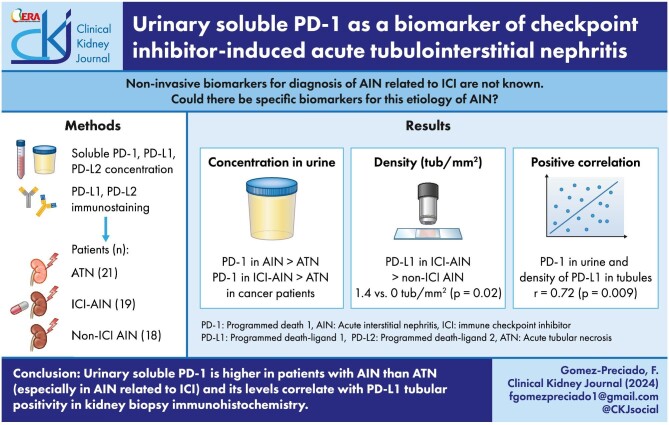
ABSTRACT
Background
Acute interstitial nephritis (AIN) related to immune checkpoint inhibitors (ICI-AIN) has a not completely understood pathophysiology. Our objectives were to analyze possible biomarkers for the differentiation between acute tubular necrosis (ATN) and AIN, especially in cancer patients, and to study the participation of the immune checkpoint pathway in ICI-AIN.
Methods
We performed an observational study. We recruited patients with incident diagnosis of ICI-AIN (n = 19). We measured soluble PD-1 (sPD-1), sPD-L1, and sPD-L2 in serum and urine at diagnosis and compared to it patients with non-ICI-related AIN (non-ICI-AIN) (n = 18) and ATN (n = 21). The findings were validated in an independent cohort from another institution (n = 30). Also, we performed PD-L1 and PD-L2 immunostaining of kidney biopsies from patients with ICI-AIN and compared to patients with non-ICI-AIN.
Results
Urinary sPD-1 (usPD-1) was higher in patients with AIN compared to ATN (P = .03). Patients with AIN also showed higher serum sPD-1 (ssPD-1) than patients with ATN (P = .021).
In cancer patients, usPD-1 <129.3 pg/ml had a 71.43% sensitivity and 94.44% specificity to differentiate ATN from ICI-AIN, with a likelihood ratio of 12.86. In the external validation cohort, the same cutoff showed a sensitivity of 80%.
In kidney biopsies, patients with ICI-AIN showed higher density of PD-L1 positive tubules than patients with non-ICI-AIN (P = .02). The proportion of patients having >2.64/mm2 PD-L2 positive tubules was higher among patients with ICI-AIN compared to non-ICI-AIN (P = .034). There was a positive correlation (P = .009, r = 0.72) between usPD-1 and the number of PD-L1 positive tubules.
Conclusions
UsPD-1 and ssPD-1 are higher in AIN than ATN. Moreover, there was a strong correlation between usPD-1 and renal tubular PD-L1 expression. Our findings suggest a role of usPD-1 as non-invasive biomarker to differentiate ICI-AIN from ATN, especially in cancer patients, which has been confirmed in an external validation cohort.
Keywords: checkpoint, nephritis, PD-1, PD-L1, PD-L2
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Immune checkpoint inhibitor induced acute interstitial nephritis has different pathophisiology from other etiologies of this condition.
PD-L1 tubular immunohistochemistry has been found positive mainly in this specific etiology.
This study adds:
Urinary PD-1 is higher in patients with immune checkpoint inhibitor induced acute interstitial nephritis, and differentiates this entity from acute tubular necrosis in cancer patients.
This correlates with the number of PD-L1 positive tubules in kidney biopsies.
Potential impact:
Urinary PD-1 is a potential non-invasive biomarker in the diagnosis of immune checkpoint inhibitor induced acute interstitial nephritis.
It could help avoid kidney biopsies in cancer patients and also aid in the follow-up of these patients.
INTRODUCTION
The immune checkpoint pathway has a key regulatory role in immunity, modulating the immune response to antigenic stimuli and preventing autoimmunity phenomena. Programmed cell death ligand 1 (PD-L1) and 2 (PD-L2) are expressed by antigen presenting cells and by some types of non-professional immune cells and binds to the programmed cell death protein 1 (PD-1), which is mainly expressed in the surface of T, B, and NK cells [1, 2]. This binding counteracts stimulatory signals and induces anergy in effector cells [3]. Some authors have described an overexpression of both molecules in renal tubular epithelial cells within an inflammatory milieu [4, 5].
PD-1, PD-L1, and PD-L2 are transmembrane proteins, but in the recent years soluble forms of these molecules (sPD-1, sPD-L1, and sPD-L2) have been identified. They are produced by cleavage of the membrane forms by proteases or by alternative splicing, thus leading to many different splice variants. The affinity of these splice variants for their ligand and their function in normal and disease conditions remain to be elucidated [6].
