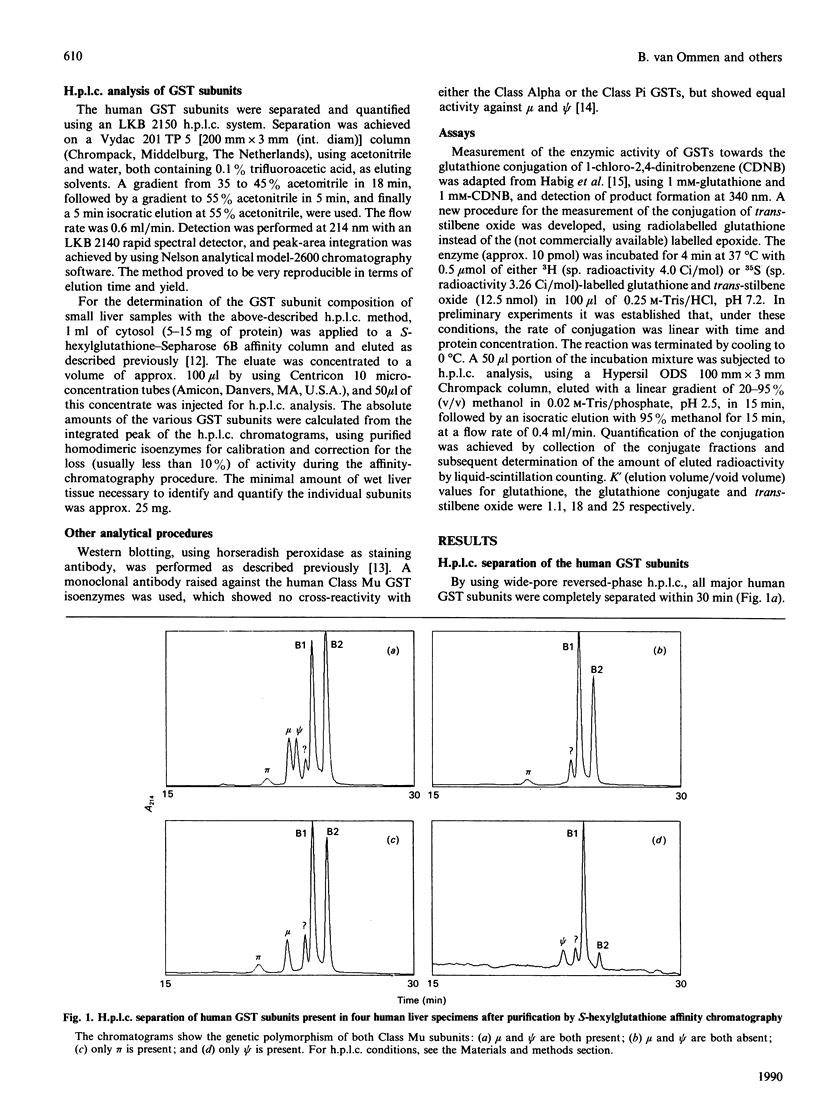
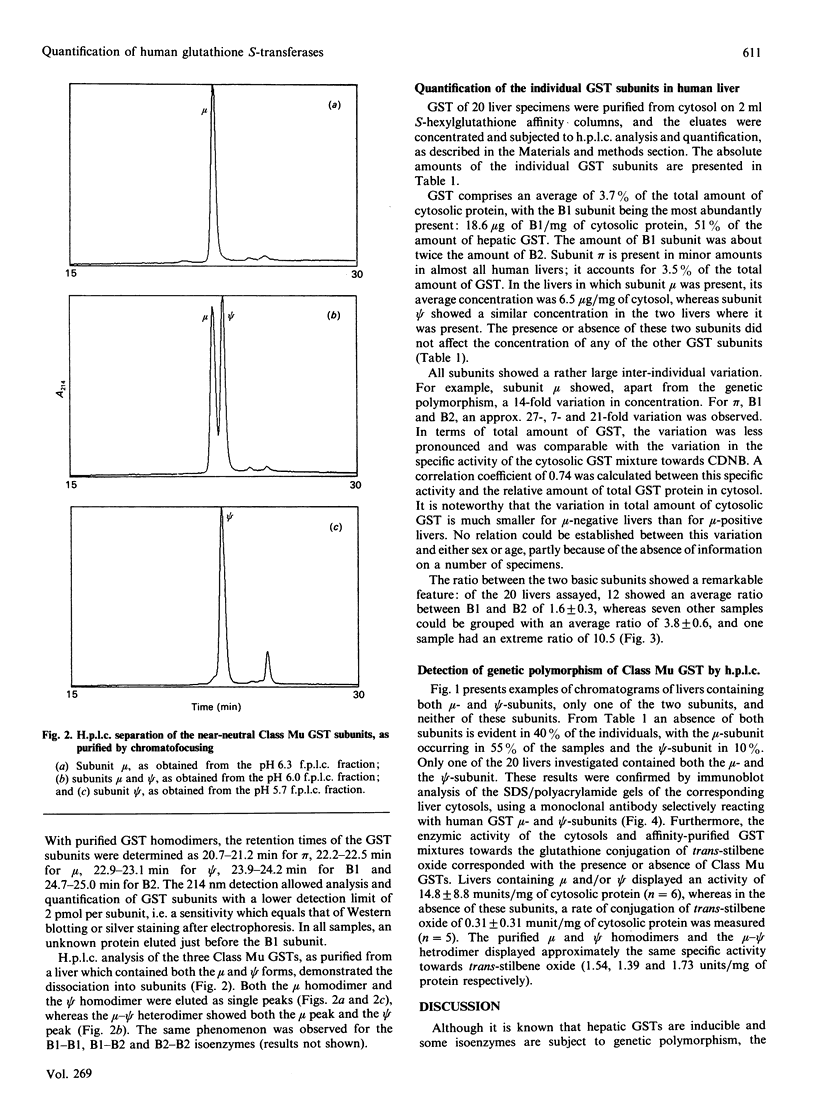
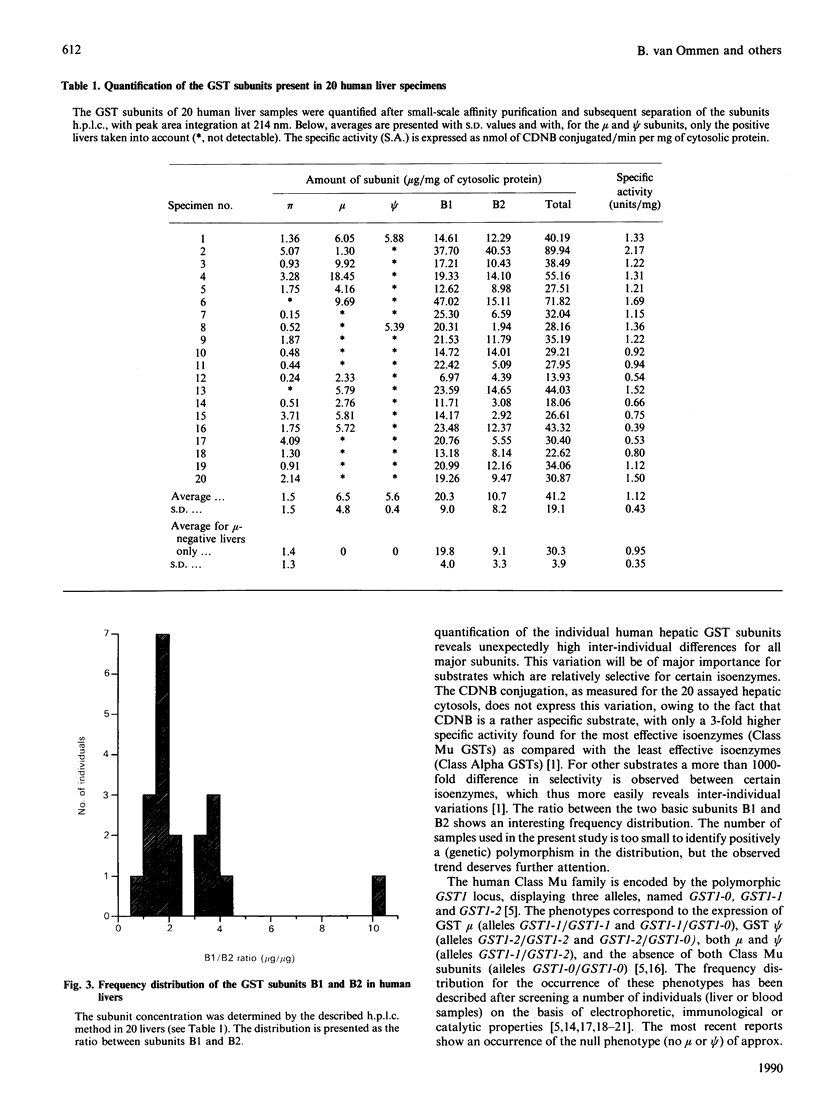
Abstract
Human hepatic glutathione S-transferase (GST) subunits were characterized and quantified with the aid of a recently developed h.p.l.c. method. In 20 hepatic tissue specimens the absolute amounts of the basic Class Alpha subunits B1 and B2, the near-neutral Class Mu subunits mu and psi and the acidic subunit pi were determined. The average total amount of GST was 37 micrograms/mg of cytosolic protein, with the Class Alpha GST being the predominant class (84% of total GSTs), and pi as the sole representative of the Class Pi GSTs present in the lowest concentration (4% of total GSTs). Large interindividual differences were observed for all subunits, with variations up to 27-fold, depending on the subunit. For the Class Alpha GST-subunits B1 and B2, a biphasic ratio was observed. The genetic polymorphism of the subunits mu and psi was confirmed by h.p.l.c. analysis, and correlated with the enzymic glutathione conjugation of trans-stilbene oxide and with Western blotting of cytosols, using a monoclonal anti-(Class Mu GST) antibody. Of the 20 livers examined, ten contained only mu, whereas the occurrence of psi alone, and the combination of mu and psi, were found in only one liver each.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Bogaards J. J., van Ommen B., van Bladeren P. J. An improved method for the separation and quantification of glutathione S-transferase subunits in rat tissue using high-performance liquid chromatography. J Chromatogr. 1989 Jul 19;474(2):435–440. doi: 10.1016/s0021-9673(01)93940-8. [DOI] [PubMed] [Google Scholar]
