Abstract
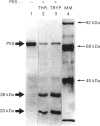
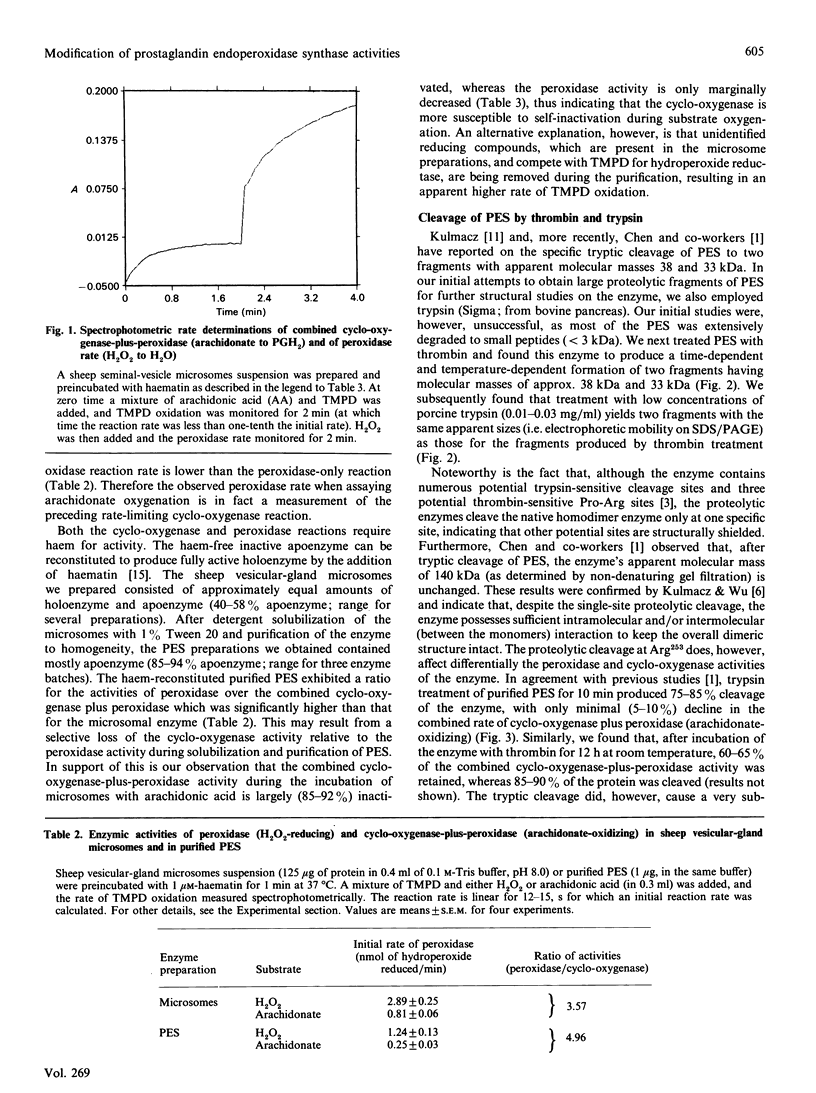
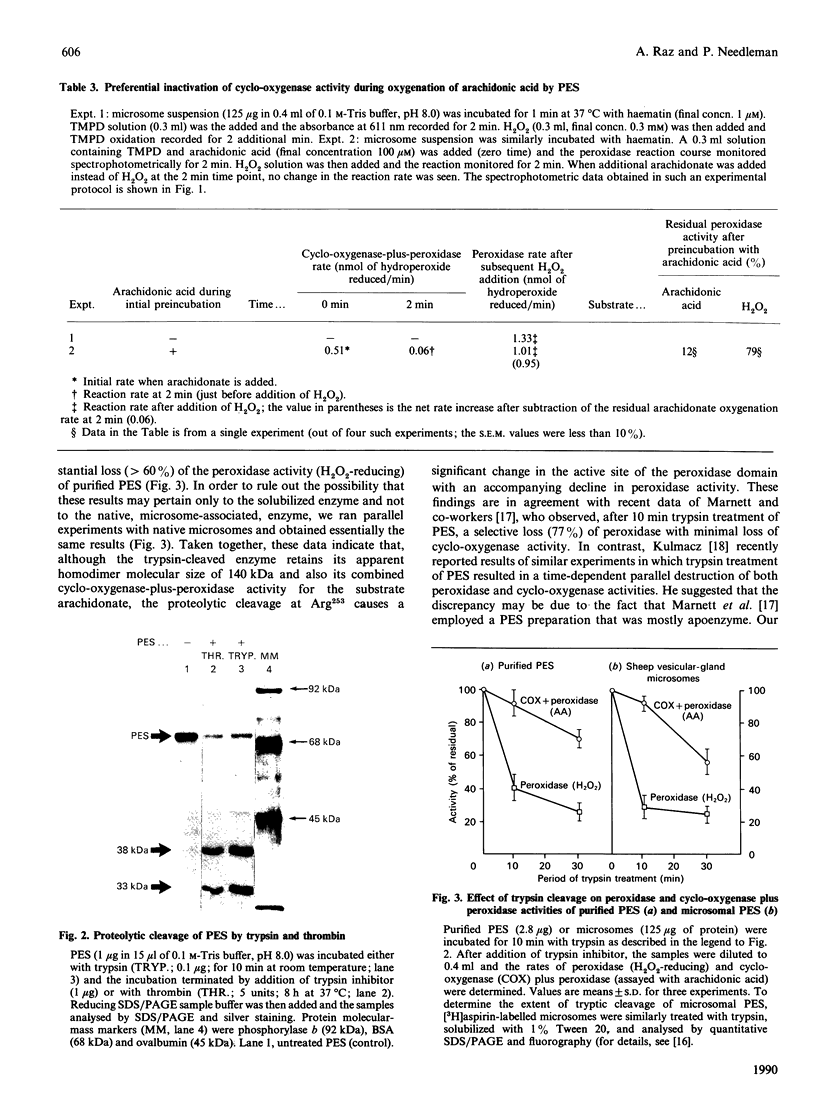
Prostaglandin endoperoxide synthase (PES, EC 1.14.99.1) catalyse the conversion of arachidonic acid into prostaglandin H2. The enzyme is a 140 kDa homodimer which contains both a cyclo-oxygenase activity (converting arachidonate into prostaglandin G2) and peroxidase activity (reducing prostaglandin G2 to H2). PES undergoes rapid self-inactivation during oxygenation of arachidonate to prostaglandin H2 in vitro. The previously reported cDNA-derived amino acid sequence indicates numerous sites for trypsin or thrombin cleavage. Most of these sites must be inaccessible, since these enzymes cleave only at Arg253. The enzyme appears to be a self-adherent and highly folded molecule, since after cleavage it retains its functional assembly and its homodimer size of 140 kDa, as well as its overall enzymic activity. Only under denaturing conditions (e.g. SDS/PAGE) can the proteolytic peptides be demonstrated: a 38 kDa C-terminal fragment containing the aspirin-derived-acetyl-binding ability, and a 33 kDa N-terminal fragment. In the present studies we investigated whether the two enzymic activities of PES can be differentially manipulated by proteolytic cleavage or by substrate (arachidonate) self-inactivation. The results indicated that, during arachidonate oxygenation by PES, the cyclooxygenase activity is selectively inactivated, whereas the peroxidase activity is essentially retained. By contrast, thrombin or trypsin cleavage of pure PES or microsomal PES (to yield the 38 and 33 kDa peptide fragments) inactivated the peroxidase, but not the cyclo-oxygenase. Taken together, these results suggest the presence of separate cyclo-oxygenase and peroxidase structural domains on the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. N., Bienkowski M. J., Marnett L. J. Controlled tryptic digestion of prostaglandin H synthase. Characterization of protein fragments and enhanced rate of proteolysis of oxidatively inactivated enzyme. J Biol Chem. 1987 Dec 15;262(35):16892–16899. [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulmacz R. J. Prostaglandin G2 levels during reaction of prostaglandin H synthase with arachidonic acid. Prostaglandins. 1987 Aug;34(2):225–240. doi: 10.1016/0090-6980(87)90246-2. [DOI] [PubMed] [Google Scholar]