As an escape mechanism from the natural immunosurveillance system, some neoplasms overexpress PD-L1 and PD-L2 to inhibit immune aggression. Immune checkpoint inhibitors (ICI) block these pathways therefore restoring the immune response that acts against the tumor cells. These recently developed drugs have improved survival in various types of cancer [7].
Owing to its mechanism of enhancing immune system, checkpoint inhibitors have well described immune-related adverse events (irAEs), which may affect various organs [8, 9]. The incidence of acute kidney injury (AKI) among patients treated with ICI ranges between 1.4% and 30% [10–12]. Acute interstitial nephritis (AIN) related to ICI (ICI-AIN) is the most reported histopathological finding in kidney biopsies [10, 13]; its exact pathomechanism is not fully understood. The second most frequent histological finding is acute tubular necrosis (ATN). ATN is usually related to other treatments frequently prescribed to these patients (non-steroidal anti-inflammatory drugs, platinum derivatives, antibiotics…) or conditions of the patient. Sometimes ATN is clinically difficult to distinguish from AIN, thus a kidney biopsy is necessary. Unfortunately, kidney biopsy is not widely available or suitable for all patients.
To overcome this limitation, our group and others have previously proposed some urinary cytokines as biomarkers that may differentiate ATN from AIN [14–16]. Some studies have been published recently about distinct biomarkers for the differentiation of ICI-AIN from other entities, showing a growing interest in this matter and its importance [17–19].
On the other hand, the concentration of serum soluble molecules of the checkpoint pathway has been studied in some autoimmune diseases [20, 21]. They have been proposed as a marker of activity. Moreover, the expression of these molecules has been studied by immunohistochemistry in kidney biopsies from patients with ICI-AIN. Previous studies showed a predominance of PD-L1 tubular staining in ICI-AIN compared to AIN from other causes (non-ICI-AIN), ATN, or controls [22–24]. Despite this finding, we do not know of any reports regarding the serum and urine concentration of these molecules in kidney irAEs. In this study, we aimed to comprehensively describe the serum and urinary concentration of sPD-1, sPD-L1, and sPD-L2 in patients diagnosed with AIN, especially focusing on the patients presenting ICI-AIN, as potential biomarkers in the distinction of AIN from ATN. In addition, we analyzed the differential renal expression of PD-L1 and PD-L2 in ICI-AIN compared non-ICI-AIN.
MATERIALS AND METHODS
Study population
Discovery cohort
We recruited all patients with clinical suspicion of AIN or ATN in the initial nephrology evaluation between 2017 and 2021 in Bellvitge University Hospital. The diagnosis of AIN was performed by kidney biopsy in all patients after evaluation by an expert pathologist. For diagnosis of ICI-AIN, patients must have received a dose of ICI 6 months prior to the diagnosis. ATN was diagnosed by kidney biopsy or by an expert nephrologist evaluation during the same period. Exclusion criteria were as follows: classification of AKI as secondary to obstructive uropathy, prerenal AKI, glomerulonephritis, and unclassified nephropathy after a complete nephrology work-up evaluation. Patients with urinary tract infection, active sepsis, or an active flare-up of an autoimmune condition with extrarenal involvement were also excluded.
All patients included in this study signed informed consent. This study was approved by Bellvitge University Hospital Ethical Committee (PR143/19).
Validation cohort
Patients diagnosed with AIN and prospectively followed up at Doctor Trueta University Hospital between 2015 and 2024 were recruited to form the validation cohort of the study. In all cases, the diagnosis was confirmed by kidney biopsy. The exclusion criteria were the same as in the discovery cohort. All samples and data from patients included in the study were provided by IDIBGI Biobank (B.0000872), which is integrated into the National Biobank Network of Spain. These samples and data were handled according to standard procedures following the approval of the Ethical Committee and the Scientific Committee.
Biomarker assays
Serum and urinary samples were collected at diagnosis of the kidney condition. Samples were centrifuged at 2000 rpm for 10 min and stored at −80°C. To determine the concentration of sPD-1 and sPD-L2, a Multiplex Immunoassay (Invitrogen ProcartaPlex, Thermofisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions was performed. A Luminex MAGPIX® reader was used to retrieve the results.
For sPD-L1, we performed an ELISA using the PD-LA/B7-H1 Quantikine ELISA commercial kit (DB7H10 from R&D Systems Minneapolis, MN, USA) following the manufacturer's instructions.
Clinical data collection
At baseline, the main demographic variables of interest (age, sex) and prior medical conditions (Diabetes, Hypertension, Chronic Kidney Disease) were recorded.
For cancer patients, we recorded the type of neoplasm and the oncologic treatments received before the obtention of the samples.
For patients with AIN, data regarding to the presence of extrarenal symptoms (rash, fever, arthralgias, or other irAEs) were obtained from medical records. Kidney biopsies were stained with hematoxylin-eosin, periodic acid-Schiff, periodic acid-methenamine silver (Jones), and Masson's trichrome stains, and they were examined by an expert kidney pathologist. The presence and extent of the inflammatory infiltrate in the kidney biopsy were assessed in non-scarred areas of the cortex and categorized as follows: absent (<25%), mild (affecting 25%–50%), moderate (affecting 50%–75%), and severe (affecting >75%).
