;8:59–73. 10.1158/2159-8290.CD-17-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Conti G, Dias MH, Bernards R. Fighting drug resistance through the targeting of drug-tolerant persister cells. Cancers (Basel). 2021;13:1118. 10.3390/cancers13051118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suda K, Mitsudomi T. Drug tolerance to EGFR tyrosine kinase inhibitors in lung cancers with EGFR mutations. Cells. 2021;10:1590. 10.3390/cells10071590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanker M, Willcutts D, Roth JA, Ramesh R. Drug resistance in lung cancer. Lung Cancer (Auckl). 2010;1:23–36. [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–93. 10.1038/s41580-018-0089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–75. 10.1038/onc.2008.302 [DOI] [PubMed] [Google Scholar]
- 12.Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HE, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22:262–9. 10.1038/nm.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terai H, Kitajima S, Potter DS, Matsui Y, Quiceno LG, Chen T, et al. ER stress signaling promotes the survival of cancer “Persister Cells” tolerant to EGFR tyrosine kinase inhibitors. Cancer Res. 2018;78:1044–57. 10.1158/0008-5472.CAN-17-1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song KA, Hosono Y, Turner C, Jacob S, Lochmann TL, Murakami Y, et al. Increased synthesis of MCL-1 protein underlies initial survival of EGFR-mutant lung cancer to EGFR inhibitors and provides a novel drug target. Clin Cancer Res. 2018;24:5658–72. 10.1158/1078-0432.CCR-18-0304 [DOI] [PubMed] [Google Scholar]
- 15.Kashima Y, Shibahara D, Suzuki A, Muto K, Kobayashi IS, Plotnick D, et al. Single-cell analyses reveal diverse mechanisms of resistance to EGFR tyrosine kinase inhibitors in lung cancer. Cancer Res. 2021;81:4835–48. 10.1158/0008-5472.CAN-20-2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Bian D, Zhang X, Zhang H, Zhu Z. Inhibition of Bcl-2 and Bcl-xL overcomes the resistance to the third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer. Mol Med Rep. 2021;23:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez Castro LN, Tirosh I, Suva ML. Decoding cancer biology one cell at a time. Cancer Discov. 2021;11:960–70. 10.1158/2159-8290.CD-20-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F, Fan J, He Y, Xiong A, Yu J, Li Y, et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat Commun. 2021;12:2540. 10.1038/s41467-021-22801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82. 10.1126/science.aaf2403 [DOI] [PubMed] [Google Scholar]
- 20.Bracht JWP, Karachaliou N, Berenguer J, Pedraz-Valdunciel C, Filipska M, Codony-Servat C, et al. Osimertinib and pterostilbene in EGFR-mutation-positive non-small cell lung cancer (NSCLC). Int J Biol Sci. 2019;15:2607–14. 10.7150/ijbs.32889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Yin K, Liu SY, Yan LX, Su J, Wu YL, et al. Multiomics analysis reveals a distinct response mechanism in multiple primary lung adenocarcinoma after neoadjuvant immunotherapy. J Immunother Cancer. 2021;9:e002312. 10.1136/jitc-2020-002312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller BF, Huang F, Atta L, Sahoo A, Fan J. Reference-free cell type deconvolution of multi-cellular pixel-resolution spatially resolved transcriptomics data. Nat Commun. 2022;13:2339. 10.1038/s41467-022-30033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. 10.1016/j.ccr.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 24.Wagner KU, Claudio E, Rucker EB 3rd, Riedlinger G, Broussard C, Schwartzberg PL, et al. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–58. 10.1242/dev.127.22.4949 [DOI] [PubMed] [Google Scholar]
- 25.Pirazzoli V, Politi K. Generation of drug-resistant tumors using intermittent dosing of tyrosine kinase inhibitors in mouse. Cold Spring Harb Protoc. 2014;2014:178–81. 10.1101/pdb.prot077842 [DOI] [PubMed] [Google Scholar]
