Abstract
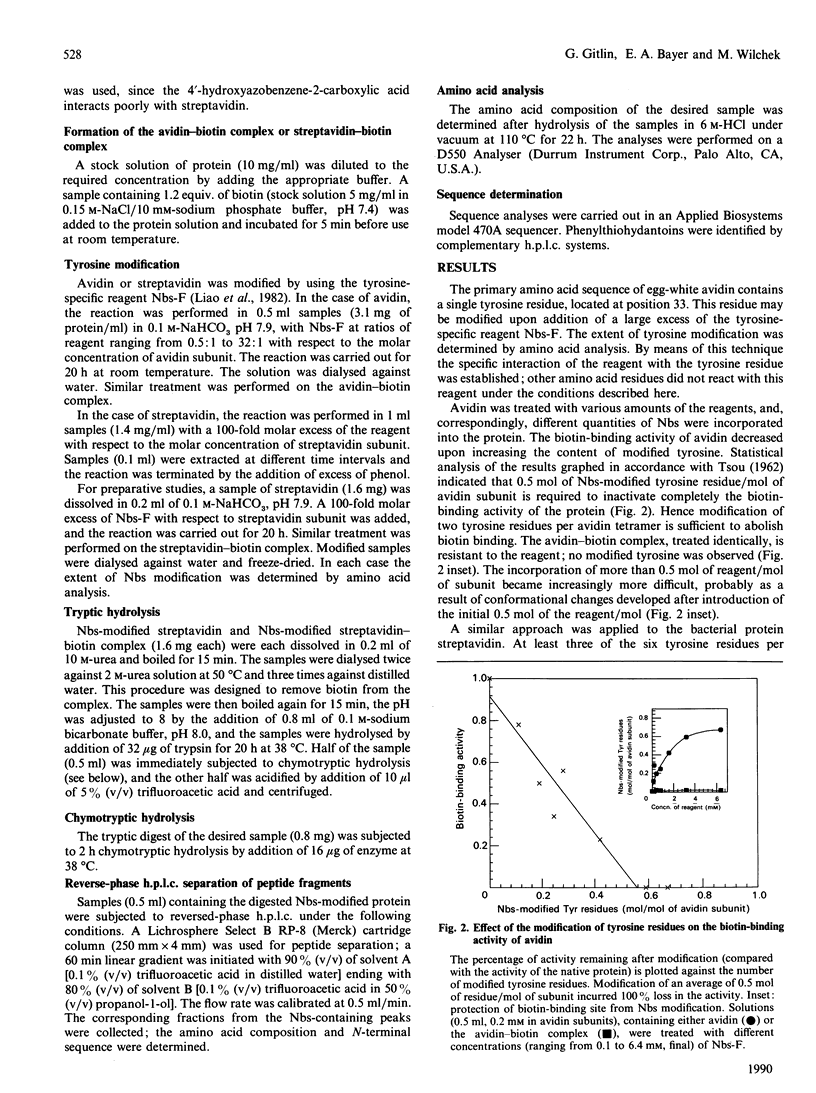
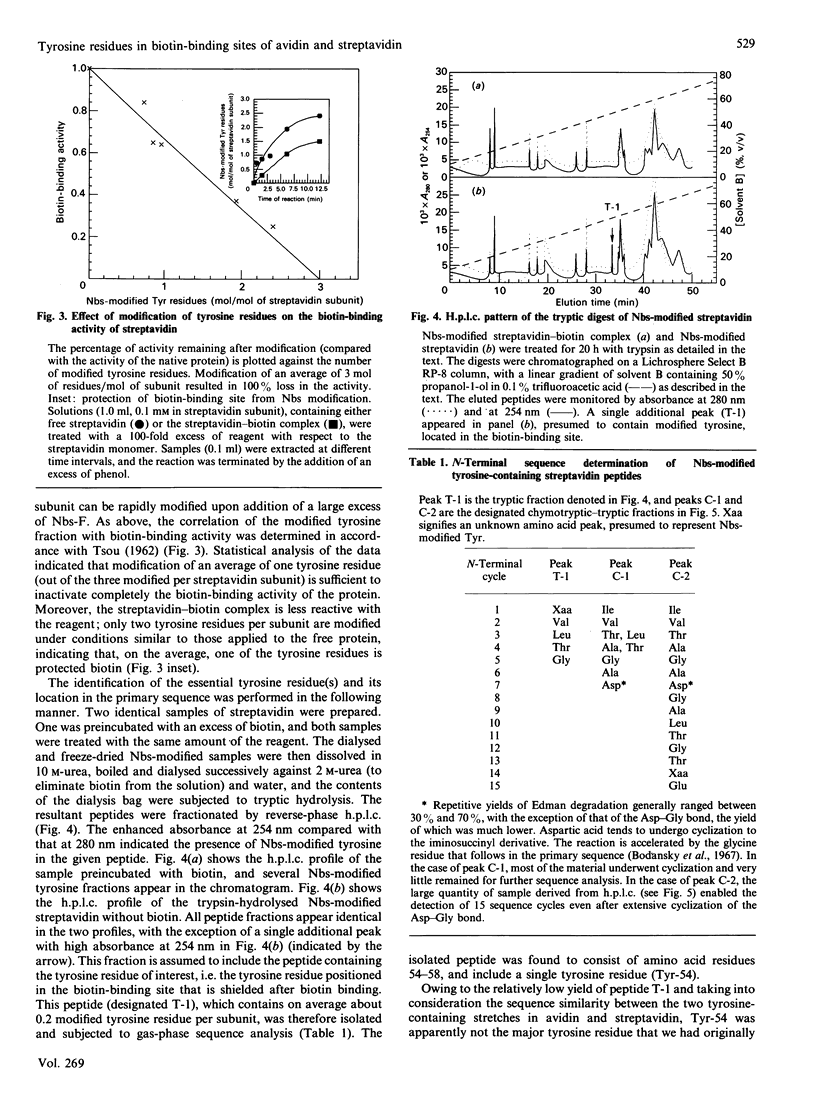
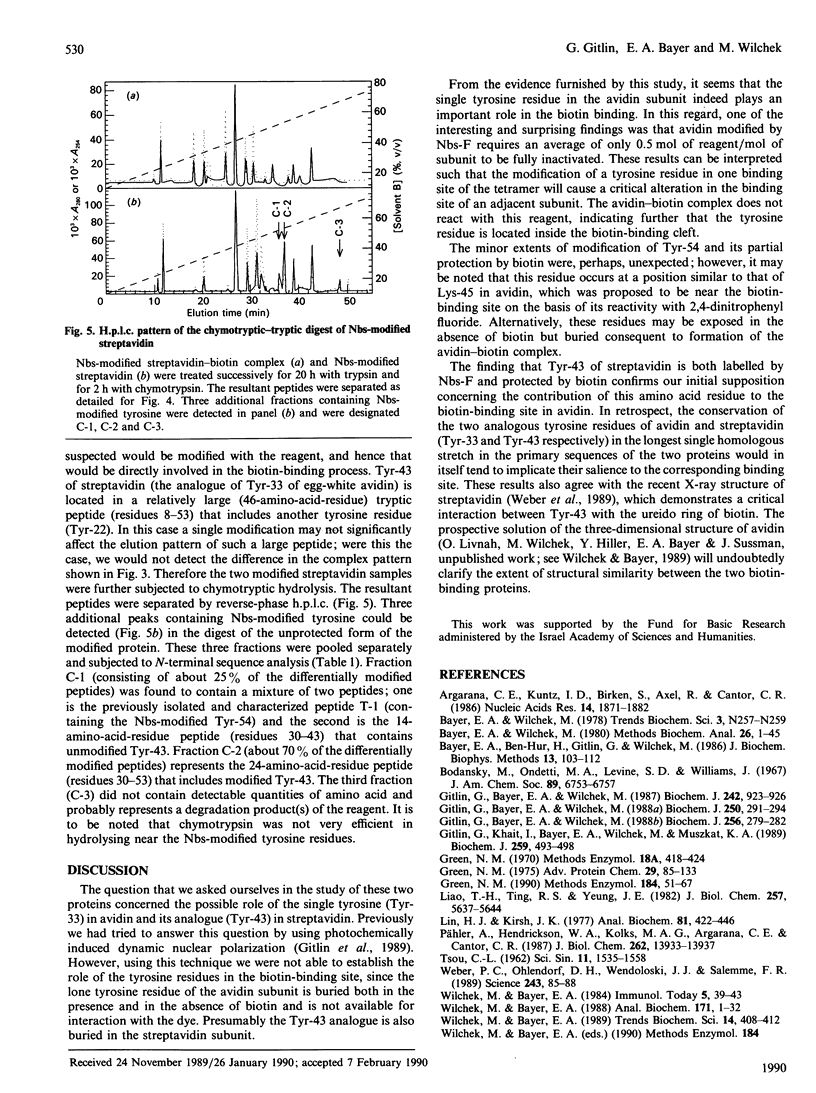
The involvement of tyrosine in the biotin-binding sites of the egg-white glycoprotein avidin and the bacterial protein streptavidin was examined by using the tyrosine-specific reagent p-nitrobenzenesulphonyl fluoride (Nbs-F). Modification of an average of about 0.5 mol of tyrosine residue/mol of avidin subunit caused the complete loss of biotin binding. This indicates that the single tyrosine residue (Tyr-33) in the avidin subunit is directly involved in the biotin-binding site and that its modification by Nbs also abolishes the binding properties of a neighbouring subunit. This suggests that the tyrosine residues of the egg-white protein may also contribute to the stabilization of the native protein structure. In streptavidin, however, the modification of an average of 3 mol of tyrosine residue/mol of subunit was required to inactivate completely the biotin-binding activity of the protein, but only 1 mol (average) of tyrosine residue/mol of subunit was protected in the presence of biotin. The difference between the h.p.l.c. elution profiles of the enzymic digests of Nbs-modified streptavidin and the Nbs-modified streptavidin-biotin complex revealed two additional fractions in the unprotected protein that contain Nbs-modified tyrosine residues. These