Central laboratory data (complete blood count, serum creatinine, C-reactive protein, proteinuria, leukocyturia, and hematuria) at baseline were obtained.
PD-L1 and PD-L2 determination in kidney biopsy
Kidney tissue biopsy specimens were collected ±8 days from the serum and urinary sampling and were paraffine embedded. For PD-L1 staining, 5 µm sections were dewaxed, and antigen was retrieved at pH 6.7. PD-L1 was stained using mouse anti-human PD-L1 antibody (Clone 22C3, Agilent-Dako) as specified in the manufacturer protocols. After 30 min of incubation with the primary antibody, the slides were treated with anti-mouse IgG and binding was demonstrated using 3,3′diaminobenzidine as a chromogen with avidin-biotin-peroxidase complex (EnVision Flex K8002 Visualization System). For PD-L2 staining, 5 µm sections were heated for 15 to 30 min at 60°C. Sections were dewaxed and rehydrated using an autostainer. Antigen retrieval was performed with a pH 6 citrate buffer. Sections were incubated in Block Buffer for 1 h at room temperature. Afterwards, they were incubated overnight at 4°C for 2 h at room temperature with primary antibody [PD-L2/B7-DC (R&D Systems MAB 1224 mouse IgG)] diluted 1:100 in TBS. Thereafter, sections were incubated for 2 h at 37°C with secondary antibody (anti-mouse IgG-cy3) diluted 1:200, and then counterstained with DAPI and cover-mounted with three drops of home-made fluorescent medium. The slides were stored flat at 4°C, and images were acquired after a maximum of 7 days using an Axio Imager M2 microscope (Zeiss; Jena, Germany). We took one cylinder and measured its area in the slide using Zeiss Zen version 3.3 and ImageJ version 1.53 software. The number and percentage of PD-L1 and PD-L2 positive tubules per slide was blindly counted afterwards. Finally, we adjusted the number of positive tubules for the area of the tissue slide, thus calculating a number of positive tubules/mm2 index.
Statistical methods
Data were analyzed using GraphPad Prism version 8.0 (GraphPad Software, La Jolla, 195 CA, USA) and IBM SPSS Statistics Version 26.0 (IBM Corp., Armonk, NY, USA). Categorical variables were expressed as total number (n) and percentage (%). Continuous variables were expressed as mean ± standard deviation. For comparison between two groups, when variables were quantitative and normally distributed, Student's t-test was used, and Mann–Whitney U was performed when the variables were not normally distributed. Chi square or Fisher's exact test were used for qualitative variables. Spearman's correlation was applied to compare not normally distributed variables. Receiver operating characteristic (ROC) curves were plotted to determine the accuracy of certain biomarkers. The optimal cutoff—defined as the cutoff presenting the highest sensitivity and specificity—was selected using the Youden method.
RESULTS
Discovery cohort
1. Urinary and serum sPD-1 differentiates ATN from AIN
We screened 72 patients with suspicion of AIN or ATN (Supplementary Figure S1). From those, we finally recruited 21 patients diagnosed with ATN and 37 patients with AIN. Table 1 depicts their clinical characteristics.
Table 1:
Baseline characteristics of the cohort.
| ATN | AIN | ||
|---|---|---|---|
| N = 21 | N = 37 | P value | |
| Age | 66.5 ± 11.9 | 68.19 ± 11.91 | P = .54 |
| Sex (male) | 15/21 (71, 42%) | 21/37 (56.76%) | P = .4 |
| Creatinine (µmol/l, median [IQR]) | 250 [162.5–585.5] | 209 [173–389.5] | P = .59 |
| Hypertension (% patients) | 14/21 (66.67%) | 23/37 (62.16%) | P = .78 |
| Diabetes (%patients) | 8/21 (38.1%) | 8/37 (21.62) | P = .23 |
| Pre-existing CKD (%patients) | 7/21 (33.33%) | 6/37 (16.22) | P = .19 |
| C-reactive protein (mg/l, median [IQR]) | 8.6 [5.33–21.28] | 34.85 [8.9–81.1] | P = .03a |
| Leukocyturiaa (%patients) | 14/21 (66.67%) | 32/37 (86.48%) | P = .097 |
| Microhaematuria (%patients) | 8/21 (38.1%) | 7/37 (18.92%) | P = .13 |
| Eosinophilia (%patients) | 0/21 (0%) | 8/37 (21.62%) | P = .041a |
| Leukocytosis (%patients) | 6/21 (28.57%) | 11/37 (29.73%) | P > .99 |
CKD: Chronic Kidney Disease.
aLeukocyturia: > 12 leukocytes/microliter.