- Kulmacz R. J. Prostaglandin H synthase and hydroperoxides: peroxidase reaction and inactivation kinetics. Arch Biochem Biophys. 1986 Sep;249(2):273–285. doi: 10.1016/0003-9861(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Kulmacz R. J. Topography of prostaglandin H synthase. Antiinflammatory agents and the protease-sensitive arginine 253 region. J Biol Chem. 1989 Aug 25;264(24):14136–14144. [PubMed] [Google Scholar]
- Kulmacz R. J., Wu K. K. Topographic studies of microsomal and pure prostaglandin H synthase. Arch Biochem Biophys. 1989 Feb 1;268(2):502–515. doi: 10.1016/0003-9861(89)90317-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefkowith J. B., Flippo V., Sprecher H., Needleman P. Paradoxical conservation of cardiac and renal arachidonate content in essential fatty acid deficiency. J Biol Chem. 1985 Dec 15;260(29):15736–15744. [PubMed] [Google Scholar]
- Markey C. M., Alward A., Weller P. E., Marnett L. J. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987 May 5;262(13):6266–6279. [PubMed] [Google Scholar]
- Marnett L. J., Chen Y. N., Maddipati K. R., Plé P., Labèque R. Functional differentiation of cyclooxygenase and peroxidase activities of prostaglandin synthase by trypsin treatment. Possible location of a prosthetic heme binding site. J Biol Chem. 1988 Nov 15;263(32):16532–16535. [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- Mevkh A. T., Sud'ina G. F., Golub N. B., Varfolomeev S. D. Purification of prostaglandin H synthetase and a fluorometric assay for its activity. Anal Biochem. 1985 Oct;150(1):91–96. doi: 10.1016/0003-2697(85)90444-0. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Brown W., Tauk N. Iothalamate stimulates hydroperoxide formation by soybean lipoxygenase. Prostaglandins. 1984 May;27(5):753–759. doi: 10.1016/0090-6980(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Reed G. A., Lasker J. M., Eling T. E., Sivarajah K. Peroxidative oxidation of bilirubin during prostaglandin biosynthesis. Prostaglandins. 1985 Jul;30(1):153–165. doi: 10.1016/s0090-6980(85)80019-8. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Siok C. J., Ozols J. Structural characteristics of prostaglandin synthetase from sheep vesicular gland. J Biol Chem. 1980 Feb 25;255(4):1301–1304. [PubMed] [Google Scholar]
- Van der Ouderaa F. J., Buytenhek M., Nugteren D. H., Van Dorp D. A. Purification and characterisation of prostaglandin endoperoxide synthetase from sheep vesicular glands. Biochim Biophys Acta. 1977 May 25;487(2):315–331. doi: 10.1016/0005-2760(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Yokoyama C., Takai T., Tanabe T. Primary structure of sheep prostaglandin endoperoxide synthase deduced from cDNA sequence. FEBS Lett. 1988 Apr 25;231(2):347–351. doi: 10.1016/0014-5793(88)80847-0. [DOI] [PubMed] [Google Scholar]