Abstract
Conditional gene targeting uses the insertion of expression cassettes for the selection of targeted embryonic stem cells. The presence of these cassettes in the final targeted chromosomal locus may affect the normal expression of the targeted gene and produce interesting knock down phenotypes. We show here that the selection cassette may then be selectively removed in vivo, using three appropriately positioned loxP sites in the targeted gene and the transgenic mouse EIIaCre. This strategy was applied to two different target genes and we demonstrated that it is reliable and reproducible. First, we generated double transgenic EIIaCre/loxP mice (F1) that showed variable degrees of mosaicism for partially Cre-recombined floxed alleles. Efficiency of EIIaCre at creating mosaicism was dependent on the target gene and on parental transmission of the transgene. The segregation of partially recombined alleles and EIIaCre transgene was obtained in the next generation using mosaic F1 males. Mosaic females were unsuitable for this purpose because they systematically generated complete excisions during oogenesis. Our strategy is applicable to other approaches based on three loxP sites. As this procedure allows generation of knock down (presence of neo), knockout (total exision of the loxP-flanked sequences) and floxed substrains (excision of the selection cassette) from a single, targeted germline mutation and in a single experiment, its use may become more widespread in conditional mutagenesis.
INTRODUCTION
Conditional gene knockout (KO) using Cre-loxP strategies is becoming a standard for in vivo loss of function studies (1,2). Given the availability of suitable replacement vectors, efficient protocols for embryonic stem (ES) cell clone selection and methods for high throughput screening, it may today be of advantage to target the gene of interest directly with a conditional KO construct. The classical KO of the gene may then be produced by Cre expression in the germline of the floxed animal, as one of a series of specific gene inactivations.
Gene replacement constructs contain expression cassettes for the positive selection of recombined ES cell clones. Often used selective markers are cassettes conferring resistance to the antibiotic neomycin. In conditional gene targeting, this neo cassette is placed in non-coding regions, most frequently introns. The bacterial neo gene contains cryptic splice sites that interfere with RNA splicing in eukaryotes (3–5). This may reduce the effective mRNA levels of the target gene, resulting in a so-called hypomorphic allele. The introduction of such attenuated alleles into the germline may cause a knock down phenotype (6–8). Therefore, to counteract the drawback of having the neo gene in the final targeted chromosomal locus, in vitro strategies have been developed for the selective elimination of resistance cassettes and applied successfully (9–11). They are based on three loxP sites, transient Cre expression in the targeted ES cell clones and isolation of clones in which the selection cassette has been specifically excised. This means additional electroporation and prolonged culture of already targeted ES cells. Although feasible under certain conditions (9–12), this may also reduce ES cell totipotency, and the probability of germline chimeras developing from a particular clone.
On the other hand, having an altered expression of the floxed allele due to the presence of neo could be of interest, especially in the case of genes with severe classical KO phenotypes. This allows study of the partial reduction of the target gene expression. As such a reduction in expression is readily obtained with hypomorphic alleles (5,8), the generation of a gene knock down should be considered when planning conditional Cre-loxP KO.
We describe herein a method that allows the combination of generation of potentially knock down mice and subsequent analysis of conditional or total KO phenotype. This method relies on the use of the EIIaCre transgenic mouse to produce in vivo partial and/or total excision of loxP flanked sequences in targeted loci of interest. In the absence of its natural E1A coactivator, the expression of genes under the control of the adenoviral EIIa promoter is known to be restricted to oocytes and preimplantation stages of the embryo (13). The EIIaCre transgene has been used previously for general deletion (14), and recently for mosaic recombinations in early embryos (15,16). Here, we report partial and total in vivo Cre recombination using two different chromosomal floxed loci as models. Our experiments involved the targeted insulin-like growth factor type I receptor (IGF-IR) locus and the targeted catalytic α2 subunit of the AMP-activated protein kinase (AMPK) locus (Fig. 1A and B). From the data presented here, specific protocols can be derived for implementation of this strategy in other contexts. In addition to obvious use in gene KO, partial Cre recombination during early embryogenesis may also be applied to the reduction of transgene copy number, controlled ablation of gene regulatory elements in vivo, or gene dosage.
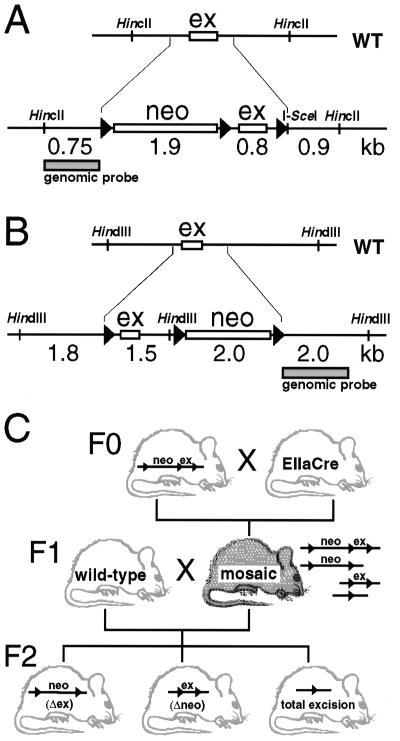
Figure 1.
