Abstract
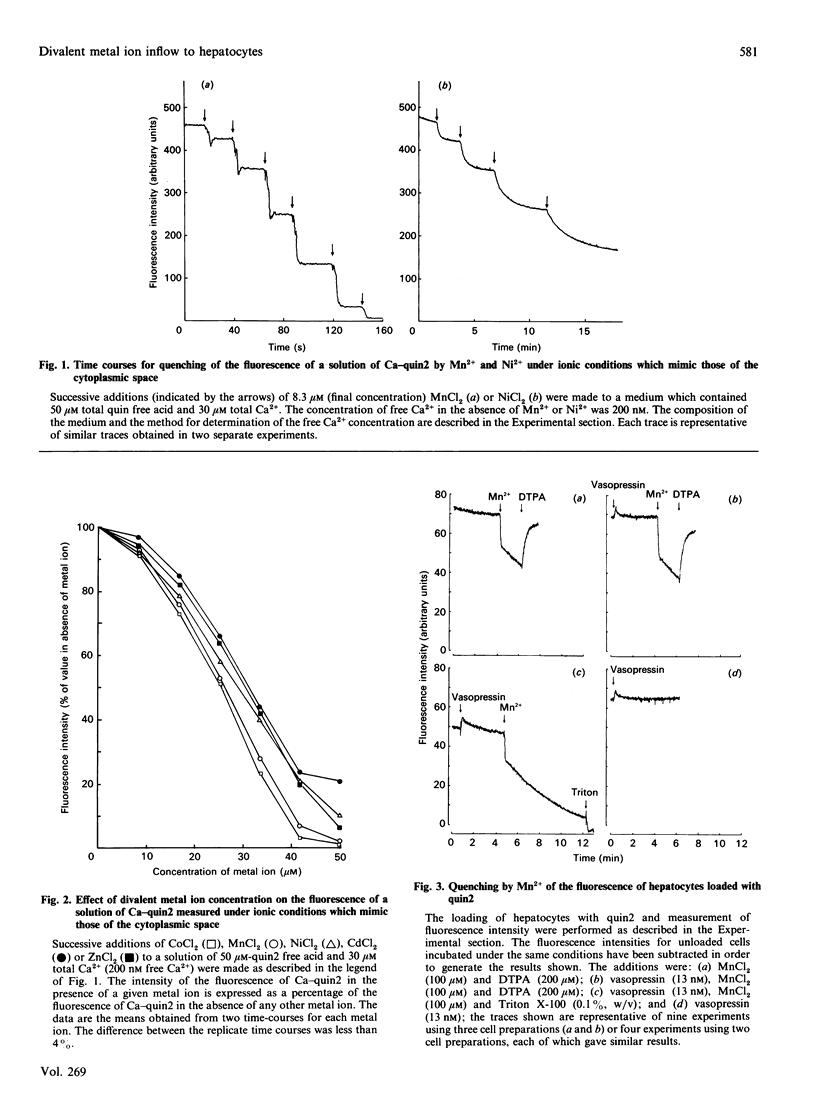
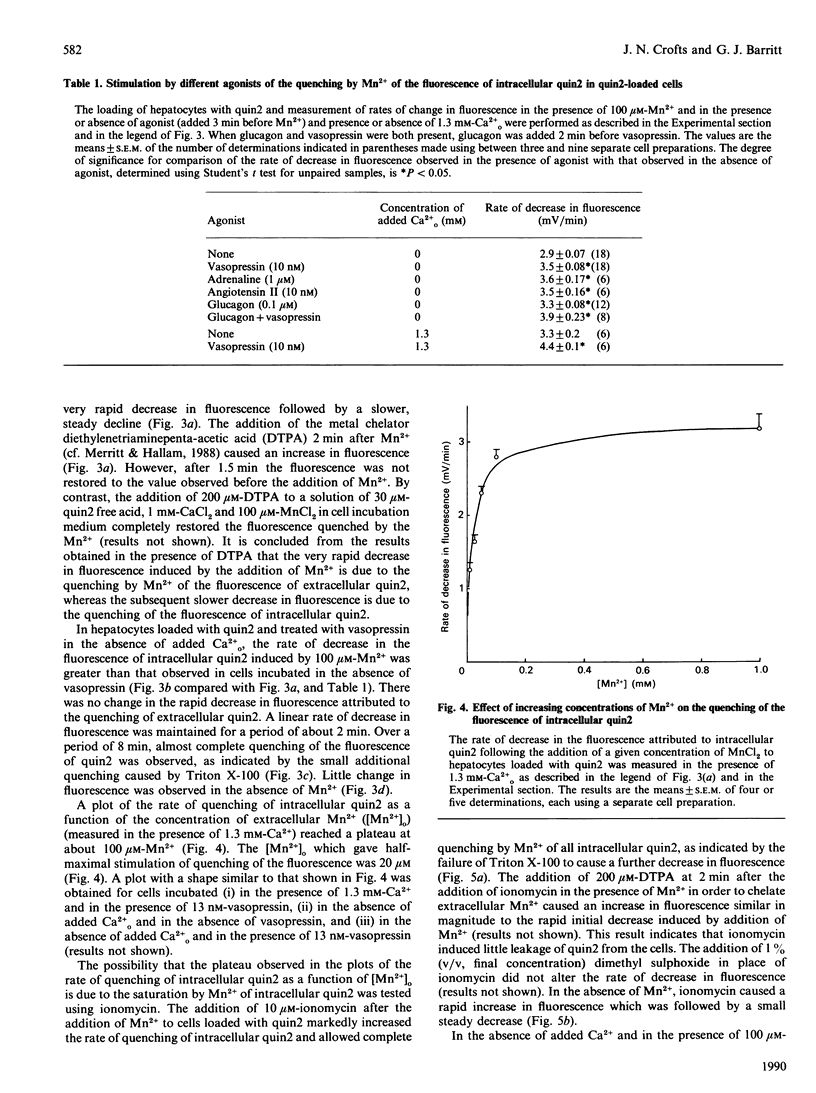
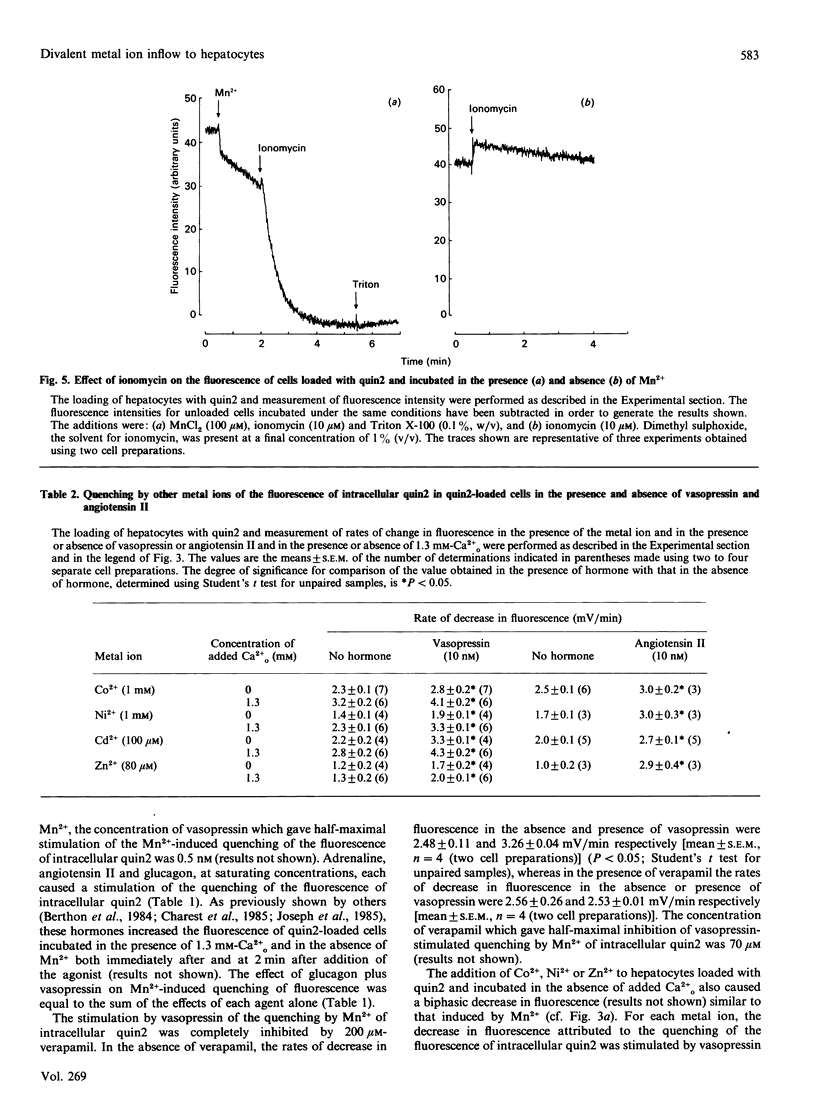
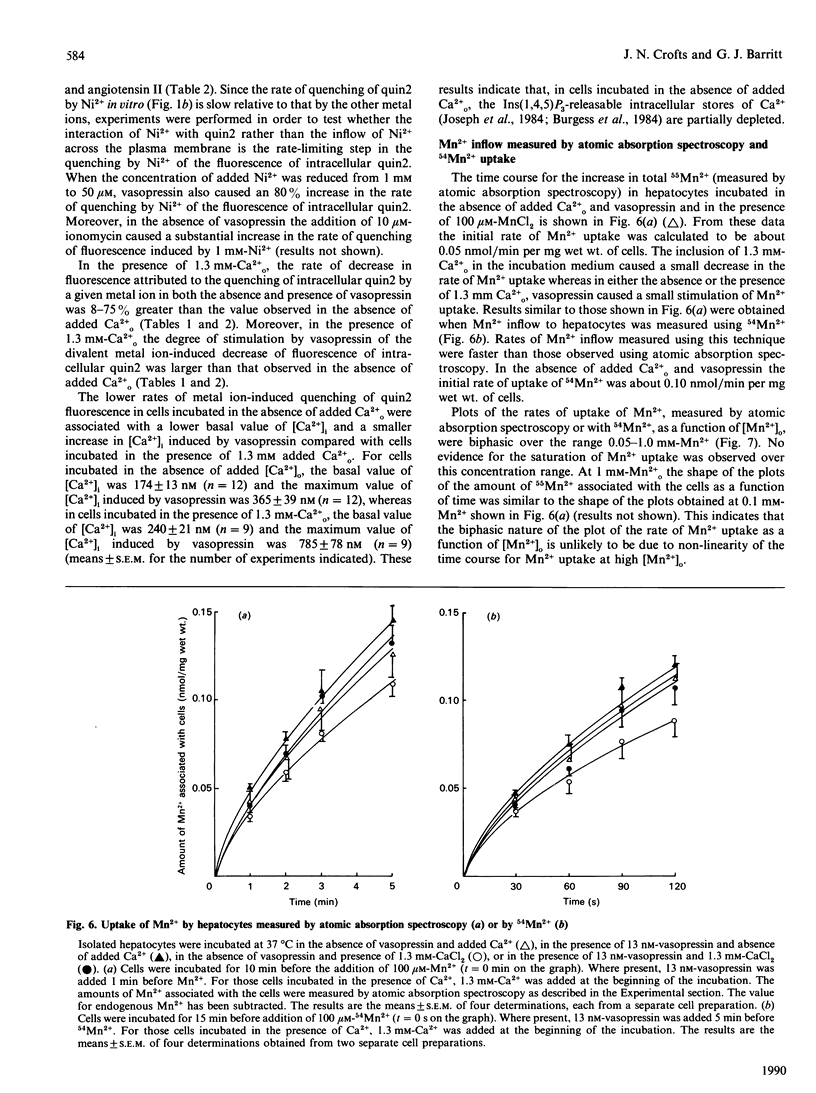
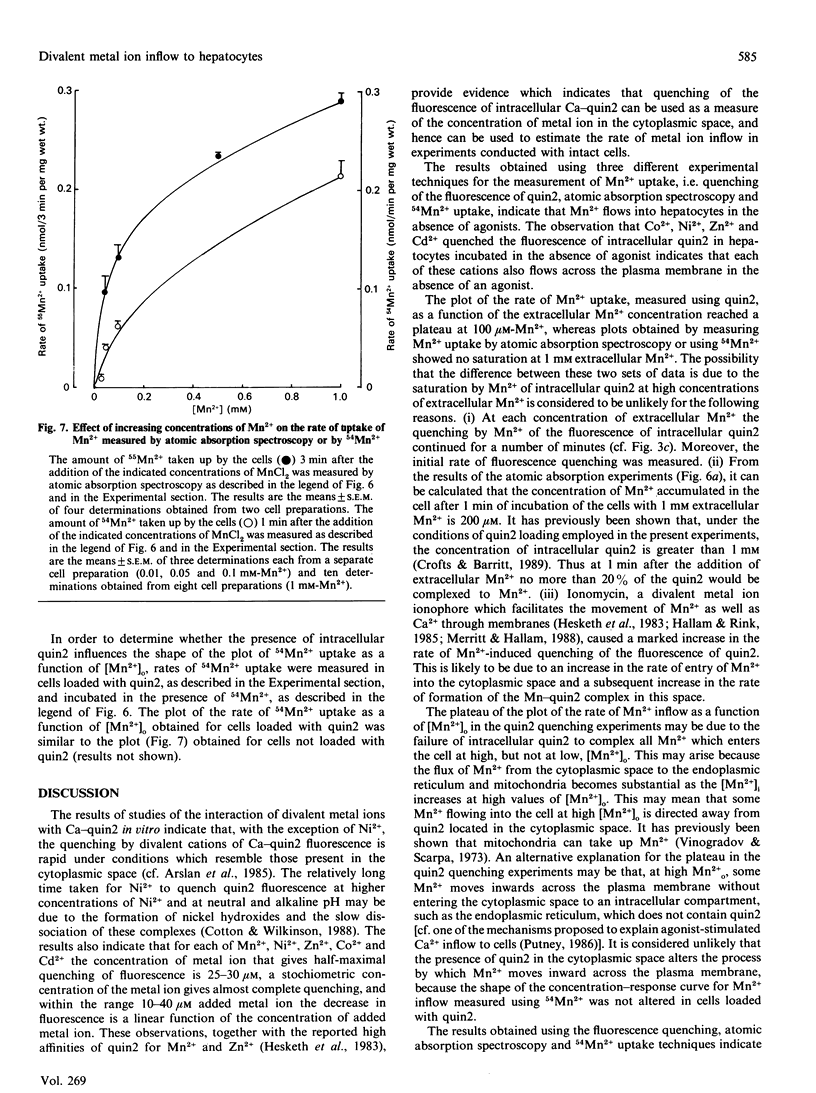
1. The inflow of Mn2+ across the plasma membranes of isolated hepatocytes was monitored by measuring the quenching of the fluorescence of intracellular quin2, by atomic absorption spectroscopy and by the uptake of 54Mn2+. The inflow of other divalent metal ions was measured using quin2. 2. Under ionic conditions which resembled those present in the cytoplasmic space, Mn2+, Zn2+, Co2+, Ni2+ and Cd2+ each quenched the fluorescence of a solution of Ca2(+)-quin2. 3. The addition of Mn2+, Zn2+, Co2+, Ni2+ or Cd2+ to cells loaded with quin2 caused a time-dependent decrease in the fluorescence of intracellular quin2. Plots of the rate of decrease in fluorescence as a function of the concentration of Mn2+ reached a plateau at 100 microM-Mn2+. 4. The rate of decrease in fluorescence induced by Mn2+ was stimulated by 20% in the presence of vasopressin. The effect of vasopressin was completely inhibited by 200 microM-verapamil. Adrenaline, angiotensin II and glucagon also stimulated the rate of decrease in the fluorescence of intracellular quin2 induced by Mn2+. 5. The rate of decrease in fluorescence induced by Zn2+, Co2+, Ni2+ or Cd2+ was stimulated by between 20 and 190% in the presence of vasopressin or angiotensin II. 6. The rates of uptake of Mn2+ measured by atomic absorption spectroscopy or by using 54Mn2+ were inhibited by about 20% by 1.3 mM-Ca2+o and stimulated by 30% by vasopressin. 