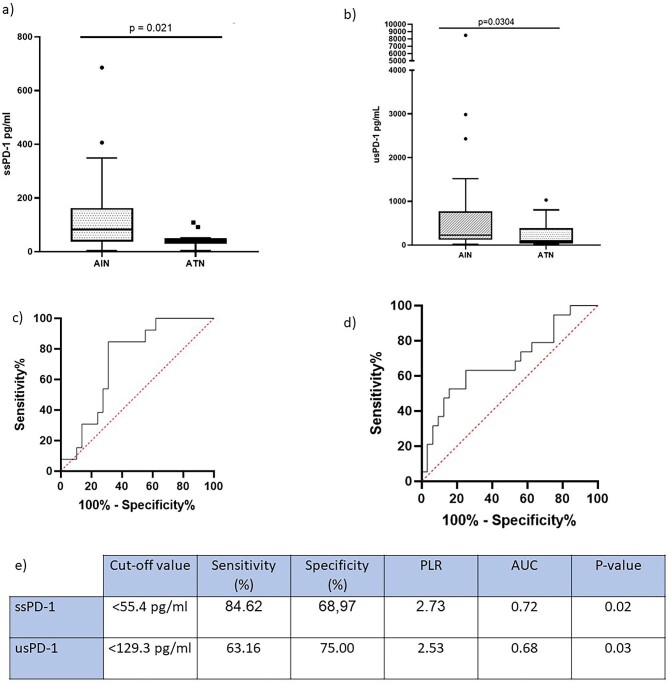
We observed higher urinary sPD-1 (usPD-1) concentration in patients with AIN compared to patients with ATN (P = .0304). There was no correlation between serum creatinine and usPD-1 in patients with AKI (r −0.091, P = .517). We then measured the level of the soluble molecules in serum. Interestingly, patients with AIN presented increased levels of serum sPD-1 (ssPD-1) compared to ATN. To identify the soluble molecule that best classified patients into AIN or ATN, we plotted ROC curves for ssPD-1 and usPD-1. Although both ssPD1 and usPD-1 appeared to be good discriminators, ssPD-1 tended to yield a higher area under the curve (AUC) (Fig. 1).
Figure 1:
(a) ssPD-1 concentration in the different study groups. (b) usPD-1 concentration in the different study groups. (c) ssPD-1 ROC curve to differentiate ATN from AIN in the whole cohort. (d) usPD-1 ROC curve to differentiate ATN from AIN in the whole cohort. (e) Statistics and optimum cutoff values of biomarkers. PLR, positive likelihood ratio.
We found no differences between ATN and AIN in serum or urinary sPD-L1 or sPD-L2 (Supplementary Table S1). We then examined the differences in usPD-1 according to the grade of the renal infiltrate present on kidney biopsies. Patients having moderate or severe renal interstitial infiltrate had higher usPD-1 concentration compared to patients with absent or mild infiltrate (238.5 [132.7–892] vs 96.28 [35.99–361] pg/ml; P = .028). We did not find differences in sPD-L1 and sPD-L2 in urine according to the grade of the renal infiltrate.
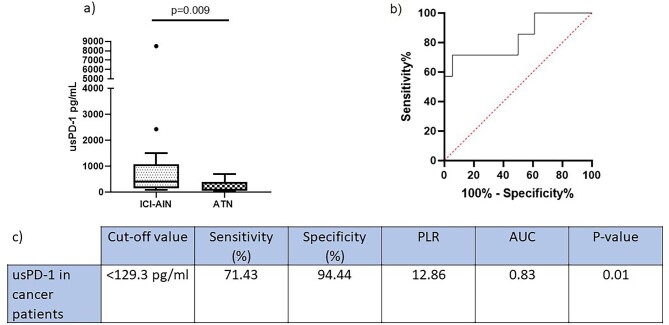
2. Urinary and serum sPD-1 differentiates ATN from AIN in cancer patients
As we were interested in ICI-AIN, we examined the ability of usPD-1 to discriminate AIN from ATN specifically in the subset of cancer patients. There were differences in usPD-1 between ICI-AIN and ATN in cancer patients. We then generated a ROC curve to assess overall diagnostic performance in this setting. Surprisingly, this ROC curve yielded a higher AUC compared to the AUC of the plotted curve for the entire study group (Fig. 2).
Figure 2:
(a) usPD-1 concentration in cancer patients. (b) ROC curve to differentiate ATN from AIN in the subset of cancer patients. (c) Statistics and optimum cutoff values of biomarkers. PLR, positive likelihood ratio.
There were no differences between groups neither in ssPD-1 nor in serum or urinary sPD-L1 and sPD-L2.
3. usPD-1 depending on the cause of AIN
To explore the differences in the concentration of the molecules between ICI-AIN and non-ICI-AIN we subdivided our patients with AIN into these two groups. The characteristics of the groups are provided in Supplementary Table S2.
Patients with ICI-AIN had significantly higher levels of usPD-1 compared to non-ICI-AIN [429.8 (169.6–976.6) pg/ml vs 160.9 (61.82–281.3) pg/ml; P = .009]. When comparing ICI-AIN with AIN related to other drugs, we also found higher levels of usPD-1 [429.8 (169.6–976.6) pg/ml vs 153.3 (41.08–281.3) pg/ml, P value .0057]. There were no differences between groups neither in ssPD-1 nor in serum or urinary sPD-L1 and sPD-L2.