Production of mice with mosaic partial Cre-loxP deletions. (A and B) An essential exon (ex) of the wild-type (WT) IGF-IR gene and of the AMPK gene was flanked with loxP sites (triangles) by gene replacement. A neomycin resistance cassette (neo), equipped with a third loxP site, was cointroduced next to a flanking loxP site. (C) Breeding scheme: heterozygous crossings between male or female EIIaCre and floxed strains were screened for double transgenic EIIaCre/IGF-IRneolox mice, which were then tested for mosaicism. Male as well as female mosaics were mated to study segregation of discrete recombinant alleles from the Cre transgene in F2. Gender of mosaics was important as detailed in the text.
MATERIALS AND METHODS
Animals and breeding
IGF-IRneolox (8) and AMPKneolox mice (S. Vaulont and A. Kahn, unpublished results) (all information concerning the targeting construct and the generation of floxed mice can be obtained upon request) were produced in our animal facilities (Paris, France). EIIaCre transgenic mice, developed in the laboratory of H. Westphal (NIH, Bethesda, MD) (13), were provided by U. Rudolph (Zürich, Switzerland) in 129/Sv and C57Bl/6 backgrounds. Mice were housed in standard conditions (25°C, 12 h light–dark cycle, water/food ad libitum).
Adult heterozygous EIIaCre transgenic mice were mated with 8-week-old IGF-IRneolox or AMPKneolox mice (F0 crossings; Fig. 1C). Separate experiments were performed for IGF-IRneolox mice previously backcrossed in 129/Sv and C57Bl/6 genetic backgrounds. Offspring (F1) was screened for the presence of EIIaCre by PCR. Partial Cre-recombination patterns of the floxed DNA segments in the targeted genes were analyzed by Southern blotting. Mice with partial and mosaic Cre-recombination patterns were then mated with wild-type mice of the same genetic background. Segregation of the EIIaCre transgene and the Cre-recombined alleles was monitored by Southern blotting and PCR in the F2 generation.
DNA extraction
Tail (5 mm) and tissue (50–100 mg) biopsies were incubated for 15 h at 55°C in 500 µl lysis buffer (100 mM Tris pH 8.5, 5 mM EDTA, 0.2% SDS, 200 mM NaCl) with 100 µg/ml Proteinase K (Eurobio, Belgium). After centrifugation at 13 000 g for 10 min, the supernatant was phenol/chloroform extracted and the DNA precipitated with isopropanol. The DNA was pelleted by centrifugation, washed with 70% ethanol, dried and resuspended in 100 µl of 10 mM Tris pH 8.0, 1 mM EDTA (TE).
Blotting of genomic DNA
Southern blot. DNA (8 µg) from each animal was digested with HindIII or HincII and I-SceI (Boehringer, Mannheim), subjected to electrophoresis in a 1.0% agarose gel, transferred to nylon membranes (Hybond+, Amersham) by capillary action, and probed with genomic DNA fragments of the target genes.
Hybridization conditions. Membranes were prehybridized for 1–3 h and hybridized overnight at 42°C in 0.03 ml/cm2 of membrane in 50% formamide, 4× SSC, 2.5× Denhardt’s solution, 0.5% SDS, 250 µg/ml sonicated salmon sperm DNA, 5% dextran sulfate and 50 mM NaPO4 in a rotating hybridization oven (Appligene, France). The membranes were then washed twice for 15 min at room temperature in 2× SSC with 0.1% SDS, and twice for 30 min at 65°C in 0.1× SSC with 0.1% SDS. Membranes were placed against X-ray film (Agfa, Curix RP-2 100 NIF) for 1–4 days at –80°C with amplifying screens. For quantification, blots were exposed to phosphorimager screens and analyzed using a STORM 850 Phosphorimager (Molecular Dynamics) and ImageQuant 5.0. Alternatively, film autoradiographs were scanned and analyzed using NIH image software.
Molecular probes. IGF-IR and AMPK alleles were detected using 0.8 and 1.5 kb genomic probes, respectively (Fig. 1). Probes were radiolabeled with [32P]dCTP using Rediprime (Amersham, UK).
