Abstract
Background
Obesity is characterized by a chronic low-grade inflammatory condition. Two emerging inflammatory biomarkers, the systemic immune-inflammation index (SII) and the systemic inflammation response index (SIRI), have gained attention. However, the relationships between obesity and SII/SRI remain unclear.
Methods
In this study, we analyzed data from the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2018 among adults. SII-SIRI/SII/SIRI were categorized into three groups based on tertiles. The association between obesity and SII-SIRI/SII/SIRI was assessed by multivariable logistic regression models. Restricted cubic spline (RCS) plots were used to examine the nonlinear association between obesity and SII/SIRI. Finally, potential independent associations between obesity and SII/SIRI were further explored using subgroup analyses.
Results
The study included 20,011 adults, of whom 7,890 (39.32%) were obesity. In model 1, participants in the high (Q3) level of SII-SIRI had a significantly association with obesity than those in the low (Q1) level group. The high level of SII and SIRI were positively associated with obesity as compared to low levels. Model 2 revealed a positive association between obesity and high levels of SII-SIRI/SII/SIRI. Model 3 demonstrated a similar trend. RCS curves revealed a nonlinear association linking obesity to SII/SIRI. Subgroup analysis showed an interaction between SII/SIRI and age.
Conclusions
Our research suggested that obesity was positively associated with SII-SIRI/SII/SIRI in U.S. adults. SII/SIRI may represent a cost-effective and direct approach to assessing obesity.
Keywords: NHANES, Obesity, Systemic immune inflammation index, Systemic inflammation response index, Population-based study
Introduction
Obesity is a chronic, recurrent and progressive disease that has become a growing public health problem worldwide. In the period 1990–2022, there was a notable increase in global obesity rates, with 2.5 billion adults aged 18 and above being overweight in 2022, of which 890 million were classified as obesity [1]. By 2030, it is estimated that over half of the global population will be overweight or obesity [2]. The clinical complications of obesity can impact multiple organ systems, including the cardiovascular, endocrine, respiratory, digestive, musculoskeletal, neurological and reproductive systems, also increase the cancer and metabolic syndrome risk [3, 4]. Moreover, obesity significantly impacts expenditures, with over 4 million deaths attributed to a high body mass index (BMI) in 2015, and global healthcare costs for obesity and its complications expected to reach 8.4% [5, 6].
In 1993, research revealed an increased levels of TNF-a have been found in the white adipose tissue (WAT) of obese mice [7]. In 2003, Weisberg SP confirmed that TNF-a in adipose tissue was secreted by macrophages [8]. As the predominant group of white blood cells in WAT, macrophages normally distribute at low densities within the stromal blood vessels of the tissue and play an anti-inflammatory role [9, 10]. Increased lipid storage in adipocytes leads to adipocyte hypertrophy, hypoxia and eventual cell death. This dysfunction within the adipose tissue promotes the release of proinflammatory cytokines such as TNF-a, MCP-1, IL-6, and IL-8 [11]. However, the continuous release of proinflammatory factors will lead to an influx of more macrophages into the adipose tissue. This prompts the conversion of macrophages from anti-inflammatory M2 cells to pro-inflammatory M1 cells, thus perpetuating a long-term chronic inflammatory state [12, 13]. As lipid accumulation increases systemically, the rise in free fatty acids in the bloodstream also regulates the number of macrophages and contributes to the inflammatory state [12]. In obesity and metabolic syndrome, there is often an excessive release of proinflammatory cytokines into the circulation, indicating that inflammatory processes serve as clinical biomarkers for the risk of metabolic disease [14].
Many studies have shown that obesity is characterized by a persistent, subclinical inflammatory condition, and the assessment of circulating pro-inflammatory indicators is pivotal for diagnosing and predicting the outcomes of various chronic diseases [15, 16]. Recently proposed markers, namely SII and SIRI, rely on the counts of three types of blood cells for assessment. These indicators offer a more comprehensive understanding of the body’s inflammatory response compared to the reliance on one or two blood cell subtypes alone. Moreover, there are significant relationships between SII/SIRI and metabolic diseases, such as hepatic steatosis [17], diabetes [18], hyperlipidemia [19], and metabolic syndrome [20]. Additionally, a study has shown the diagnostic potential of SII in distinguishing metabolic syndrome in pediatric obesity, correlating with biomarkers of cardiovascular risk [21].
NHANES is a cross-sectional, population-based study that designed to assess Americans’ health and nutritional status. For a representative sample of the entire US population, NHANES employs a multistage, stratified random sampling method [22]. To date, few studies have analyzed the association between obesity and SII/SIRI in adults. Therefore, this study utilized the large sample data from NHANES to explore potential links between adult obesity and SII/SIRI, aiming to provide insights for the prevention and treatment of obesity.
Materials and methods
Study population
Data from the NHANES, a comprehensive database containing demographic, dietary, examination, laboratory and questionnaire data. Informed consent was provided by all participants. Detailed statistical information can be accessed at https://www.cdc.gov/nchs/nhanes/.
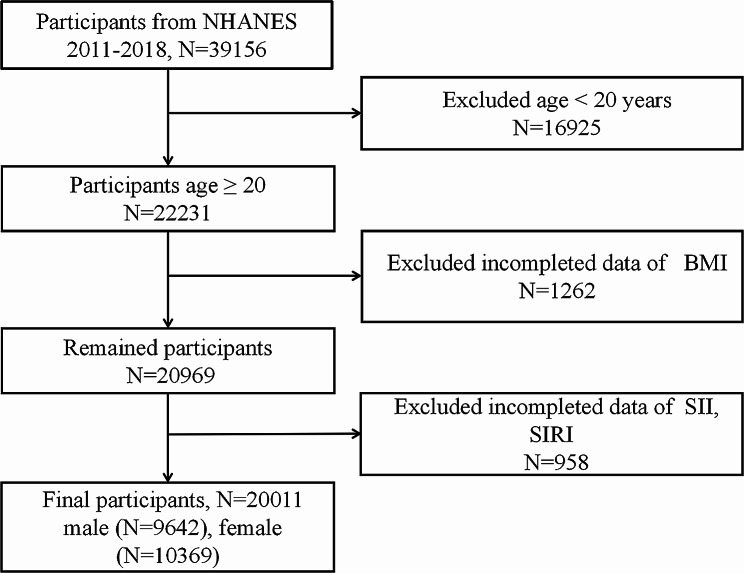
We recruited 39,156 participants from NHANES (2011–2018). The exclusion criteria comprised: (1) missing data on obesity diagnosis, (2) age less than 20 years old, and (3) absence of platelet, neutrophil, monocyte and lymphocyte data. Ultimately, a total of 20,011 participants were analyzed (Fig. 1).