- Faulder C. G., Hirrell P. A., Hume R., Strange R. C. Studies of the development of basic, neutral and acidic isoenzymes of glutathione S-transferase in human liver, adrenal, kidney and spleen. Biochem J. 1987 Jan 1;241(1):221–228. doi: 10.1042/bj2410221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Ishikawa T., Tsuchida S., Satoh K., Sato K. The subunit structure of a major glutathione S-transferase form, MT, in rat testis. Evidence for a heterodimer consisting of subunits with different isoelectric points. Eur J Biochem. 1988 Oct 1;176(3):551–557. doi: 10.1111/j.1432-1033.1988.tb14313.x. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Idle J. R., Hirom P. C. Differential expression of glutathione transferases by native and cultured human lymphocytes. Biochem Pharmacol. 1988 Dec 1;37(23):4586–4590. doi: 10.1016/0006-2952(88)90679-x. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. H., Jansen P. L. Immunocharacterization of UDP-glucuronyltransferase isoenzymes in human liver, intestine and kidney. Biochem Pharmacol. 1988 Feb 1;37(3):564–567. doi: 10.1016/0006-2952(88)90234-1. [DOI] [PubMed] [Google Scholar]
- Peters W. H., Kock L., Nagengast F. M., Roelofs H. M. Immunodetection with a monoclonal antibody of glutathione S-transferase mu in patients with and without carcinomas. Biochem Pharmacol. 1990 Feb 1;39(3):591–597. doi: 10.1016/0006-2952(90)90068-v. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W. The hereditary transmission of high glutathione transferase activity towards trans-stilbene oxide in human mononuclear leukocytes. Hum Genet. 