PCR genotyping
Genomic DNA was extracted from 1 mg tail biopsy by 2 h digestion in 56°C GNT-K buffer (17). After brief centrifugation, 2 µl of the supernatant was transferred to 50 µl PCR reactions. Amplification conditions were 94°C for 2 min initial strand separation, 40 cycles at 94°C for 45 s, 55°C for 45 s and 72°C for 1 min, and a 7 min final elongation step at 72°C. A Mastercycler 5330 was used (Eppendorf, Hamburg, Germany), and Taq DNA-polymerase from Gibco BRL (Life Technologies, France). Four oligonucleotides were used to simultanously detect EIIaCre and Gabra1 (positive control). Primers 5′-AACACACACTGGCAGGACTGGCTAGG-3′ and 5′-CAATGGTAGGCTCACTCTGGGAGATGATA-3′ (Genset, Paris, France) amplified a 290 bp fragment from the Gabra1 gene, primer 5′-CCTGGAAAATGCTTCTGTCCG-3′ and 5′-CAGGGTGTTATAAGCAATCCC-3′ amplify a 400 bp fragment from Cre. Amplification products were separated on 2.0% agarose gels and stained with ethidium bromide.
RESULTS
Production of mosaic mice
Two different gene-targeted mouse lines, each equipped with three loxP sites in genomic regions of interest, were used as the starting material. The structure of the targeted loci, namely the IGF-receptor and the catalytic α2 subunit of AMPK loci (8; S. Vaulont and A. Kahn, unpublished results), is presented in Figure 1A and B. These floxed-targeted lines were mated with the EIIaCre transgenic mouse line to obtain partial and total germline excision of the loxP-flanked DNA sequences at early stages of development. The breeding scheme is illustrated in Figure 1C. Fifteen breeding pairs were used for the IGF-IRneolox experiment and 10 breeding pairs for the AMPK experiment. Experiments were conducted independently in the 129/Sv, C57Bl/6 and mixed genetic backgrounds. The results are presented together, since genetic background did not have significant impact. In total, 190 F1 pups were born, the mean litter size was normal, and 64 of them were double transgenics, positive for EIIaCre and the floxed allele (Table 1). Cre-recombination was detectable in 23 of the 41 double transgenics for the IGF-IR experiment and in 21 of the 23 double transgenic in the case of the AMPK experiment (56 and 91%, respectively; Table 1). As expected, mating to EIIa-Cre resulted in first generation progeny harboring mosaicism due to Cre action past the zygote stage (13). The genetic mosaicism of all double transgenics was evaluated by Southern blotting using DNA from tail biopsy samples (Fig. 2). Mosaicism in tail biopsies of a given individual was very similar to the mosaicism found in other tissues, like skin, muscle, heart, lung, liver, kidney, testis or brain (not shown).
Table 1. Generation of mosaic mice.
| Targeted gene | Mating pairs | Total offspring | Double transgenics | Mosaic mice |
|---|---|---|---|---|
| IGF-IR | 15 | 105 | 41a | 23 |
| AMPK | 10 | 85 | 23 | 21 |
aExperiments were carried out using heterozygous loxP and heterozygous EIIaCre mice, except for some homozygous IGF-IRneolox F0 females, which explains the higher frequency of double transgenics in F1 (see also Fig. 1).
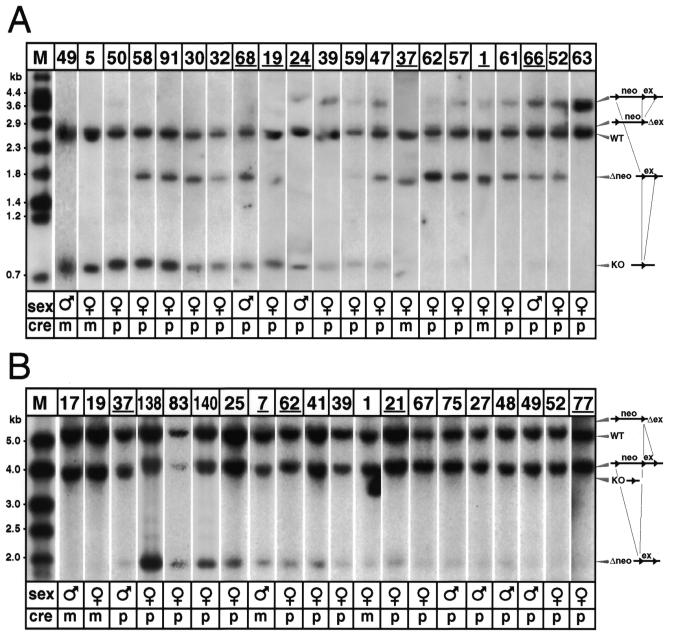
Figure 2.
