Abstract
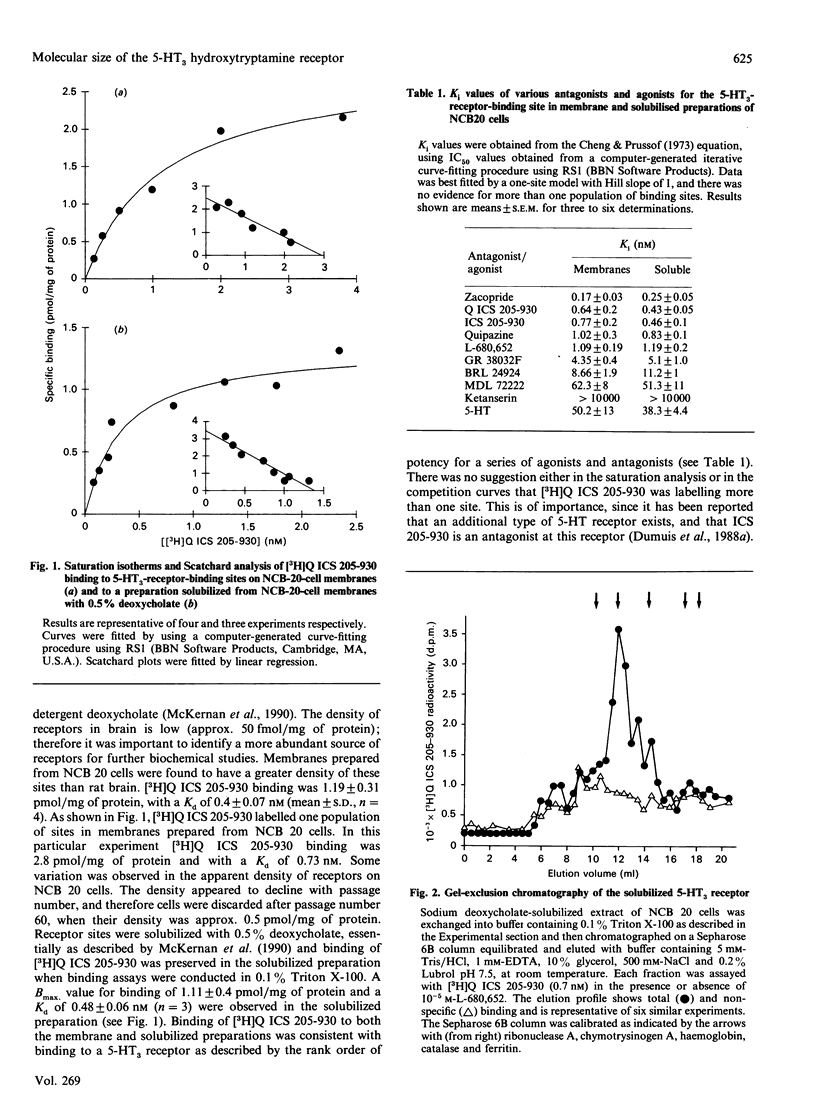
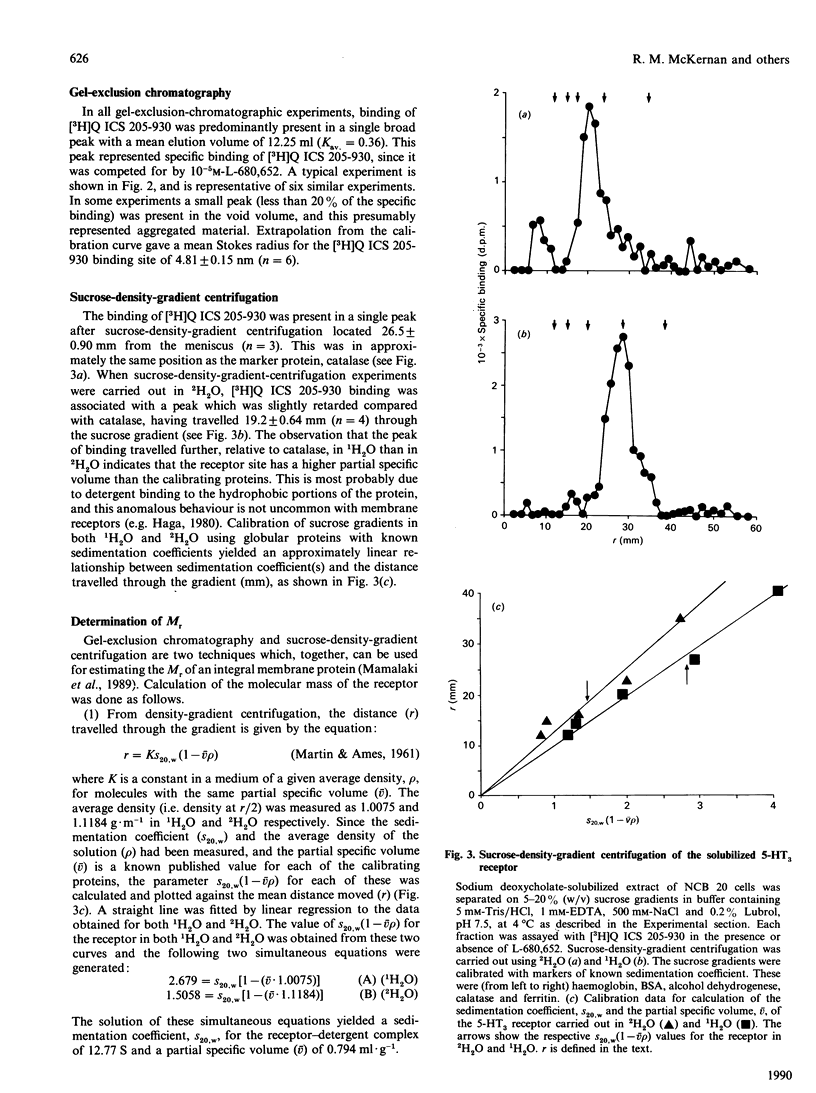
The 5-HT3 hydroxytryptamine receptor from NCB 20 cells was solubilized and the molecular and hydrodynamic properties of the receptor were investigated. The receptor was identified by binding of the radioligand 3-NN'-[3H]dimethyl-8-azabicyclo[3.2.1]octanyl indol-3-yl carboxylate ester [( 3H]Q ICS 205-930) to NCB 20 membranes (Bmax = 1.19 +/- 0.31 pmol/mg of protein; Kd = 0.43 +/- 0.076 nM) and was optimally solubilized with 0.5% deoxycholate. [3H]Q ICS 205-930 labelled one population of sites in solution (Bmax = 1.11 +/- 0.4 pmol/mg of protein; Kd = 0.48 +/- 0.06 nM; n = 4). The characteristics of [3H]Q ICS 205-930 binding were essentially unchanged by solubilization, and competition for [3H]Q ICS 205-930 binding by a series of 5-HT3 agonists and antagonists was consistent with binding to a 5-HT3 receptor site and was similar to that observed for 5-HT3 receptors solubilized from rat brain [McKernan, Quirk, Jackson & Ragan (1990) J. Neurochem. 54, 924-930]. Some physical properties of the solubilized receptor were investigated. The molecular size (Stokes radius) of the [3H]Q ICS 205-930-binding site was measured by gel-exclusion chromatography in a buffer containing 0.2% Lubrol and 0.5 M-NaCl and was determined as 4.81 +/- 0.15 nm (mean +/- S.E.M.; n = 6). Sucrose-density-gradient centrifugation was also performed under the same detergent and salt conditions to determine the partial specific volume (v) of the detergent-receptor site complex. This was found to be 0.794 ml.g-1. Sucrose-density-gradient centrifugation was carried out in both 1H2O and 2H2O to allow correction for detergent binding to the receptor. The Mr of the 5-HT3 receptor under these conditions was calculated as 249,000 +/- 18,000 (n = 3). The size and physical properties of the 5-HT3 receptor are similar to those observed for members of the family of ligand-gated ion channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes N. M., Costall B., Ironside J. W., Naylor R. J. Identification of 5-HT3 recognition sites in human brain tissue using [3H]zacopride. J Pharm Pharmacol. 1988 Sep;40(9):668–668. doi: 10.1111/j.2042-7158.1988.tb05338.x. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Derkach V., Surprenant A., North R. A. 5-HT3 receptors are membrane ion channels. Nature. 1989 Jun 29;339(6227):706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Bouhelal R., Sebben M., Bockaert J. A 5-HT receptor in the central nervous system, positively coupled with adenylate cyclase, is antagonized by ICS 205 930. Eur J Pharmacol. 