7. Plots of Mn2+ uptake, measured by atomic absorption spectroscopy or with 54Mn2+, as a function of the extracellular concentration of Mn2+ were biphasic over the range 0.05-1.0 mM added Mn2+ and did not reach a plateau at 1.0 mM-Mn2+. 8. It is concluded that (i) hepatocytes possess both a basal and a receptor-activated divalent cation inflow system, each of which has a broad specificity for metal ions, and (ii) the receptor-activated divalent cation inflow system is the receptor-operated Ca2+ channel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. T., Sanchez A., García-Sancho J. Monitoring of the activation of receptor-operated calcium channels in human platelets. Biochem Biophys Res Commun. 1989 Jul 14;162(1):24–29. doi: 10.1016/0006-291x(89)91956-6. [DOI] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The influx of Ca2+ induced by the administration of glucagon and Ca2+-mobilizing agents to the perfused rat liver could involve at least two separate pathways. Biochem J. 1987 Feb 15;242(1):43–50. doi: 10.1042/bj2420043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Assessment of effects of vasopressin, angiotensin II, and glucagon on Ca2+ fluxes and phosphorylase activity in liver. Methods Enzymol. 1985;109:550–558. doi: 10.1016/0076-6879(85)09113-3. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittadini A., Van Rossum G. D. Properties of the calcium-extruding mechanism of liver cells. J Physiol. 