Partial Cre-recombinations of targeted IGF-IR (A) and AMPK (B) genes as detected by genomic Southern blotting. (A) In HincII/I–SceI double digested DNA, the WT IGF-IR allele and all four targeted allelic forms were detected by Southern blotting. From top to bottom: targeted allele with floxed neo-cassette and floxed exon (3.6 kb allele), exon excised (Δex, 2.7 kb allele), wild-type (WT, 2.4 kb allele), neo-cassette excised (Δneo, 1.7 kb allele) and total excision (KO, 0.8 kb allele). Males 24, 66 and 68, and females 1, 19 and 37 (underlined) were used to segregate the allelic forms. Mouse 63 (the final lane) was EIIaCre-positive, but did not recombine, so that only WT and unmodified IGF-IRneolox alleles were detected. (B) AMPK allelic forms in HindIII digested DNA, from top to bottom: exon excised (Δex, 5.7 kb allele; of apparently very low abundance), wild-type (WT, 5.3 kb), targeted allele with floxed neo-cassette and floxed exon (4.0 kb), total excision (KO, 3.8 kb) and neo-cassette excised (Δneo, 2.0 kb allele). Males 7 and 37, and females 21, 62, and 77 (underlined) were used for segregation. Abbreviations: ex, exon; M, DNA molecular weight marker; m, maternal Cre transmission; neo, neomycin resistance cassette; p, paternal Cre transmission; WT, wild-type.
At the IGF-IR locus (Fig. 2A), double transgenics had inherited one wild-type allele (WT, 2.4 kb), and one IGF-IRneolox allele (neo + ex, 3.6 kb). The IGF-IRneolox allele either remained intact (mouse 63), or Cre recombined into a 2.7 (Δex) or 1.7 kb (Δneo) intermediate product, or was converted to the completely excised form (KO, 0.8 kb). At the AMPK locus (Fig. 2B), double transgenics had inherited one wild-type allele (WT, 5.3 kb), and one AMPKneolox allele (neo + ex, 4.0 kb). The AMPKneolox allele either remained intact (mouse 77), or Cre recombined into a 2.0 kb (Δneo) intermediate product, or was converted to the completely excised form (KO, 3.8 kb).
For both experiments, recombination patterns were shown to differ substantially between individuals. This is illustrated in Figure 2, where individual Southern blot autoradiographs were arranged according to the allelic pattern, from almost complete excision of the entire floxed region (left side of the figure) to partial excision (right side of the figure). For the IGF-IR, we did not observe mice with slight mosaicism. All the different patterns of excision were represented. However, the larger floxed region (the neo-cassette, 1.9 kb) was more frequently excised than the shorter, exonic region (0.8 kb). Thirteen of the patterns in Figure 2 show greater excision of the larger region, whereas only two show greater excision of the smaller region. The other five mice had equal excision of both regions or did not produce intermediate recombination products. In the AMPK experiment, although the mosaicism rate in F1 double transgenics was >90%, the intensity of the mosaicism is predominantly very low. Furthermore, there was virtually no representation of the exon excision event (Δex, band expected at 5.7 kb), suggesting that excision either stopped at Δneo (2.0 kb) or ended in total excision (3.9 kb).
The efficacy of maternal and paternal EIIaCre transgene transmission for the production of mosaic individuals was assessed by using either male or female EIIaCre transgenic mice to mate with the floxed mice. For the IGF-IR experiment, among the 37 double transgenics with paternal transmission, only 19 were mosaic. Interestingly, in contrast, all four double transgenics with maternally transmitted EIIaCre were mosaic. Thus, maternal EIIaCre was more efficient than paternal EIIaCre in generating detectable mosaicism. The same was true with the AMPK experiment where the total excision was obtained only with maternally transmitted EIIaCre (mice 17 and 19). Finally, the recombination patterns analyzed shortly after birth persisted during postnatal development and identical patterns were observed when mosaic mice were analyzed again at 6 months of age (results not shown).
Choice of mosaic breeders
To eliminate the neomycin resistance cassette and to produce total KO mice in the subsequent segregation experiments, we selected mosaic mice with high prevalence of neo-excision (Δneo; 1.7 and 2.0 kb alleles in Fig. 2A and B, respectively), and others with almost complete recombination (0.8 and 3.9 kb alleles). To test whether the recombination patterns detected in tail DNA reflected closely the distribution of recombined alleles in the germline, we analyzed DNA from various tissues by Southern blotting. We found similar loxP recombination patterns in skin, kidney, liver, muscle, heart and testis (not shown), indicating that Cre-recombination occurred early during the morula and blastula stages, among totipotent embryonic cells.