Fig. 1.
Flowchart of participant selection. NHANES: National Health and Nutrition Examination Survey; BMI: body mass index; SII, systemic immune inflammation index; SIRI, systemic inflammation response index
Exposure and outcome variable
In this study, exposure variables were SII and SIRI. The blood samples were analyzed by Beckman Coulter HMX hematology analyzers. As follows are the SII and SIRI calculations [23, 24]: SII = (platelet count × neutrophil count) / lymphocyte count, SIRI = (neutrophil count × monocyte count) / lymphocyte count. Based on examination data, obesity was defined as a BMI ≥ 30.
Covariables
This research assessed potential confounders that may influence obesity. A range of covariables were examined, including age, gender, race, marital status, education level, family poverty income ratio (PIR), waist circumference (WC), smoking, alcohol intake, type 2 diabetes (T2D), hypertension, and hyperlipidemia. T2D is diagnosed with glycosylated hemoglobin (HbA1c) ≥ 6.5%, fasting blood glucose (FBG) greater than 7.0 mmol/L (126 mg/dL) [25], self-report diabetes, or the use of hypoglycemic medications. Diagnosis of hypertension: in a resting state, mean systolic pressure ≥ 140 mmHg or mean diastolic pressure ≥ 90 mmHg on three consecutive measurements, self-report hypertension, or the use of antihypertensive medications. Hyperlipidemia is diagnosed if the total cholesterol (TC) ≥ 200 mg/dL, triglyceride (TG) ≥ 150 mg/dL, low density lipoprotein (LDL) ≥ 130 mg/dL, high density lipoprotein (HDL) ≤ 40 mg/dL for male and ≤ 50 mg/dL for female [26], self-report hyperlipidemia, or the use of cholesterol-lowering medications.
Statistical analysis
Given the complex, multistage sampling design of the NHANES database, this study employed sample-specific weights to ensure representative analysis. Sampling weight is calculated as follows [27]: 8-year MEC weight = 2-year MEC weight/4. Categorical covariables were depicted through weighted percentages, while non-normally distributed continuous covariables were depicted by the mean ± standard error. Statistical differences of the baseline characteristics in different groups were analyzed using weighted chi-square tests, ANOVA, or nonparametric tests. In order to explore the association between obesity and different levels of SII/SIRI, we stratified SII-SIRI/SII/SIRI into three groups based on tertiles (low group: Q1; medium group: Q2; high group: Q3). Then we used multivariable logistic regression model to evaluate the association of obesity with SII-SIRI/SII/SIRI. Additionally, we used a 3-knot (25th, 50th, and 75th quantile) restricted cubic spline (RCS) to examine the exposure-response relationship between obesity and SII/SIRI. Finally, we performed subgroup analyses based on age, gender, T2D, hypertension and hyperlipidemia. The interaction analyse was conducted for each subgroup with SII/SIRI. R Studio (version 4.3.1) and SPSS 27.0 were used for all analyses.
Results
Participant baseline characteristics
Among the 20,011 participants included in our research, 7,890 individuals (39.32%) were classified as obesity. There was a meaningful difference between obesity and non-obesity group regarding gender, age, race, marital status, education level, family PIR, smoking, alcohol intake, hypertension, T2D, hyperlipidemia, WC, SII, SIRI, platelets, neutrophils, monocytes, and lymphocytes (P < 0.001) (Table 1).
Table 1.
Baseline characteristics of participants in the NHANES from 2011–2018 (n = 20011)
| Characteristics | Participants | P - value | ||
|---|---|---|---|---|
| Total | Non obesity | Obesity | ||
| N | 20,011 | 12,121 (60.68%) | 7890 (39.32%) | |
| Gender | < 0.001 | |||
| Male | 9642 (47.91%) | 6216 (29.74%) | 3426 (18.17%) | |
| Female | 10,369 (52.09%) | 5905 (30.94%) | 4464 (21.15%) | |
| Age | < 0.001 | |||
| 20–60 | 13,636 (73.86%) | 8209 (44.78%) | 5427 (29.08%) | |
| > 60 | 6375 (26.14%) | 3912 (15.90%) | 2463 (10.24%) | |
| Race/ethnicity | < 0.001 | |||
| Mexican American | 2727 (8.58%) | 1403 (4.39%) | 1324 (4.19%) | |
| Non-Hispanic White | 7476 (65.35%) | 4577 (40.30%) | 2899 (25.05%) | |