Validation cohort
To validate our findings, we recruited an external cohort consisting of 27 patients diagnosed with AIN who were prospectively followed up in the Nephrology Unit of the Doctor Josep Trueta University Hospital. In all cases, the diagnosis was confirmed by kidney biopsy, evaluated by an expert pathologist. Supplementary Table S3 presents the main characteristics of patients with AIN included in the validation cohort and a comparison with those from the discovery cohort. Overall, there were no significant differences in the evaluated variables, with the exception of the proportion of patients presenting leukocyturia, which was significantly higher in the discovery cohort compared to the validation cohort. In addition, three patients diagnosed with ATN from this institution were also recruited.
In the validation cohort, we confirmed higher levels of usPD-1 in patients diagnosed with AIN compared to those with ATN [280.8 (132.4–493.5) pg/ml vs 64.765 (18.56–71.67) pg/ml, P = .045]. The usPD-1 levels in patients with AIN from the validation cohort were consistent with those in the discovery cohort [225.7 (121.5–771) pg/ml vs 280.8 (132.4–493.5) pg/ml, P = .79].
We subsequently aimed to examine the performance of usPD-1 as a biomarker of AIN in the validation cohort. Considering the optimal cutoff value established in the discovery cohort (129.3 pg/ml), usPD-1 exhibited 76.92% sensitivity and 100% specificity in discriminating AIN from ATN in the validation cohort. Moreover, focusing on cancer patients, the sensitivity increased to 80% in the validation cohort.
Regarding ssPD-1, we observed a slightly worse performance as a biomarker for diagnosing AIN in the validation cohort compared to the discovery cohort, with a sensitivity of 57.69% and a specificity of 33.33%.
PD-L1 and PD-L2 immunostaining of kidney tissue
We aimed to explore the differences in the renal tubular expression of transmembrane checkpoint proteins between patients with ICI-AIN and non-ICI-AIN. We studied PD-L1 by immunohistochemistry and PD-L2 by immunofluorescence in 17 kidney biopsies from patients with ICI-AIN and 11 patients with non-ICI-AIN. Optical microscopy characteristics of the biopsies are shown in Table 2.
Table 2:
Kidney biopsy characteristics.
| Non-ICI-AIN | ICI-AIN | |||
|---|---|---|---|---|
| N = 11 | N = 17 | P value | ||
| Infiltrate intensity | Mild | 1/11 (9.1%) | 3/17 (17.6%) | P = .63 |
| Moderate | 4/11 (36.4%) | 6/17 (35.3%) | ||
| Severe | 6/11 (54.5%) | 8/17 (47.1%) | ||
| Granulomas (% patients) | 1/11 (9.1%) | 4/17 (23.5%) | P = .62 | |
| Eosinophils (% patients) | 6/11 (54.5%) | 13/17 (76.5%) | P = .41 | |
| Plasma cells (% patients) | 5/11 (45.5%) | 5/17 (29.4%) | P = .044 | |
| ATN (% patients) | 4/11 (36.4%) | 3/17 (17.6%) | P = .38 | |
| Fibrosis (% patients) | 6/11 (54.5%) | 10/17 (58.8%) | P ≥ .99 | |
| Tubulitis (% patients) | 6/11 (54.5%) | 10/17 (58.8%) | P ≥ .99 |
ATN: Patients showing any degree of acute tubular necrosis. Non-ICI-AIN—cute interstitial nephritis not related to checkpoint inhibitor therapy.
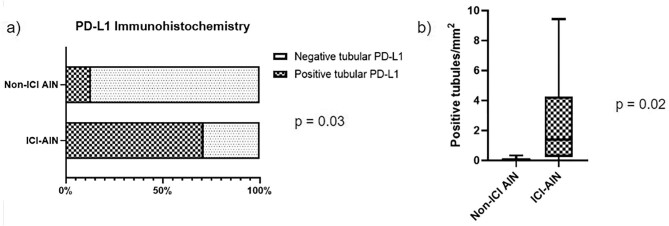
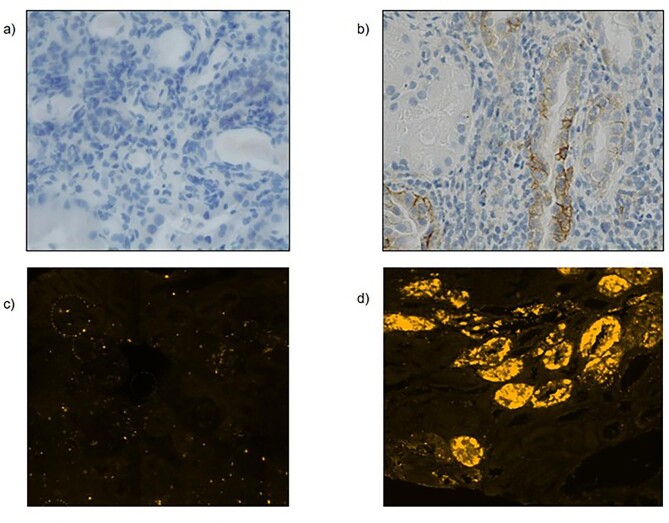
Of note, 71.14% patients with ICI-AIN showed any grade of PD-L1 positive tubular staining compared to only 12.5% of patients with non-ICI-AIN (P = .03). We found higher density of PD-L1 positive tubules in ICI-AIN compared to non-ICI-AIN (0 tubules/mm2 [0–0.165] vs 1.4 tubules/mm2 [0.23–4.26]; P = .02) (Fig. 3).