- 26.Trisciuoglio D, Tupone MG, Desideri M, Di Martile M, Gabellini C, Buglioni S, et al. BCL-X(L) overexpression promotes tumor progression-associated properties. Cell Death Dis. 2017;8:3216. 10.1038/s41419-017-0055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Guo M, Wei H, Chen Y. Targeting MCL-1 in cancer: current status and perspectives. J Hematol Oncol. 2021;14:67. 10.1186/s13045-021-01079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah KN, Bhatt R, Rotow J, Rohrberg J, Olivas V, Wang VE, et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat Med. 2019;25:111–8. 10.1038/s41591-018-0264-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurppa KJ, Liu Y, To C, Zhang T, Fan M, Vajdi A, et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell. 2020;37:104–122.e112. 10.1016/j.ccell.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farnsworth DA, Chen YT, de Rappard Yuswack G, Lockwood WW. Emerging molecular dependencies of mutant EGFR-driven non-small cell lung cancer. Cells. 2021;10:3553. 10.3390/cells10123553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirai S, Tada M, Yamaguchi M, Niki T, Sakuma Y. EGFR-independent EGFR-mutant lung adenocarcinoma cells depend on Bcl-xL and MCL1 for survival. Biochem Biophys Res Commun. 2020;526:417–23. 10.1016/j.bbrc.2020.03.116 [DOI] [PubMed] [Google Scholar]
- 32.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–9. 10.1038/nature09732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M, Zhang Y, Zhang X, Zhang J, Jing P, Cao L, et al. Targeting FBW7 as a strategy to overcome resistance to targeted therapy in non-small cell lung cancer. Cancer Res. 2017;77:3527–39. 10.1158/0008-5472.CAN-16-3470 [DOI] [PubMed] [Google Scholar]
- 34.Widden H, Placzek WJ. The multiple mechanisms of MCL1 in the regulation of cell fate. Commun Biol. 2021;4:1029. 10.1038/s42003-021-02564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolomsky A, Vogler M, Kose MC, Heckman CA, Ehx G, Ludwig H, et al. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J Hematol Oncol. 2020;13:173. 10.1186/s13045-020-01007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer A, Rosenberg AS, Jakubowiak A, Raje N, Chatterjee M, Trudel S, et al. A phase 1, first-in-human study of AMG 176, a selective MCL-1 inhibitor, in patients with relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e53–e54. 10.1016/j.clml.2019.09.081 [DOI] [Google Scholar]
- 37.Bertino EM, Gentzler RD, Clifford S, Kolesar J, Muzikansky A, Haura EB, et al. Phase IB study of osimertinib in combination with navitoclax in EGFR-mutant NSCLC following resistance to initial EGFR therapy (ETCTN 9903). Clin Cancer Res. 2021;27:1604–11. 10.1158/1078-0432.CCR-20-4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogino A, Kitao H, Hirano S, Uchida A, Ishiai M, Kozuki T, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–14. 10.1158/0008-5472.CAN-07-0681 [DOI] [PubMed] [Google Scholar]
- 39.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20. 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama S, Sng N, Carretero J, Welner R, Hayashi Y, Yamamoto M, et al. Beta-catenin contributes to lung tumor development induced by EGFR mutations. Cancer Res. 2014;74:5891–902. 10.1158/0008-5472.CAN-14-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergenstråhle J, Larsson L, Lundeberg J. Seamless integration of image and molecular analysis for spatial transcriptomics workflows. BMC Genomics. 2020;21:482. 10.1186/s12864-020-06832-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franzen O, Gan LM, Bjorkegren JLM. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxf). 2019;2019:baz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:W191–W198. 10.1093/nar/gkz369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1-12 and Table 1, 3, 4
Data Availability Statement
Datasets generated or analyzed during the current study are available from the corresponding author on reasonable request. The public single-cell sequencing dataset applied in this study is on the GEO database with number GSE146100.
The version and parameters for the R packages used in this study are available in the Material and Methods section. Any additional information required to reanalyze the data reported in this study is available from the corresponding author on reasonable request.