1988 Jan 27;146(1):187–188. doi: 10.1016/0014-2999(88)90503-1. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Bouhelal R., Sebben M., Cory R., Bockaert J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol. 1988 Dec;34(6):880–887. [PubMed] [Google Scholar]
- Haga T. Molecular size of muscarinic acetylcholine receptors of rat brain. FEBS Lett. 1980 Apr 21;113(1):68–72. doi: 10.1016/0014-5793(80)80497-2. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Neijt H. C. Identification of serotonin 5-HT3 recognition sites in membranes of N1E-115 neuroblastoma cells by radioligand binding. Mol Pharmacol. 1988 Mar;33(3):303–309. [PubMed] [Google Scholar]
- Jones B. J., Costall B., Domeney A. M., Kelly M. E., Naylor R. J., Oakley N. R., Tyers M. B. The potential anxiolytic activity of GR38032F, a 5-HT3-receptor antagonist. Br J Pharmacol. 1988 Apr;93(4):985–993. doi: 10.1111/j.1476-5381.1988.tb11489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick G. J., Jones B. J., Tyers M. B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987 Dec 24;330(6150):746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Hales T. G., Dempster J. The properties of 5-HT3 receptors in clonal cell lines studied by patch-clamp techniques. Br J Pharmacol. 1989 May;97(1):27–40. doi: 10.1111/j.1476-5381.1989.tb11920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mamalaki C., Barnard E. A., Stephenson F. A. Molecular size of the gamma-aminobutyric acidA receptor purified from mammalian cerebral cortex. J Neurochem. 1989 Jan;52(1):124–134. doi: 10.1111/j.1471-4159.1989.tb10906.x. [DOI] [PubMed] [Google Scholar]
- McClintock T. S., Ache B. W. Histamine directly gates a chloride channel in lobster olfactory receptor neurons. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8137–8141. doi: 10.1073/pnas.86.20.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan R. M., Quirk K., Jackson R. G., Ragan C. I. Solubilisation of the 5-hydroxytryptamine3 receptor from pooled rat cortical and hippocampal membranes. J Neurochem. 1990 Mar;54(3):924–930. doi: 10.1111/j.1471-4159.1990.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Milburn C. M., Peroutka S. J. Characterization of [3H]quipazine binding to 5-hydroxytryptamine3 receptors in rat brain membranes. J Neurochem. 1989 Jun;52(6):1787–1792. doi: 10.1111/j.1471-4159.1989.