| Non-Hispanic Black | 4416 (10.83%) | 2256 (5.48%) | 2160 (5.35%) | |
| Other | 5392 (15.24%) | 3885 (10.51%) | 1507 (4.73%) | |
| Education level | < 0.001 | |||
| Below high school | 4409 (14.24%) | 2627 (8.50%) | 1782 (5.73%) | |
| High School or above | 15,602 (85.76%) | 9494 (52.18%) | 6108 (33.58%) | |
| Marital status | < 0.001 | |||
| Married/living with partner | 11,992 (64.06%) | 7375 (39.15%) | 4617 (24.91%) | |
| Widowed/divorced/separated/never married | 8019 (35.94%) | 4746 (21.53%) | 3273 (14.41%) | |
| Family PIR | < 0.001 | |||
| PIR ≤ 2 | 8830 (32.58%) | 5145 (18.86%) | 3685 (13.72%) | |
| PIR > 2 | 9266 (59.70%) | 5800 (37.01%) | 3466 (22.69%) | |
| Smoking | < 0.001 | |||
| ≤ 20 cigarettes/day | 3561 (17.07%) | 2294 (10.99%) | 1267 (6.08%) | |
| > 20 cigarettes/day | 233 (1.39%) | 148 (0.85%) | 85 (0.54%) | |
| Alcohol intake | < 0.001 | |||
| ≤ 5 glasses/day | 11,205 (63.32%) | 6905 (39.24%) | 4300 (24.08%) | |
| > 5 glasses/day | 1175 (6.00%) | 662 (3.27%) | 513 (2.74%) | |
| T2D | 2934 (10.82%) | 1234 (3.99%) | 1700 (6.83%) | < 0.001 |
| Hypertension | 3885 (16.71%) | 2150 (8.87%) | 1735 (7.84%) | < 0.001 |
| Hyperlipidemia | 12,302 (62.38%) | 6762 (33.91%) | 5540 (28.47%) | < 0.001 |
| BMI | 29.37 ± 0.00 | 25.00 ± 0.00 | 36.12 ± 0.00 | < 0.001 |
| WC | 100.18 ± 0.00 | 90.49 ± 0.00 | 115.30 ± 0.00 | < 0.000 |
| SII (103 cells/µL) | 528.32 ± 0.02 | 512.32 ± 0.03 | 553.01 ± 0.03 | < 0.001 |
| SIRI (103 cells/µL) | 1.30 ± 0.00 | 1.25 ± 0.00 | 1.37 ± 0.00 | < 0.001 |
| Peripheral platelet (103 cells/µL) | 238.57 ± 0.00 | 233.43 ± 0.01 | 246.50 ± 0.01 | < 0.001 |
| Neutrophil (103 cells/µL) | 4.34 ± 0.00 | 4.12 ± 0.00 | 4.67 ± 0.00 | < 0.001 |
| Monocyte (103 cells/µL) | 0.57 ± 0.00 | 0.55 ± 0.00 | 0.60 ± 0.00 | < 0.001 |
| Lymphocyte (103 cells/µL) | 2.15 ± 0.00 | 2.08 ± 0.00 | 2.27 ± 0.00 | < 0.001 |
PIR, poverty income ratio; BMI, body mass index; WC, waist circumference; T2D, type 2 diabetes; SII, systemic immune inflammation index; SIRI, systemic inflammation response index
Participants were categorized into three subgroups according to the levels of SII-SIRI/SII/SIRI. These subgroups exhibited differences in gender, age, race, marital status, education level, family PIR, smoking, alcohol intake, hypertension, T2D, and hyperlipidemia (P < 0.001). The counts of BMI, WC, SII, SIRI, platelets, monocytes, neutrophils and lymphocytes showed significant differences among the three subgroups (P < 0.001). Additionally, in the three subgroups, an increasing trend was observed in the counts of BMI, WC, SII, SIRI, platelets, monocytes, and neutrophils, while the lymphocytes showed a decline trend (Table 2).
Table 2.
Baseline characteristics of participants
| Characteristics | SII-SIRI pattern | SII (103 cell/µL) | SIRI (103 cell/µL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 |
P- value |
Q1 | Q2 | Q3 |
P- value |
Q1 | Q2 | Q3 |
P- value |
|
| N | 6669 | 6671 | 6670 | 6672 | 6671 | 6668 | 6787 | 6563 | 6661 | |||
| SII-SIRI pattern | < 0.001 | < 0.001 | ||||||||||
| Low | - | - | - | 6665 (29.85%) |
4 (0.01%) |
0 (0.00%) |
4700 (20.37%) |
1702 (8.29%) |
267 (1.20%) |
|||
| Medium | - | - | - |
6 (0.04%) |
6661 (34.85%) |
4 (0.02%) |
1782 (8.62%) |
3201 (17.62%) |
1688 (8.66%) |
|||
| High | - | - | - |
0 (0.00%) |
6 (0.02%) |
6664 (35.21%) |
304 (1.33%) |
1660 (8.26%) |
4706 (25.64%) |
|||
| Gender | < 0.001 | < 0.001 | < 0.001 | |||||||||
| Male |
3522 (15.92%) |
3186 (16.89%) |
2933 (15.10%) |
3522 (15.92%) |
3191 (16.90%) |
2929 (15.10%) |
2908 (13.06%) |
3082 (15.97%) |
3652 (18.88%) |
|||
| Female |
3147 (13.94%) |
3485 (18.02%) |
3737 (20.13%) |
3150 (13.97%) |
3480 (17.98%) |
3739 (20.13%) |
3879 (17.26%) |
3481 (18.20%) |
3009 (16.62%) |
|||
| Age | < 0.001 | < 0.001 | < 0.001 | |||||||||
| 20–60 |
4536 (22.41%) |
4640 (26.13%) |
4460 (25.32%) |
4536 (22.43%) |
4641 (26.11%) |
4459 (25.32%) |
5020 (24.30%) |
4578 (25.62%) |
4038 (23.94%) |
|||
| > 60 |
2133 (7.45%) |
2031 (8.78%) |
2210 (9.92%) |
2136 (7.46%) |
2030 (8.77%) |
2209 (9.91%) |
1767 (6.02%) |
1985 (8.56%) |
2623 (11.56%) |