Segregation of partial recombinant alleles and the EIIaCre transgene
Female mosaic mice. We mated double transgenic mosaic females (IGF-IR: mice 1, 19 and 37; AMPK: mice 21, 62 and 77) with wild-type males and screened 48 newborns. Despite the high prevalence of partially recombined alleles in these mosaic mothers, none of these alleles was transmitted intact to these 48 pups. Instead, we obtained 21 total excisions of the floxed regions (the other 27 were wild-type). We conclude, that significant EIIaCre expression occurred during oogenesis, resulting in the excision of all floxed segments from mature oocytes. Several of the completely Cre-lox recombined newborns had not inherited EIIaCre, suggesting that the transgene was expressed before or during meiosis. For both genes, we selected newborns that were EIIaCre-negative and that carried one completely recombined allele (the equivalent of the classical KO in the heterozygous state), and intercrossed them to produce the homozygous classical KOs in the next generation (see below).
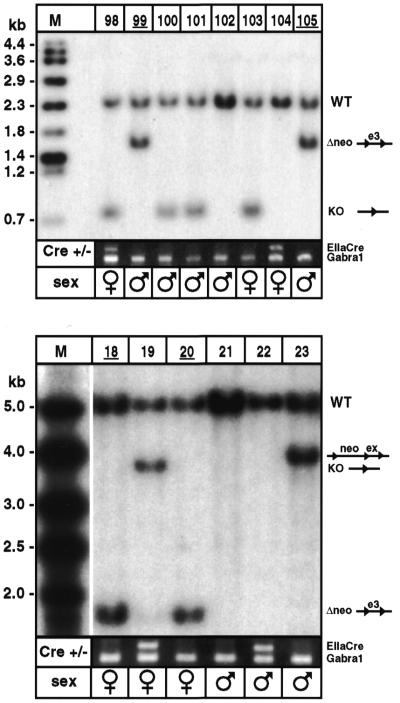
Male mosaic mice. Five mosaic males with partial recombinations of interest for the segregation experiment (males 24, 66 and 68 from Fig. 2A; males 7 and 37 from Fig. 2B) were mated with wild-type females. We genotyped a total of 91 pups for the transmission of partial recombinations and segregation of EIIaCre. The prevalence of the various alleles in F2 offspring correlated well with the prevalence of corresponding alleles revealed by Southern blotting in F1 (r2 = 0.81; P = 0.0001). We readily obtained the expected final mice (e.g. neo-less and EIIaCre-negative mice 99, 105, 18 and 20; Fig. 3). The final mice included several males, from which new colonies of IGF-IRlox and AMPKlox mice were produced.
Figure 3.
Mouse genotypes after segregation. (Top) F2 mice were genotyped by Southern blot probing with an IGF-IR genomic fragment. EIIaCre was detected using genomic PCR. Males 99 and 105 carried the required 1.7 kb (Δneo) allele, and were EIIaCre-negative. Both were used to establish the final mouse colony. (Bottom) Similarly, AMPK F2 mice were probed with an AMPK genomic fragment. Females 18 and 20 were used to establish the final colony.
Production of the classical phenotype and recovery of the knock down phenotype
We recently reported the knock down phenotype of the IGF-IR due to the presence of the neomycin cassette in the IGF-IR chromosomal locus. By selective elimination of the floxed neomycin resistance cassette, we expected to recover this knock down phenotype. Whereas the attenuated, hypomorphic IGF-IRneolox allele caused a deficit in postnatal growth, the selective elimination of neo in turn restored growth to wild-type levels. Nine-week-old mice from the IGF-IRlox line had the same mean body weight as wild-type littermates (31.1 ± 0.8 g versus 31.6 ± 0.4 g for males, 27.7 ± 0.3 g versus 27.3 ± 0.6 g for females; mean ± SEM).
The constitutional IGF-IR KO was previously reported. By eliminating the entire floxed regions, we expected to reproduce the complete, constitutional KO of the IGF-IR gene (18,19). The complete excision, brought to the homozygous state, indeed generated the previously described phenotype (8,18,19), namely, homozygous newborns showed severe growth retardation, were unable to breathe and died within the first few minutes of extrauterine life.
We generated 307 mice for these experiments. If homozygous EIIaCre and homozygous neolox had been used, the number could have been reduced to ~200. Given the low abundance of males in one of our F1 populations, then 50 mice per experiment could be sufficient to obtain the expected result, with high probability. Three homozygous mating pairs could produce approximately 20 double transgenics with at least 10 mosaic mice, five of which would be expected to be males. At least one of these males should have both an acceptable degree of mosaicism and a useful distribution of recombined alleles. Depending on partial recombination pattern, the expected final genotype should be obtained within the first 20 offspring from such a mosaic male.