1978 Aug;281:29–43. doi: 10.1113/jphysiol.1978.sp012407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts J. N., Barritt G. J. The measurement of Ca2+ inflow across the liver cell plasma membrane by using quin2 and studies of the roles of Na+ and extracellular Ca2+ in the mechanism of Ca2+ inflow. Biochem J. 1989 Nov 15;264(1):61–70. doi: 10.1042/bj2640061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. Voltage-gated calcium channels: direct observation of the anomalous mole fraction effect at the single-channel level. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5207–5211. doi: 10.1073/pnas.86.13.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Kawa K. Permeation of manganese, cadmium, zinc, and beryllium through calcium channels of an insect muscle membrane. Science. 1977 Apr 15;196(4287):309–311. doi: 10.1126/science.847472. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Membrane biophysics of calcium currents. Fed Proc. 1981 Jun;40(8):2220–2225. [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Kinsella P. A., Osterhoudt K. C. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem. 1987 Dec 5;262(34):16333–16337. [PubMed] [Google Scholar]
- Hughes B. P., Auld A. M., Barritt G. J. Effect of extracellular Ca2+ on plasma membrane Ca2+ inflow and cytoplasmic free Ca2+ in isolated hepatocytes. Biochim Biophys Acta. 1987 Apr 22;928(2):208–216. doi: 10.1016/0167-4889(87)90123-6. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Evidence that guanosine 5'-[gamma-thio]triphosphate stimulates plasma membrane Ca2+ inflow when introduced into hepatocytes. Biochem J. 1989 Jan 15;257(2):591–598. doi: 10.1042/bj2570591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Inhibition of the liver cell receptor-activated Ca2+ inflow system by metal ion inhibitors of voltage-operated Ca2+ channels but not by other inhibitors of Ca2+ inflow. Biochim Biophys Acta. 1989 Oct 9;1013(3):197–205. doi: 10.1016/0167-4889(89)90135-3. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. The stimulation by sodium fluoride of plasma-membrane Ca2+ inflow in isolated hepatocytes. Evidence that a GTP-binding regulatory protein is involved in the hormonal stimulation of Ca2+ inflow. Biochem J. 1987 Jul 1;245(1):41–47. doi: 10.1042/bj2450041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Crofts J. N., Auld A. M., Read L. C., Barritt G. J. Evidence that a pertussis-toxin-sensitive substrate is involved in the stimulation by epidermal growth factor and vasopressin of plasma-membrane Ca2+ inflow in hepatocytes. Biochem J. 1987 Dec 15;248(3):911–918. doi: 10.1042/bj2480911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Milton S. E., Barritt G. J., Auld A. M. Studies with verapamil and nifedipine provide evidence for the presence in the liver cell plasma membrane of two types of Ca2+ inflow transporter which are dissimilar to potential-operated Ca2+ channels. Biochem Pharmacol. 1986 Sep 15;35(18):3045–3052. doi: 10.1016/0006-2952(86)90384-9. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Milton S. E., Barritt G. J. Effects of vasopressin and La3+ on plasma-membrane Ca2+ inflow and Ca2+ disposition in isolated hepatocytes. Evidence that vasopressin inhibits Ca2+ disposition. Biochem J. 1986 Sep 15;238(3):793–800. doi: 10.1042/bj2380793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz L. Pharmacology of calcium channels and smooth muscle. Annu Rev Pharmacol Toxicol. 