|||
| Race/ethnicity | < 0.001 | < 0.001 | < 0.001 | |||||||||
| Mexican American |
815 (2.57%) |
974 (3.02%) |
938 (2.99%) |
814 (2.56%) |
977 (3.03%) |
936 (2.99%) |
876 (2.86%) |
951 (2.98%) |
900 (2.75%) |
|||
| Non-Hispanic White |
1907 (17.21%) |
2592 (23.45%) |
2976 (24.70%) |
1911 (17.24%) |
2589 (23.40%) |
2976 (24.71%) |
1679 (16.59%) |
2538 (22.83%) |
3259 (25.93%) |
|||
| Non-Hispanic Black |
2123 (5.15%) |
1234 (3.07%) |
1059 (2.61%) |
2123 (5.15%) |
1235 (3.07%) |
1058 (2.60%) |
2201 (5.46%) |
1224 (3.02%) |
991 (2.36%) |
|||
| Other |
1824 (4.93%) |
1871 (5.37%) |
1697 (4.94%) |
1824 (4.93%) |
1870 (5.37%) |
1698 (4.94%) |
2031 (5.42%) |
1850 (5.35%) |
1511 (4.47%) |
|||
| Education level | < 0.001 | < 0.001 | < 0.001 | |||||||||
| Below high school |
1529 (4.54%) |
1443 (4.61%) |
1437 (5.08%) |
1529 (4.55%) |
1443 (4.61%) |
1437 (5.08%) |
1406 (4.12%) |
1496 (4.87%) |
1507 (5.24%) |
|||
| High School or above |
5140 (25.31%) |
5228 (30.30%) |
5233 (30.15%) |
5143 (25.34%) |
5228 (30.27%) |
5231 (30.15%) |
5381 (26.20%) |
5067 (29.30%) |
5154 (30.26%) |
|||
| Marital status | < 0.001 | < 0.001 | < 0.001 | |||||||||
| Married/living with partner |
4019 (19.36%) |
4089 (22.85%) |
3883 (21.85%) |
4020 (19.37%) |
4089 (22.83%) |
3883 (21.86%) |
4113 (19.61%) |
4013 (22.47%) |
3866 (21.98%) |
|||
| Widowed/divorced/separated/never married |
2650 (10.49%) |
2582 (12.07%) |
2787 (13.38%) |
2652 (10.52%) |
2582 (12.05%) |
2785 (13.37%) |
2674 (10.71%) |
2550 (11.70%) |
2795 (13.52%) |
|||
| Family PIR | < 0.001 | < 0.001 | < 0.001 | |||||||||
| PIR ≤ 2 |
2890 (9.72%) |
2887 (11.02%) |
3053 (11.84%) |
2892 (9.75%) |
2888 (11.00%) |
3050 (11.83%) |
2855 (9.72%) |
2856 (10.74%) |
3119 (12.12%) |
|||
| PIR > 2 |
3123 (17.93%) |
3149 (21.23%) |
2994 (20.54%) |
3124 (17.94%) |
3149 (21.23%) |
2993 (20.54%) |
3286 (18.44%) |
3056 (20.78%) |
2924 (20.49%) |
|||
| Smoking | < 0.001 | < 0.001 | < 0.001 | |||||||||
| ≤ 20 cigarettes/day |
1114 (4.51%) |
1144 (5.78%) |
1303 (6.78%) |
1116 (4.53%) |
1144 (5.76%) |
1301 (6.78%) |
1010 (4.25%) |
1124 (5.35%) |
1427 (7.47%) |
|||
| > 20 cigarettes/day |
48 (0.27%) |
78 (0.49%) |
107 (0.62%) |
48 (0.27%) |
78 (0.49%) |
107 (0.62%) |
37 (0.20) |
76 (0.52%) |
120 (0.67%) |
|||
| Alcohol intake | < 0.001 | < 0.001 | < 0.001 | |||||||||
| ≤ 5 glasses/day |
3721 (18.89%) |
3809 (22.63%) |
3675 (21.80%) |
3722 (18.90%) |
3808 (22.61%) |
3675 (21.81%) |
3877 (19.60%) |
3678 (21.89%) |
3650 (21.84%) |
|||
| > 5 glasses/day |
388 (1.75%) |
391 (2.09%) |
396 (2.17%) |
389 (1.76%) |
392 (2.08%) |
394 (2.16%) |
331 (1.51%) |
396 (2.01%) |
448 (2.49%) |
|||
| T2D |
884 (2.57%) |
961 (3.72%) |
1089 (4.53%) |
< 0.001 |
884 (2.57%) |
960 (3.72%) |
1090 (4.53%) |
< 0.001 |
804 (2.34%) |
897 (3.17%) |
1233 (5.32%) |
< 0.001 |
| Hypertension |
1276 (4.58%) |
1221 (5.42%) |
1388 (8.71%) |
< 0.001 |
1277 (4.59%) |
1221 (5.41%) |
1387 (8.71%) |
< 0.001 |
1099 (3.77%) |
1260 (5.63%) |
1526 (7.31%) |
< 0.001 |
| Hyperlipidemia |
3909 (17.79%) |
4192 (21.98%) |
4201 (22.60%) |
< 0.001 |
3910 (17.80%) |
4191 (21.97%) |
4201 (22.61%) |
< 0.001 |
4124 (18.35%) |
4094 (21.65%) |
4084 (22.37%) |
< 0.001 |
| BMI | 28.37 ± 0.00 | 29.19 ± 0.00 | 30.40 ± 0.00 | < 0.001 | 28.36 ± 0.00 | 29.20 ± 0.00 | 30.39 ± 0.00 | < 0.001 | 28.19 ± 0.00 | 29.38 ± 0.00 | 30.37 ± 0.00 | < 0.001 |
| WC (cm) | 97.54 ± 0.00 | 99.92 ± 0.00 | 102.72 ± 0.00 | < 0.001 | 97.53 ± 0.00 | 99.93 ± 0.00 | 102.71 ± 0.00 | < 0.001 | 96.14 ± 0.00 | 100.22 ± 0.00 | 103.67 ± 0.00 | < 0.001 |
|
SII (103 cells/µL) |
266.04 ± 0.01 | 448.09 ± 0.01 | 830.13 ± 0.04 | < 0.001 | 266.11 ± 0.01 | 448.15 ± 0.01 | 830.11 ± 0.04 | < 0.001 | 319.80 ± 0.02 | 468.82 ± 0.02 | 763.71 ± 0.04 | < 0.001 |
|
SIRI (103 cells/µL) |
0.71 ± 0.00 | 1.11 ± 0.00 | 1.98 ± 0.00 | < 0.001 | 0.71 ± 0.00 | 1.11 ± 0.00 | 1.98 ± 0.00 | < 0.001 | 0.58 ± 0.00 | 1.04 ± 0.00 | 2.16 ± 0.00 | < 0.001 |
|
Peripheral platelet (103 cells/µL) |
204.75 ± 0.01 | 235.29 ± 0.01 | 270.48 ± 0.01 | < 0.001 | 204.76 ± 0.01 | 235.27 ± 0.01 | 270.51 ± 0.01 | < 0.001 |
230.29 ± 0.01 |
237.32 ± 0.01 |
246.84 ± 0.01 |