DISCUSSION
Regions containing multiple loxP sites can be partially eliminated in vitro by transient Cre-expression in ES cells (9). Here we explored an alternative strategy, based on ubiquitous partial in vivo Cre-recombinations, and allelic segregation in the next generation. We used the EIIaCre mouse for in vivo Cre expression (13). This enabled us to derive various mouse lines from two different mouse models, each targeted with three loxP site constructs. One of them, the IGF-IRneolox mouse, had a knock down growth deficiency phenotype due to the presence of a floxed neo cassette (8) (for IGF-IR reviews see 20 and 21). As expected, neo excision resulted in phenotype reversion and generated a line in which IGF-IR was solely flanked by two loxP sites. The same experiment also produced a complete receptor KO line with a homozygous phenotype identical to that of the published classical KO. Knock down alleles and KO alleles were also precious substrates for subsequent gene dosage experiments, because they can be combined to produce almost complete invalidations of the target gene (M. Holzenberger, unpublished results). When applied to the second gene, this strategy yielded readily the strain devoid of neo. For both genes we had the possibility to derive the complete homozygous KO directly from the F1, because among F1 mosaic mice were already heterozygous KOs. In our hands, this entire in vivo strategy is less time-consuming, labor-intensive and expensive than the generation and microinjection of various ES cell clones obtained by in vitro excision of neo. The minimum time from the production of mosaic mice to detection of the expected segregation was ~14 weeks, no longer than the time required to achieve partial Cre recombination in vitro (ES cell culture, transfection, screening and microinjection), and to evaluate postinjection mosaicism. The in vivo approach is not labor-intensive, consisting essentially in two rounds of genotyping. It does not require the repetitive use of a microinjection facility, and the materials, including a few EIIaCre transgenics, are inexpensive. Moreover, in this mosaic strategy, the initial ES cell clones, obtained by homologous recombination, are available for microinjection at an earlier timepoint, without additional in vitro manipulations, so that unexpected difficulties with the germline transmission of particular clones or mutations will be detected sooner. The utility of gene knock down combined with conditional strategies, and the possible introduction of point mutations, have recently been explored elsewhere (5,22,23).
Characteristics of EIIaCre
Our results demonstrated the feasibility of controlled partial in vivo recombination and some important traits of EIIaCre. (i) Many, but not all, of the double transgenic mice developed mosaicism. Some mice may have generated only slight recombinations, not detectable by Southern analysis, or their copy of the transgene may have been silent, although variegation has not been reported from EIIaCre (14). Our results indicate that the frequency and extent of mosaicism differ depending on the target gene. In the case where stronger Cre expression is necessary to excise floxed segments, maternal Cre transmission may be useful. (ii) EIIaCre produced highly variable individual recombination patterns. Several of them suggested that single-cell recombination events during early development are amplified via the expansion of cell lines in the embryo. Thus, the creation of mosaicisms with very high levels of representation of one specific recombination product is possible, though less frequent. (iii) When crossing mosaic males with wild-type females for allelic segregation, Southern analysis predicted the distribution of discrete alleles among offspring. This suggests that mosaicism develops during preimplantation stages, and does not substantially change thereafter in germline cells. This may however be different in those cases where the target gene plays a significant role in gametogenesis. (iv) In our system, the larger floxed DNA fragment (neo) excised more often than the smaller floxed exons. For the IGF-IR, the excision of the exon produces a null allele, while the loss of the larger floxed region results in a gain of function (loss of the hypomorphic neo cassette). As the IGF-IR positively regulates the cell cycle and proliferation, IGFs are probably not an ideal system for studying the kinetics of loxP recombination in vivo. Partial Cre recombination of the targeted AMPK gene confirmed that loxP sites differ in their susceptibility to recombine. This may, at least in part, depend on local factors, like structural effects from surrounding DNA or the size of the floxed segments. Results on the sequential excision of a LacZ reporter transgene (24) that contained multiple floxed segments of different size, indicated that 5 kb floxed regions are more readily excised than 1.5 kb floxed segments (25). Thus, to facilitate the selective excision of multiple floxed regions it may be advisable to keep the disposable floxed segment relatively large. However, since mosaicism starts early in development, many other factors may come into play.
Utility of mosaicism in conditional gene KO
Our results indicate that male mosaics are most useful for generating offspring with partial excisions and that female mosaics efficiently produce complete excisions. However, EIIaCre has many more potential applications. To study the effects of PIGA gene KO on the life span of blood cells, Tremml, Keller and colleagues recently generated mosaic mice by crossing PIGAlox mice with EIIaCre (15,16). PIGAlox mice had only two loxP sites, ~3 kb apart, properties that we have found to facilitate loxP recombination. Under these conditions, maternal EIIaCre produced mice with stronger mosaicism than paternally transmitted EIIaCre (15). This is consistent with our findings from AMPK and IGF-IR experiments. The floxed PIGA gene, however, has an X-linked inheritance pattern, and embryonic cells with one recombined (null) PIGA allele are subject to strong negative selection. Thus, PIGA mosaicisms are biased depending on the inheritance of PIGA and the EIIaCre transgene. The production of EIIaCre-induced mosaicism, even under such conditions, indicates that this strategy is robust. EIIaCre has also recently been used to produce mosaic gene dosage of a floxed insulin-like growth factor I gene (6; distance between loxP sites: 2.5 kb), an approach that revealed elegantly that postnatal mouse development depends on the dose of circulating growth factor. In terms of potential applications, EIIaCre mice may also be useful for reducing the copy number of transgenes equipped with loxP sites, and also in strategies in which endogenous genes have been equipped with multiple loxP sites delimiting selected exons, or distinct promoter regions, to create various degrees or types of genomic deletion.