residues, Tyr-43 (major fraction) and Tyr-54 (minor fraction), appear to contribute to the biotin-binding site in streptavidin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argaraña C. E., Kuntz I. D., Birken S., Axel R., Cantor C. R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Gitlin G., Wilchek M. An improved method for the single-step purification of streptavidin. J Biochem Biophys Methods. 1986 Sep;13(2):103–112. doi: 10.1016/0165-022x(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Bodanszky M., Ondetti M. A., Levine S. D., Williams N. J. Synthesis of secretin. II. The stepwise approach. J Am Chem Soc. 1967 Dec 6;89(25):6753–6757. doi: 10.1021/ja01001a063. [DOI] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Lysine residues involved in the active site. Biochem J. 1987 Mar 15;242(3):923–926. doi: 10.1042/bj2420923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Tryptophan residues involved in the active site. Biochem J. 1988 Feb 15;250(1):291–294. doi: 10.1042/bj2500291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of streptavidin. Tryptophan residues involved in the active site. Biochem J. 1988 Nov 15;256(1):279–282. doi: 10.1042/bj2560279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Khait I., Bayer E. A., Wilchek M., Muszkat K. A. Studies on the biotin-binding sites of avidin and streptavidin. A chemically induced dynamic nuclear polarization investigation of the status of tyrosine residues. Biochem J. 1989 Apr 15;259(2):493–498. doi: 10.1042/bj2590493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Liao T. H., Ting R. S., Yeung J. E. Reactivity of tyrosine in bovine pancreatic deoxyribonuclease with p-nitrobenzenesulfonyl fluoride. J Biol Chem. 1982 May 25;257(10):5637–5644. [PubMed] [Google Scholar]
- Lin H. J., Kirsch J. F. A sensitive fluorometric assay for avidin and biotin. Anal Biochem. 1977 Aug;81(2):442–446. doi: 10.1016/0003-2697(77)90715-1. [DOI] [PubMed] [Google Scholar]
- Pähler A., Hendrickson W. A., Kolks M. A., Argaraña C. E., Cantor C. R. Characterization and crystallization of core streptavidin. J Biol Chem. 1987 Oct 15;262(29):13933–13937. [PubMed] [Google Scholar]
- TSOU C. L. Relation between modification of functional groups of proteins and their biological activity. I.A graphical method for the determination of the number and type of essential groups. Sci Sin. 1962 Nov;11:1535–1558. [PubMed] [Google Scholar]
- Weber P. C., Ohlendorf D. H., Wendoloski J. J., Salemme F. R. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989 Jan 6;243(4887):85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Avidin-biotin technology ten years on: has it lived up to its expectations? Trends Biochem Sci. 1989 Oct;14(10):408–412. doi: 10.1016/0968-0004(89)90289-2. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]