< 0.001 |
|
Neutrophil (103 cells/µL) |
3.06 ± 0.00 | 4.10 ± 0.00 | 5.66 ± 0.00 | < 0.001 | 3.06 ± 0.00 | 4.10 ± 0.00 | 5.65 ± 0.00 | < 0.001 | 2.98 ± 0.00 | 4.08 ± 0.00 | 5.75 ± 0.00 | < 0.001 |
|
Monocyte (103 cells/µL) |
0.53 ± 0.00 | 0.56 ± 0.00 | 0.62 ± 0.00 | < 0.001 | 0.53 ± 0.00 | 0.56 ± 0.00 | 0.62 ± 0.00 | < 0.001 | 0.44 ± 0.00 | 0.55 ± 0.00 | 0.71 ± 0.00 | < 0.001 |
|
Lymphocyte (103 cells/µL) |
2.43 ± 0.00 | 2.15 ± 0.00 | 1.92 ± 0.00 | < 0.001 | 2.44 ± 0.00 | 2.15 ± 0.00 | 1.92 ± 0.00 | < 0.001 | 2.32 ± 0.00 | 2.16 ± 0.00 | 2.00 ± 0.00 | < 0.001 |
PIR, poverty income ratio; BMI, body mass index; WC, waist circumference; T2D, type 2 diabetes; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; SII: Q1 (< 358.00), Q2 (358.00-555.79), Q3 (> 555.79). SIRI: Q1 (< 0.80), Q2 (0.80-1.32), Q3 (> 1.32)
The relationship between obesity and SII-SIRI/SII/SIRI
In this study, multivariable logistic regression was employed to analyze the relationship between obesity and SII-SIRI/SII/SIRI (Table 3). In model 1, high level of SII-SIRI was associated with the obesity [1.58 (1.42, 1.75)]. After multivariate adjustment, the statistical significance of the results persisted in the model 2 and 3 (P < 0.001). Compared with low levels of SII/SIRI, moderate and high levels of SII/SIRI showed a positive association with obesity (P < 0.001).
Table 3.
Odds ratios (95%CI) of obesity and SII-SIRI/SII/SIRI
| Categories | Model 1 OR (95% CI) | Model 2 OR(95% CI) | Model 3 OR(95% CI) |
|---|---|---|---|
| SII-SIRI pattern | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 1.22 (1.10, 1.36) | 1.22 (1.04, 1.41) | 1.13 (0.97, 1.33) |
| Q3 | 1.58 (1.42, 1.75) | 1.57 (1.34, 1.84) | 1.47 (1.25, 1.73) |
| p-value for trend | < 0.001 | < 0.001 | < 0.001 |
| SII levels | |||
| Q1 (< 358.00) | Ref. | Ref. | Ref. |
| Q2 (358.00-555.79) | 1.23 (1.10, 1.36) | 1.29 (1.10, 1.50) | 1.20 (1.02, 1.41) |
| Q3 (> 555.79) | 1.58 (1.43, 1.75) | 1.68 (1.43, 1.97) | 1.57 (1.33, 1.86) |
| p-value for trend | < 0.001 | < 0.001 | < 0.001 |
| SIRI levels | |||
| Q1 (< 0.80) | Ref. | Ref. | Ref. |
| Q2 (0.80-1.32) | 1.42 (1.29, 1.57) | 1.49 (1.29, 1.72) | 1.45 (1.24, 1.70) |
| Q3 (> 1.32) | 1.74 (1.59, 1.90) | 1.87 (1.62, 2.16) | 1.79 (1.53, 2.09) |
| p-value for trend | < 0.001 | < 0.001 | < 0.001 |
Model 1 adjusted no covariables. Model 2 adjusted adjusted age, gender, race, marital status, education level, PIR, and alcohol intake. Model 3 adjusted age, gender, race, marital status, education level, PIR, alcohol intake, hypertension, T2D, and hyperlipidemia
In the pattern of SII-SIRI, we conducted a detailed analysis of the relationship between obesity and SII/SIRI (Table 4). Specifically, when the SII ranged between 358.00 × 103 cells/µL to 555.79 × 103 cells/µL and SIRI exceeded 1.32 × 103 cells/µL, there was a significant association with obesity [1.87 (1.43, 2.44)] (P < 0.001).
Table 4.
Odds ratios (95%CI) of obesity according to subgroups of SIRI levels stratified by SII levels
| SII levels | |||
|---|---|---|---|
| Q1 (< 358.00) | Q2 (358.00-555.79) | Q3 (> 555.79) | |
| SIRI levels | |||
| Q1 (< 0.80) | Ref. | Ref. | Ref. |
| Q2 (0.80-1.32) | 1.42 (1.14, 1.77) | 1.36 (1.06, 1.76) | 0.92 (0.59, 1.45) |
| Q3 (> 1.32) | 2.20 (1.32, 3.67) | 1.87 (1.43, 2.44) | 0.95 (0.62, 1.47) |
| p-value for trend | 0.003 | < 0.001 | 0.826 |
ORs were adjusted for age, gender, race, marital status, education level, PIR, alcohol intake, hypertension, T2D, and hyperlipidemia
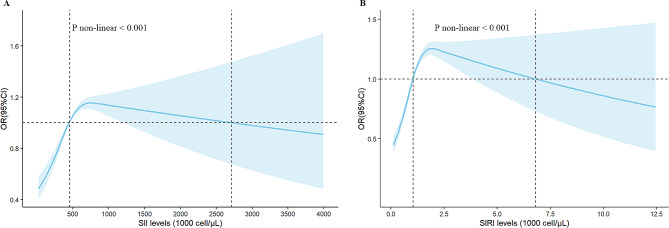
Dose-response association between obesity and SII/SIRI
In the fully adjusted model (model 3), obesity and SII/SIRI exhibit a nonlinear relationship (Fig. 2). When SII levels increased from 456.66 × 103 cells/µL to 753.02 × 103 cells/µL, the OR increased from 1.01 [1.01, 1.02] to 1.16 [1.11, 1.20], suggesting that obesity is positively associated with SII in this rage. Similarly, when SIRI levels increased from 1.04 × 103 cells/µL to 1.91 × 103 cells/µL, the OR surged from 1.01 [1.01, 1.01] to 1.25 [1.20, 1.31], indicating that obesity positively associated with SIRI in this range.