EIIaCre has been used by others as a general deletor (14,26). In the original publication (14), EIIaCre’s efficiency of loxP excision has been tested using a single floxed genomic segment and paternal transmission (13). In half of the F1 double transgenics, EIIaCre caused total excision, whereas the other half was mosaic. One reason for the slightly stronger Cre-mediated recombination in these experiments may have been that the floxed target locus (a 1.4 kb DNA segment) was more susceptible to recombination than the floxed locus used in our experiment. It is also possible that simply the number of loxP sites available for Cre has an impact on the frequency of recombination, by reducing, for example, the chance that any two sites are simultaneously occupied by sufficient Cre molecules.
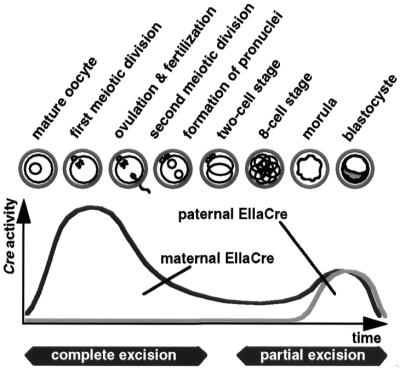
Finally, observed mosaic and general EIIaCre-mediated deletions are consistent with the EIIa promoter activity described in the literature (27). EIIaCre probably has a biphasic expression profile, starting during oogenesis and early embryogenesis, with a second peak of expression during the morula and blastocyste stages, that ends before implantation (represented schematically in Fig. 4). Oocyte EIIaCre expression and the concomitant accumulation of the Cre enzyme seemingly lead to the efficient recombination of any floxed genomic fragment present in the mature oocyte. Cre produced by the oocyte may be active beyond the two-cell stage, but does not have the same strong effect on paternally derived floxed alleles introduced via fertilization. Mosaicism proper is generated after the two-cell stage, when these events are mostly independent of whether Cre is of maternal or paternal origin.
Figure 4.
Cre-recombinant activity and developmental stage. EIIaCre of maternal origin produced excision patterns suggesting that the transgene is expressed during mature oocyte stages, and, to a much lesser extent, after ovulation. This Cre-activity is strong enough to complete recombination of floxed segments when they are also maternally transmitted. EIIaCre is re-expressed from maternal and paternal transgene copies during the morula and blastocyst stages. In the presence of multiple loxP sites, this late EIIaCre expression is able to produce mosaic mice with partial excision patterns.
Recent advances in development of the FLP-frt system (4,28) show that flanking selection cassettes by frt sites while flanking the exon by loxP sites is an alternative to the three-loxP-sites strategy. In addition, combinatory use of Cre-lox and FLP-frt opens new perspectives, for example the generation of tissue-specific reversion from the knock down to the wild-type allele. However, use of the FLP-frt system requires a second (FLP) transgenic mouse line (29) and introduction of frt sites into the target gene. Moreover, the use of FLP-frt needs a decision at the time when the targeting construct is designed.
Acknowledgments
ACKNOWLEDGEMENTS
Heiner Westphal generously provided the EIIaCre transgenic mouse. IGF-IR genomic clone S/B5.4 was a kind gift from Argiris Efstratiadis. M.H. is supported by a European Community Research Grant and a Novo Nordisk (France) Fellowship. This work was supported by Aventis, INSERM and the University of Paris VI.