Figure 3:
PD-L1 immunostaining findings. (a) PD-L1 tubular positivity staining frequencies depending on the ethology of the AIN. (b) Mean differences of the number of positive tubules indexed by mm2.
For PD-L2 immunofluorescence, we calculated an optimal cutoff point of 2.6 tubules/mm2 to determine high or low density of positive tubules using the Youden's J statistic. Whereas up to 80% of patients with ICI-AIN presented high density of PD-L2 tubular staining, it was only observed in 30% of patients with non-ICI-AIN (P = .034).
Finally, we integrated the results from the soluble molecules studies with the results obtained in the immunostaining studies. usPD-1 in patients with >25% of positive PD-L1 tubules in the kidney biopsy was higher in comparison to those patients who presented a low percentage of PD-L1 positive tubules (<25%) [118.6 mg/ml (78.7–254.8) vs 429.8 mg/ml (346.3–976.6); P = .007]. Accordingly, we found a strong positive correlation between PD-L1 positive tubules per mm2 of kidney tissue and usPD-1 concentration (r = 0.72 and P = .009). We found no direct correlation between renal PD-L2 staining and the soluble urinary molecule levels.
Images of the immunostaining are shown in Fig. 4 and Supplementary Figure S2.
Figure 4:
(a) Image representative from a non-ICI-AIN kidney biopsy, negative for tubular PD-L1 staining (magnification ×40). (b) Image representative from an ICI-AIN kidney biopsy, positive for PD-L1 staining (magnification ×40). (c) Image representative from a non-ICI-AIN kidney biopsy, negative for tubular PD-L2 staining. (d) Image representative from a ICI-AIN kidney biopsy, positive for PD-L2 staining. Original magnification ×400 for panels (c) and (d).
DISCUSSION
The development of AKI in patients on ICI therapy is a clinically relevant event, as it is associated with increased mortality rate [25, 26]. Evolution into CKD is the factor most associated with death, probably because most drugs in oncology are not approved for patients with certain degrees of CKD.
With proper diagnosis and treatment, >80% of patients with ICI-AIN have a complete or partial recovery [27]. As a result, accurate diagnosis and treatment of AKI is of paramount importance. ICI-AIN is the most common histologic pattern seen in renal biopsies from these patients, but not the only one [13, 28]. Some of these individuals have comorbidities that contraindicate renal biopsy, which highlights the urgent need for non-invasive biomarkers to diagnose this etiology.
Our main finding showed that patients with AIN had higher serum and urine levels of sPD-1 compared to ATN, demonstrating a good performance as biomarkers for the differential diagnosis between AIN and ATN. This observation is particularly pronounced for usPD-1 in the cancer population. To validate these results, we recruited an independent cohort of patients from another hospital in our area. Consistent with our initial findings in the discovery cohort, usPD-1 in the validation cohort demonstrated even higher sensitivity for this differential diagnosis, which was maintained when the analysis focused on cancer patients with AIN. The confirmation of our initial findings in a validation cohort adds robustness and validity to our study. Conversely, the sensitivity and specificity of ssPD-1 in the validation cohort were lower compared to those in the discovery cohort. We hypothesize that increasing the number of patients included may improve these results.
In kidney tubule, PD-L1 and PD-L2 are expressed mainly in proximal segments [4]. It has been proved that their proteomic and mRNA expression in healthy kidneys is low, but this expression can be upregulated by some inflammatory cytokines [5].
Given the inducible expression of PD-L1 and PD-L2 in the renal tubular cells, we assessed and quantified this expression by immunostaining of kidney biopsies. Consistent with previous work, we found that tubular positive staining of PD-L1 was more frequent in patients with ICI-AIN than in non-ICI-AIN.
We performed PD-L2 immunofluorescence staining and found that ICI-AIN showed a greater density of PD-L2 positive tubules. This finding has not been previously described. Although PD-L2 did not better classify ICI-AIN from non-ICI-AIN than PD-L1 in our cohort of patients, its utility and pathologic implication could be further explored.
We found that there is a linear correlation between usPD-1 and tubular PD-L1 positivity. This reinforces that the determination of the soluble molecule indicates activation of the pathway at the histologic level and makes biologically plausible its use as a biomarker.