Fig. 2.
Dose-response curves of SII and SIRI levels and obesity. (A) SII-obesity. (B) SIRI-obesity. Adjusting for age, gender, race, marital status, educational level, PIR, alcohol intake, hypertension, T2D, and hyperlipidemia. SII, systemic immune inflammation index; SIRI, systemic inflammation response index
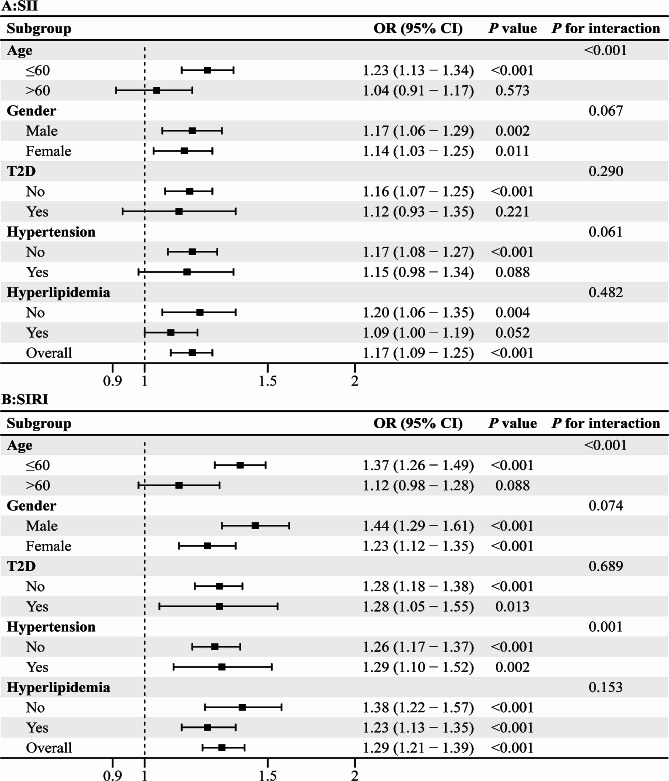
Subgroup analysis
In order to further explore the link between obesity and SII/SIRI, this study stratified participants into subgroups based on age, gender, T2D, hypertension, and hyperlipidemia (Fig. 3). Based on the SII subgroup analysis, there was a statistically significant connection with age, especially aged below 60 years. Based on the SIRI subgroup analysis, there were interactions between SIRI and age, hypertension.
Fig. 3.
Subgroup analysis for the relationship of obesity with SII (A) and SIRI (B). SII, systemic immune inflammation index; SIRI, systemic inflammation response index; T2D, type 2 diabetes
Discussion
Our research investigated the potential relationship of SII-SIRI/SII/SIRI with obesity. The key findings were as follows: (1) There was a significant difference in SII/SIRI levels between the obesity and non-obesity groups. (2) A high level of SII-SIRI/SII/SIRI was more significantly associated with obesity. (3) Obesity and SII/SIRI exhibited a nonlinear dose-response relationship. Therefore, the SII/SIRI can be the importent indicators for early assessment of obesity individuals and provide a reference for the management of obesity from the perspective of inflammation.
Studies have shown that the chronic inflammatory state is closely related to the development of chronic diseases. Our study also showed that platelets, neutrophils, monocytes, SII and SIRI levels were significantly higher in the obesity group than in the non-obesity group. In the process of weight gain, the proliferation and hypertrophy of adipocytes trigger the activation and recruitment of innate immune cells, fostering a proinflammatory adipose tissue microenvironment [28]. Within this milieu, heightened inflammatory and metabolic demands induce alterations in proinflammatory cytokines, adipokines, free fatty acids and other markers, consequently disrupting organ or tissue homeostasis [29]. The increase in peripheral leukocytes serves as a marker of inflammation, and changes in platelets, neutrophils, monocytes and lymphocytes have been associated with obesity. For instance, studies have demonstrated that neutrophils and platelets counts tend to rise with increasing BMI, whereas lymphocytes counts exhibit a negative correlation with BMI [30], consistent with our findings. Monocytes serve as a vital source of neutrophil chemokines during the progression of obesity [31]. SII/SIRI is a comprehensive prognostic parameter that combines peripheral platelets, neutrophils, monocytes and lymphocytes, providing a more comprehensive measure of the body’s inflammatory process and immune function compared to a single inflammatory index. Research indicates that elevated SII levels are linked to metabolic syndrome [20], hyperlipidemia [32], diabetic nephropathy [33], and non-alcoholic fatty liver disease (NAFLD) [34]. Therefore, we hypothesized that SII/SIRI could not only assess the inflammatory status of obesity individuals, but also play an important role in the health management of obesity and related complications.
The results of multivariable logistic regression analysis demonstrated that obesity is potentially associated with SII/SIRI, suggesting that inflammation may play an important role in the development of obesity, and conversely, the state of obesity may also lead to a persistent inflammatory state. Furthermore, the introduction of the SII-SIRI provided us with a new perspective to assess the relationship between obesity and inflammation. Compared to the single indicator (SII or SIRI), the SII-SIRI offered more comprehensive information to understand the complex relationship between obesity and inflammation. So, the SII-SIRI could be a potential biomarker for the health management of obesity.
There was a nonlinear, dose-dependent relationship between obesity and SII/SIRI, reflecting the complex relationship between obesity and inflammation. Our research showed that obesity was closely associated with SII/SIRI within a specific range. We hypothesized that the development of obesity is associated with the inflammatory state of adipose tissue and the activation of immune cells. Studies have shown that adipocytes increase with weight gain, and enlarged adipocytes or adipose tissue release pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and MCP-1 [35]. The homeostatic functions of immune cells in adipose tissue are also affected by obesity, thus inducing complications of obesity, such as diabetes and dyslipidemia [36]. Therefore, it is necessary to consider different ranges of BMI and levels of inflammation when assessing the relationship between obesity and inflammation biomarkers.