REFERENCES
- 1.Kühn R., Schwenk,F., Aguet,M. and Rajewsky,K. (1995) Science, 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- 2.Marth J.D. (1996) J. Clin. Invest., 97, 1999–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacks T., Shih,T.S., Schmitt,E.M., Bronson,R.T., Bernards,A. and Weinberg,R.A. (1994) Nature Genet., 7, 353–361. [DOI] [PubMed] [Google Scholar]
- 4.Meyers E.N., Lewandoski,M. and Martin,G.R. (1998) Nature Genet., 18, 136–141. [DOI] [PubMed] [Google Scholar]
- 5.Nagy A., Moens,C., Ivanyi,E., Pawling,J., Gertsenstein,M., Hadjantonakis,A.K., Pirity,M. and Rossant,J. (1998) Curr. Biol., 8, 661–664. [DOI] [PubMed] [Google Scholar]
- 6.Liu J.L., Grinberg,A., Westphal,H., Sauer,B., Accili,D., Karas,M. and LeRoith,D. (1998) Mol. Endocrinol., 12, 1452–1462. [DOI] [PubMed] [Google Scholar]
- 7.Yakar S., Liu,J.-L., Stannard,B., Butler,A., Accili,D., Sauer,B. and Le Roith,D. (1999) Proc. Natl Acad. Sci. USA, 96, 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzenberger M., Leneuve,P., Hamard,G., Ducos,B., Perin,L., Binoux,M. and Le Bouc,Y. (2000) Endocrinology, 141, 2557–2566. [DOI] [PubMed] [Google Scholar]
- 9.Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Science, 265, 103–106. [DOI] [PubMed] [Google Scholar]
- 10.Brüning J.C., Michael,M.D., Winnay,J.N., Hayashi,T., Horsch,D., Accili,D., Goodyear,L.J. and Kahn,C.R. (1998) Mol. Cell, 2, 559–569. [DOI] [PubMed] [Google Scholar]
- 11.Tronche F., Kellendonk,C., Kretz,O., Gass,P., Anlag,K., Orban,P.C., Bock,R., Klein,R. and Schütz,G. (1999) Nature Genet., 23, 99–103. [DOI] [PubMed] [Google Scholar]
- 12.Abuin A. and Bradley,A. (1996) Mol. Cell. Biol., 16, 1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakso M., Pichel,J.G., Gorman,J.R., Sauer,B., Okamoto,Y., Lee,E., Alt,F.W. and Westphal,H. (1996) Proc. Natl Acad. Sci. USA, 93, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams-Simons L. and Westphal,H. (1999) Transgenic Res., 8, 253–254. [DOI] [PubMed] [Google Scholar]
- 15.Tremml G., Dominguez,C., Rosti,V., Zhang,Z., Pandolfi,P.P., Keller,P. and Bessler,M. (1999) Blood, 94, 2945–2954. [PubMed] [Google Scholar]
- 16.Keller P., Tremml,G., Rosti,V. and Bessler,M. (1999) Proc. Natl Acad. Sci. USA, 96, 7479–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malumbres M., Mangues,R., Ferrer,N., Lu,S. and Pellicer,A. (1997) Biotechniques, 22, 1114–1119. [DOI] [PubMed] [Google Scholar]
- 18.Liu J.-P., Baker,J., Perkins,A.S., Robertson,E.J. and Efstratiadis,A. (1993) Cell, 75, 59–72. [PubMed] [Google Scholar]
- 19.Baker J., Liu,J.-P., Robertson,E.J. and Efstratiadis,A. (1993) Cell, 75, 73–82. [PubMed] [Google Scholar]
- 20.Blakesley V.A., Butler,A.A., Koval,A.P., Okubo,Y. and Le Roith,D. (1999) In Rosenfeld,R. and Roberts,C.,Jr (eds), The IGF System. Humana Press, Totowa, NJ, pp. 143–164.
- 21.D’Ercole A.J. (1999) In Rosenfeld,R. and Roberts,C.,Jr (eds), The IGF System. Humana Press, Totowa, NJ, pp. 545–577.
- 22.McDevitt M.A., Shivdasani,R.A., Fujiwara,Y., Yang,H. and Orkin,S.H. (1997) Proc. Natl Acad. Sci. USA, 94, 6781–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postic C., Shiota,M., Niswender,K.D., Jetton,T.L., Chen,Y., Moates,J.M., Shelton,K.D., Lindner,J., Cherrington,A.D. and Magnuson,M.A. (1999) J. Biol. Chem., 274, 305–315. [DOI] [PubMed] [Google Scholar]
- 24.Araki K., Araki,M., Miyazaki,J. and Vassalli,P. (1995) Proc. Natl Acad. Sci. USA, 92, 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzenberger M., Zaoui,R., Leneuve,P., Hamard,G. and Le Bouc,Y. (2000) Genesis, 26, 157–159. [PubMed] [Google Scholar]
- 26.Forlino A., Porter,F.D., Lee,E.J., Westphal,H. and Marini,J.C. (1999) J. Biol. Chem., 274, 37923–37931. [DOI] [PubMed] [Google Scholar]
- 27.Dooley T.P., Miranda,M., Jones,N.C. and DePamphilis,M.L. (1989) Development, 107, 945–956. [DOI] [PubMed] [Google Scholar]
- 28.Sun X., Lewandoski,M., Meyers,E.N., Liu,Y.H., Maxson,R.E.,Jr and Martin,G.R. (2000) Nature Genet., 25, 83–86. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez C.I., Buchholz,F., Galloway,J., Sequerra,R., Kasper,J., Ayala,R., Stewart,A.F. and Dymecki,S.M. (2000) Nature Genet., 25, 139–140. [DOI] [PubMed] [Google Scholar]