1985;69(1):66–68. doi: 10.1007/BF00295531. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. V., Kurosky A., Awasthi Y. C. Human liver glutathione S-transferase psi. Chemical characterization and secondary-structure comparison with other mammalian glutathione S-transferases. Biochem J. 1987 Apr 1;243(1):61–67. doi: 10.1042/bj2430061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Davis B. A., Faulder C. G., Cotton W., Bain A. D., Hopkinson D. A., Hume R. The human glutathione S-transferases: developmental aspects of the GST1, GST2, and GST3 loci. Biochem Genet. 1985 Dec;23(11-12):1011–1028. doi: 10.1007/BF00499944. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Matsushima A., Li N. Q., Rhoads D. M., Srikumar K., Reddy A. P., Reddy C. C. Immunological and sequence interrelationships between multiple human liver and rat glutathione S-transferases. J Biol Chem. 1986 Jul 15;261(20):9540–9545. [PubMed] [Google Scholar]
- Vos R. M., Snoek M. C., van Berkel W. J., Müller F., van Bladeren P. J. Differential induction of rat hepatic glutathione S-transferase isoenzymes by hexachlorobenzene and benzyl isothiocyanate. Comparison with induction by phenobarbital and 3-methylcholanthrene. Biochem Pharmacol. 1988 Mar 15;37(6):1077–1082. doi: 10.1016/0006-2952(88)90513-8. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- van Ommen B., den Besten C., Rutten A. L., Ploemen J. H., Vos R. M., Müller F., van Bladeren P. J. Active site-directed irreversible inhibition of glutathione S-transferases by the glutathione conjugate of tetrachloro-1,4-benzoquinone. J Biol Chem. 1988 Sep 15;263(26):12939–12942. [PubMed] [Google Scholar]