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Neijt H. C., Karpf A., Schoeffter P., Engel G., Hoyer D. Characterisation of 5-HT3 recognition sites in membranes of NG 108-15 neuroblastoma-glioma cells with [3H]ICS 205-930. Naunyn Schmiedebergs Arch Pharmacol. 1988 May;337(5):493–499. doi: 10.1007/BF00182721. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Papp M. Similar effects of diazepam and the 5-HT3 receptor antagonist ICS 205-930 on place aversion conditioning. Eur J Pharmacol. 1988 Jul 7;151(2):321–324. doi: 10.1016/0014-2999(88)90816-3. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988 Nov;11(11):496–500. doi: 10.1016/0166-2236(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Hamik A. [3H]quipazine labels 5-HT3 recognition sites in rat cortical membranes. Eur J Pharmacol. 1988 Mar 29;148(2):297–299. doi: 10.1016/0014-2999(88)90579-1. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. Species variations in 5-HT3 recognition sites labeled by 3H-quipazine in the central nervous system. Naunyn Schmiedebergs Arch Pharmacol. 1988 Nov;338(5):472–475. doi: 10.1007/BF00179316. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Lambert J. J. Electrophysiology of 5-HT3 receptors in neuronal cell lines. Trends Pharmacol Sci. 1989 May;10(5):172–175. doi: 10.1016/0165-6147(89)90230-7. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Stephenson F. A. Understanding the GABAA receptor: a chemically gated ion channel. Biochem J. 1988 Jan 1;249(1):21–32. doi: 10.1042/bj2490021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange P. G. The structure and mechanism of neurotransmitter receptors. Implications for the structure and function of the central nervous system. Biochem J. 1988 Jan 15;249(2):309–318. doi: 10.1042/bj2490309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Crist J. Electrophysiological characterization of functionally distinct 5-hydroxytryptamine receptors on guinea-pig submucous plexus. Neuroscience. 1988 Jan;24(1):283–295. doi: 10.1016/0306-4522(88)90331-4. [DOI] [PubMed] [Google Scholar]
- Watling K. J., Aspley S., Swain C. J., Saunders J. [3H]quaternised ICS 205-930 labels 5-HT3 receptor binding sites in rat brain. Eur J Pharmacol. 1988 May 10;149(3):397–398. doi: 10.1016/0014-2999(88)90677-2. [DOI] [PubMed] [Google Scholar]
- Whiting P. J., Lindstrom J. M. Purification and characterization of a nicotinic acetylcholine receptor from chick brain. Biochemistry. 1986 Apr 22;25(8):2082–2093. doi: 10.1021/bi00356a037. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]