In subgroup analyses, we identified an interaction between age and SII/SIRI, especially among individuals aged 20 to 60 years. Adiposity, defined as the proportion of body weight accounted by fat, increases with age, while the relative decrease in muscle and bone mass reflects a lower basal metabolic rate [37]. Studies have shown that metabolic rate peaks in infancy, gradually declines to adulthood levels by the age of 20, remains relatively stable from 20 to 60 years, and begins to decline in the elderly [38]. Research has indicated that more than half of the females experience a rapid increase in BMI from early adulthood (18 to 23 years) to mid-adulthood (37 to 42 years) [39]. Furthermore, obesity rates in older adults have shown a steady increase due to population aging [40]. Consequently, the relationship of SII/SIRI with obesity may be influenced by age, and the potential mechanisms of age differences on obesity need to be further explored.
This study demonstrated that SII/SIRI are potential markers of obesity, reflecting the association between obesity and body’s immune status. The SII/SIRI could be a new measurement tool for assessing inflammatory status in obese individuals. It indicated the potential benefits of dynamically monitoring SII/SIRI for understanding obesity’s progression and developing health management strategies. Furthermore, further in-depth study of the relationship between SII/SIRI and obesity-related diseases, such as hyperlipidemia and diabetes mellitus, can help to reveal the pathophysiological mechanism of obesity and advance the progress towards personalized treatment approaches.
Several strengths are apparent in our research. First, the extensive sample supported the credibility and inclusivity of its conclusions. Next, our research employed the SII-SIRI pattern and SII/SIRI to comprehensive evaluate their respective impacts on the development of obesity. Furthermore, we explored the association of threshold range changes in SII/SIRI and obesity, potentially providing value for early health management of obesity. Our study also has drawbacks. Firstly, this cross-sectional study was unable to establish a causal link between obesity and SII/SIRI. Secondly, considering the authority of evaluation indicators, we used the widely used BMI to evaluate indicators [41], which may have certain limitations. Thirdly, the stability of the models is affected when we added the covariable of WC. Despite the close relationship between WC and obesity, considering the stability and accuracy of the results, we did not include WC in the analysis. Lastly, our study focused solely on the U.S. population, thus the results may not be generalizable to other populations.
Conclusion
Our study demonstrated a significant nonlinear association between obesity and SII/SIRI. Further observation found that higher levels of SII/SIRI exhibited a stronger relationship with obesity. As non-invasive biomarkers, SII/SIRI are easily obtainable and are expected to become potential indicators for obesity management. However, considering the limitations of this study, well-designed and large-scale prospective studies are needed to verify these findings.
Acknowledgements
Not applicable.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- SII
systemic immune inflammation index
- SIRI
systemic inflammation response index
- RCS
restricted cubic spline
- BMI
body mass index
- PIR
poverty income ratio
- WC
waist circumference
- T2D
type 2 diabetes
Author contributions
ZYY: Writing – original draft, Conceptualization, Methodology, Formal Analysis, Visualization. WYQ: Writing – original draft, Methodology, Formal Analysis, Software. WTT: Writing – review & editing, Software, Methodology, Formal Analysis. ZAQ: Writing – review & editing, Software, Methodology, Visualization. LYS: Writing – review & editing, Conceptualization, Supervision, Project administration, Funding acquisition.
Funding
This project was supported by High level Key Discipline of National Administration of Traditional Chinese Medicine-Traditional Chinese constitutional medicine (No. zyyzdxk-2023251), Beijing University of Traditional Chinese Medicine Campus level Project (No. 90010961020140), and Special Project of State Key Laboratory of Dampness Syndrome of Traditional Chinese Medicine jointly established by the Ministry and the Province (No. SZ2022KF05).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Consent for publication
All authors approved the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The studies involving human participants were reviewed and approved by the National Center for Health Statistics (NCHS). The patients/participants provided written informed consent to participate in this study. All authors agreed to participate in the research and writing of the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaoyao Zhou and Yaqi Wang contributed equally to this work.
References
- 1.World Health Organization, Obesity. and overweight. WHO. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed May 30, 2024.
- 2.Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7:276–88. 10.1007/s13679-018-0317-8. 10.1007/s13679-018-0317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans M, de Courcy J, de Laguiche E, Faurby M, Haase CL, Matthiessen KS, Moore A, Pearson-Stuttard J. Obesity-related complications, healthcare resource use and weight loss strategies in six European countries: the RESOURCE survey. Int J Obes (Lond). 2023;47:750–57. 10.1038/s41366-023-01325-1. 10.1038/s41366-023-01325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson SC, Burgess S. Causal role of high body mass index in multiple chronic diseases: a systematic review and meta-analysis of mendelian randomization studies. BMC Med. 2021;19:320. 10.1186/s12916-021-02188-x. 10.1186/s12916-021-02188-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. 10.1056/NEJMoa1614362. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.OECD. The heavy burden of obesity: the Economics of Prevention. OECD Publishing; 2019.