1986;26:225–258. doi: 10.1146/annurev.pa.26.040186.001301. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Thomas A. P., Rubin R., Williamson J. R. The role of extracellular Ca2+ in the response of the hepatocyte to Ca2+-dependent hormones. J Biol Chem. 1985 Oct 15;260(23):12508–12515. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kawa K. Zinc-dependent action potentials in giant neurons of the snail, Euhadra quaestia. J Membr Biol. 1979 Sep 14;49(4):325–344. doi: 10.1007/BF01868990. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Hallam T. J. Platelets and parotid acinar cells have different mechanisms for agonist-stimulated divalent cation entry. J Biol Chem. 1988 May 5;263(13):6161–6164. [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Parker J. C., Barritt G. J. Evidence that lanthanum ions stimulate calcium inflow to isolated hepatocytes. Biochem J. 1981 Oct 15;200(1):109–114. doi: 10.1042/bj2000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C., Barritt G. J., Wadsworth J. C. A kinetic investigation of the effects of adrenaline on 45Ca2+ exchange in isolated hepatocytes at different Ca2+ concentrations, at 20 degrees C and in the presence of inhibitors of mitochondrial Ca2+ transport. Biochem J. 1983 Oct 15;216(1):51–62. doi: 10.1042/bj2160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Takemura H., Hughes A. R., Horstman D. A., Thastrup O. How do inositol phosphates regulate calcium signaling? FASEB J. 1989 Jun;3(8):1899–1905. doi: 10.1096/fasebj.3.8.2542110. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The action of alpha-adrenergic agonists on plasma-membrane calcium fluxes in perfused rat liver. Biochem J. 1984 May 15;220(1):43–50. doi: 10.1042/bj2200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Prpić V., Exton J. H., Blackmore P. F. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in hepatocytes by vasopressin. J Biol Chem. 1983 Mar 10;258(5):2770–2773. [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage A. L., Biffen M., Martin B. R. Vasopressin-stimulated Ca2+ influx in rat hepatocytes is inhibited in high-K+ medium. Biochem J. 1989 Jun 15;260(3):821–827. doi: 10.1042/bj2600821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawanobori T., Takanashi H., Hiraoka M., Iida Y., Kamisaka K., Maezawa H. Electrophysiological properties of isolated rat liver cells. J Cell Physiol. 1989 Jun;139(3):580–585. doi: 10.1002/jcp.1041390318. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. M., van de Pol E., van Helden D. F., Reinhart P. H., Bygrave F. L. Effect of depolarizing concentrations of potassium on calcium uptake and metabolism in rat liver. FEBS Lett. 1985 Apr 8;183(1):70–74. doi: 10.1016/0014-5793(85)80956-x. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov A., Scarpa A. The initial velocities of calcium uptake by rat liver mitochondria. J Biol Chem. 1973 Aug 10;248(15):5527–5531. [PubMed] [Google Scholar]