Altogether, the findings of our study suggest that the checkpoint pathway is involved in the pathogenesis of AIN, especially when it is related to treatment with ICI. Some authors have hypothesized that ICI-AIN is caused by loss of regulatory T-cell tolerance to a previously or concurrently administered drug; if ICI enhances the immune response to that drug, AIN develops [29]. We hypothesize that by blocking the pathway with checkpoint inhibitors, patients with tubulointerstitial injury due to drugs and/or an unknown personal predisposition may attempt to activate the protective pathway without success in preventing inflammation because of this blockade. This hypothesis could explain the findings of increased pathway activation in patients with ICI-AIN in our study.
We were not able to demonstrate differences in sPD-L1 or sPD-L2 levels neither between AIN and ATN nor between ICI-AIN and non-ICI-AIN, despite the observed differences in the histologic immunostaining of these molecules. The mechanisms that enhance the soluble or the transmembrane synthesis of the different forms of these proteins in different settings are unknown. In fact, in the field of oncology the attempts to correlate sPD-L1 and tumor PD-L1 positivity have been lackluster. Another possible explanation could be that there are several isoforms of PD-L1 and PD-L2, synthesized by alternative splicing, so our assay may not be able to detect the precise secreted form in this type of inflammation.
To summarize, we found that usPD-1 and ssPD-1 could be suitable biomarkers to differentiate AIN from ATN, especially in cancer patients, and that its concentration is related to the local tubulointerstitial activation. If confirmed, this could be of the utmost utility for reaching the diagnosis in cancer patients with contraindication for kidney biopsy.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge HUB-ICO-IDIBELL Biobank, funded by Instituto de Salud Carlos III (PT17/0015/0024) and by Xarxa de Bancs de Tumors de Catalunya sponsored by Pla Director d'Oncologia de Catalunya (XBTC). We wish to extend special recognition to the patients and the IDIBGI Biobank (IDIBGI Biobank, B.0000872), integrated into the National Biobank Network of Spain, for their collaboration. We thank CERCA Programme/Generalitat de Catalunya for institutional support. We thank ISCIII RETICS RedinRen RD16/0009/0003. We also thank Silvia Barceló, head of Molecular Interactions IDIBELL, for her support in Luminex procedures; Benjamin Torrejon from Serveis Científico-Tècnics (UB, Campus Bellvitge) for technical support; and Lola Mulero and Jose Antonio Llamas from Histology Platform of IDIBELL for the immunohistochemistry and immunofluorescence performance and help. This study was approved by Bellvitge University Hospital Ethical Committee.
Contributor Information
Francisco Gomez-Preciado, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain.
Laura Martinez-Valenzuela, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain.
Paula Anton-Pampols, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain.
Xavier Fulladosa, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain; Department of Clinical Sciences, University of Barcelona, Hospitalet de Llobregat, Barcelona, Spain.
Marina Gomez Tena, Department of Pathology, Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona, Spain.
Montserrat Gomà, Department of Pathology, Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona, Spain.
María Jove, Department of Medical Oncology, Catalan Institute of Oncology, and Clinical Research in Solid Tumors Group, Oncobell, l’Institut d’Investigació Biomèdica de Bellvitge, L’Hospitalet, Barcelona, Spain.
Ernest Nadal, Department of Medical Oncology, Catalan Institute of Oncology, and Clinical Research in Solid Tumors Group, Oncobell, l’Institut d’Investigació Biomèdica de Bellvitge, L’Hospitalet, Barcelona, Spain.
Ana Merino-Ribas, Department of Nephrology, Dr Josep Trueta University Hospital, Girona Biomedical Research Institute (IDIBGI), Girona, Spain.
Nadia Martin-Alemany, Department of Nephrology, Dr Josep Trueta University Hospital, Girona Biomedical Research Institute (IDIBGI), Girona, Spain.
Josep María Cruzado, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain; Department of Clinical Sciences, University of Barcelona, Hospitalet de Llobregat, Barcelona, Spain.
Joan Torras, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain; Department of Clinical Sciences, University of Barcelona, Hospitalet de Llobregat, Barcelona, Spain.
Juliana Draibe, Department of Nephrology, Bellvitge University Hospital, Bellvitge Biomedical Research Institute (IDIBELL), Hospitalet de Llobregat, Barcelona, Spain.
FUNDING
This study has been funded by Instituto de Salud Carlos III through the grant CM21/00170 and JR21/00059 [co-funded by European Social Fund (ESF investing in your future)] and through the project PI20/00812 (Co-funded by European Regional Development Fund. ERDF, a way to build Europe).