- 7.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. 10.1126/science.7678183. 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. 10.1172/jci19246. 10.1172/jci19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. 10.1038/ni.2705. 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW. Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–30. 10.1016/j.cmet.2013.11.001. 10.1016/j.cmet.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci (Lond). 2021;135:731–52. 10.1042/cs20200895. 10.1042/cs20200895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira WA, Sadie-Van Gijsen H, Ferris WF. Free fatty acid G-protein coupled receptor signaling in M1 skewed white adipose tissue macrophages. Cell Mol Life Sci. 2016;73:3665–676. 10.1007/s00018-016-2263-5. 10.1007/s00018-016-2263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, Wang Y, Lam KSL, Xu A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–49. 10.1172/jci123069. 10.1172/jci123069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. 10.1016/j.diabres.2004.11.007. 10.1016/j.diabres.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 15.Haybar H, Pezeshki SMS, Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: an early indicator of prognosis? Exp Mol Pathol. 2019;110:104267. 10.1016/j.yexmp.2019.104267. 10.1016/j.yexmp.2019.104267 [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic Immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause Mortality and Cardiovascular Mortality: a 20-Year Follow-Up Cohort Study of 42,875 US adults. J Clin Med. 2023;12. 10.3390/jcm12031128. [DOI] [PMC free article] [PubMed]
- 17.Song Y, Guo W, Li Z, Guo D, Li Z, Li Y. Systemic Immune-inflammation index is Associated with hepatic steatosis: evidence from Nhanes 2015–2018. Front Immunol. 2022;3:1058779. 10.3389/fimmu.2022.1058779. 10.3389/fimmu.2022.1058779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, Zhao Y, Shu Y, Zhang L, Cheng W, Wang L, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more Valuable for Predicting Peripheral arterial disease in type 2 Diabetic patients: a cross-sectional study. Front Endocrinol. 2023;14:1100453. 10.3389/fendo.2023.1100453. 10.3389/fendo.2023.1100453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahemuti N, Jing X, Zhang N, Liu C, Li C, Cui Z, et al. Association between systemic immunity-inflammation index and hyperlipidemia: a Population-based study from the Nhanes (2015–2020). Nutrients. 2023;15:5. 10.3390/nu15051177. 10.3390/nu15051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Shao W, Zhu Q, Zhang R, Sun T, Wang B, et al. Association between systemic Immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011–2016. J Translational Med. 2023;21:691. 10.1186/s12967-023-04491-y. 10.1186/s12967-023-04491-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicoară DM, Munteanu AI, Scutca AC, Mang N, Juganaru I, Brad GF, Mărginean O. Assessing the relationship between systemic Immune-inflammation index and metabolic syndrome in children with obesity. Int J Mol Sci. 2023;24. 10.3390/ijms24098414. [DOI] [PMC free article] [PubMed]
- 22.Hartwell ML, Khojasteh J, Wetherill MS, Croff JM, Wheeler D. Using Structural equation modeling to examine the influence of social, behavioral, and Nutritional Variables on Health outcomes based on NHANES Data: addressing Complex Design, Nonnormally distributed variables, and missing information. Curr Dev Nutr. 2019;3:nzz010. 10.1093/cdn/nzz010. 10.1093/cdn/nzz010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–222. 10.1158/1078-0432.Ccr-14-0442. 10.1158/1078-0432.Ccr-14-0442 [DOI] [PubMed] [Google Scholar]
- 24.Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, Liu L, Meng Z, Wang P, Chen Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122:2158–167. 10.1002/cncr.30057. 10.1002/cncr.30057 [DOI] [PubMed] [Google Scholar]
- 25.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–9. 10.1001/jama.2015.10029. 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 26.National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 27.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 28.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent manner. Cell. 2019;178:686–98. 10.1016/j.cell.2019.05.054. 10.1016/j.cell.2019.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–60. 10.1016/j.cell.2015.02.022. 10.1016/j.cell.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purdy JC, Shatzel JJ. The hematologic consequences of obesity. Eur J Haematol. 2021;106:306–19. 10.1111/ejh.13560. 10.1111/ejh.13560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDowell SAC, Milette S, Doré S, Yu MW, Sorin M, Wilson L, Desharnais L, Cristea A, Varol O, Atallah A, et al. Obesity alters monocyte developmental trajectories to enhance metastasis. J Exp Med. 2023;220. 10.1084/jem.20220509. [DOI] [PMC free article] [PubMed]
- 32.Mahemuti N, Jing X, Zhang N, Liu C, Li C, Cui Z, Liu Y, Chen J. Association between systemic immunity-inflammation index and hyperlipidemia: a Population-based study from the NHANES (2015–2020). Nutrients. 2023;15. 10.3390/nu15051177. [DOI] [PMC free article] [PubMed]
- 33.Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, Fu H, Shao L. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011–2018. Front Endocrinol (Lausanne). 2022;13:1071465. 10.3389/fendo.2022.1071465. 10.3389/fendo.2022.1071465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao E, Cheng Y, Yu C, Li H, Fan X. The systemic Immune-inflammation index was non-linear Associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med. 2023;55:2197652. 10.1080/07853890.2023.2197652. 10.1080/07853890.2023.2197652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315. 10.1016/j.biopha.2021.111315. 10.1016/j.biopha.2021.111315 [DOI] [PubMed] [Google Scholar]
- 36.Schleh MW, Caslin HL, Garcia JN, Mashayekhi M, Srivastava G, Bradley AB, Hasty AH. Metaflammation in obesity and its therapeutic targeting. Sci Transl Med. 2023;15:eadf9382. 10.1126/scitranslmed.adf9382. 10.1126/scitranslmed.adf9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buch A, Marcus Y, Shefer G, Zimmet P, Stern N. Approach to obesity in the older Population. J Clin Endocrinol Metab. 2021;106:2788–805. 10.1210/clinem/dgab359. 10.1210/clinem/dgab359 [DOI] [PubMed] [Google Scholar]
- 38.Pontzer H, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, Bedu-Addo K, Blaak EE, et al. Daily energy expenditure through the human life course. Science. 2021;373:808–12. 10.1126/science.abe5017. 10.1126/science.abe5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo J, Hodge A, Hendryx M, Byles JE. Age of obesity onset, cumulative obesity exposure over early adulthood and risk of type 2 diabetes. Diabetologia. 2020;63:519–27. 10.1007/s00125-019-05058-7. 10.1007/s00125-019-05058-7 [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Wong PY, Chung YL, Chow SK, Cheung WH, Law SW, Chan JCN, Wong RMY. Deciphering the obesity paradox in the elderly: a systematic review and meta-analysis of sarcopenic obesity. Obes Rev. 2023;24:e13534. 10.1111/obr.13534. 10.1111/obr.13534 [DOI] [PubMed] [Google Scholar]
- 41.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. 10.1001/jama.2016.645837. 10.1001/jama.2016.645837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.