AUTHORS’ CONTRIBUTIONS
F.G., L.M., J.T., and J.D. had the original idea for the study. F.G. and L.M. extracted clinical data, performed the statistical analysis, performed the laboratory techniques, interpreted the immunostaining, reviewed the literature, and wrote the first draft of the manuscript. A.M. and N.M. extracted clinical data in the validation cohort. J.T. is the principal investigator and provided oversight of the project. M.G.T. and M.G. performed histological diagnosis. P.A., X.F., M.J., E.N., and J.M.C. provided critical input to the analysis design and discussion. All authors reviewed and revised the manuscript, and approved it as submitted. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–42. 10.1111/j.1600-065X.2010.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 2015;125:3335–7. 10.1172/JCI83871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol 2005;115:184–91. 10.1016/j.clim.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Chen Y, Li J et al. Renal tubular epithelial expression of the coinhibitory molecule B7-DC (programmed death-1 ligand). J Nephrol 2006;19:429–38. [PubMed] [Google Scholar]
- 6. Mahoney KM, Shukla SA, Patsoukis N et al. A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression. Cancer Immunol Immunother 2019;68:421–32. 10.1007/s00262-018-2282-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol Mech Dis 2021;16:223–49. 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 8. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306–16. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 10. Cortazar FB, Marrone KA, Troxell ML et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanchoo R, Karam S, Uppal NN et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017;45:160–9. 10.1159/000455014 [DOI] [PubMed] [Google Scholar]
- 12. Manohar S, Kompotiatis P, Thongprayoon C et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant 2019;34:108–17. 10.1093/ndt/gfy105 [DOI] [PubMed] [Google Scholar]
- 13. Xu L-Y, Zhao H-Y, Yu X-J et al. Clinicopathological features of kidney injury related to immune checkpoint inhibitors: a systematic review. J Clin Med 2023;12:1349–62. 10.3390/jcm12041349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez Valenzuela L, Draibe J, Bestard O et al. Urinary cytokines reflect renal inflammation in acute tubulointerstitial nephritis: a multiplex bead-based assay assessment. J Clin Med 2021;10:2986–98. 10.3390/jcm10132986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez Valenzuela L, Draibe J, Fulladosa X et al. New biomarkers in acute tubulointerstitial nephritis: a novel approach to a classic condition. Int J Mol Sci 2020;21:4690–99. 10.3390/ijms21134690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moledina DG, Obeid W, Smith RN et al. Identification and validation of urinary CXCL9 as a biomarker for diagnosis of acute interstitial nephritis. J Clin Invest 2023;133:e168950. 10.1172/JCI168950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isik B, Alexander MP, Manohar S et al. Biomarkers, clinical features, and rechallenge for Immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep 2021;6:1022–31. 10.1016/j.ekir.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farooqui N, Zaidi M, Vaughan L et al. Cytokines and immune cell phenotype in acute kidney injury associated with immune checkpoint inhibitors. Kidney Int Rep 2023;8:628–41. 10.1016/j.ekir.2022.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sise ME, Wang Q, Seethapathy H et al. Soluble and cell-based markers of immune checkpoint inhibitor-associated nephritis. J Immunother Cancer 2023;11:e006222. 10.1136/jitc-2022-006222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukasawa T, Yoshizaki A, Ebata S et al. Contribution of soluble forms of programmed death 1 and programmed death ligand 2 to disease severity and progression in systemic sclerosis. Arthritis Rheumatol 2017;69:1879–90. 10.1002/art.40164 [DOI] [PubMed] [Google Scholar]
- 21. Tong M, Fang X, Yang J et al. Abnormal membrane-bound and soluble programmed death ligand 2 (PD-L2) expression in systemic lupus erythematosus is associated with disease activity. Immunol Lett 2020;227:96–101. 10.1016/j.imlet.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 22. Hakroush S, Kopp SB, Tampe D et al. Variable expression of programmed cell death protein 1-ligand 1 in kidneys independent of immune checkpoint inhibition. Front Immunol 2021;11:624547. 10.3389/fimmu.2020.624547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cassol C, Satoskar A, Lozanski G et al. Anti-PD-1 immunotherapy may induce interstitial nephritis with increased tubular epithelial expression of PD-L1. Kidney Int Rep 2019;4:1152–60. 10.1016/j.ekir.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tampe D, Kopp SB, Baier E et al. Compartmentalization of intrarenal programmed cell death protein 1-ligand 1 and its receptor in kidney injury related to immune checkpoint inhibitor nephrotoxicity. Front Med (Lausanne) 2022;9:902256. 10.3389/fmed.2022.902256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanbay M, Copur S, Siriopol D et al. The association between acute kidney injury and outcomes in cancer patients receiving immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Clin Kidney J 2022;16:sfac194. 10.1093/ckj/sfac194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schadendorf D, Wolchok JD, Hodi FS et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with Nivolumab and Ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. JCO 2017;35:3807–14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortazar FB, Kibbelaar ZA, Glezerman IG et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. JASN 2020;31:435–46. 10.1681/ASN.2019070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palamaris K, Alexandris D, Stylianou K et al. Immune checkpoint inhibitors’ associated renal toxicity: a series of 12 cases. JCM 2022;11:4786. 10.3390/jcm11164786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016;68:287–91. 10.1053/j.ajkd.2016.02.057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.