Abstract
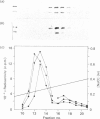
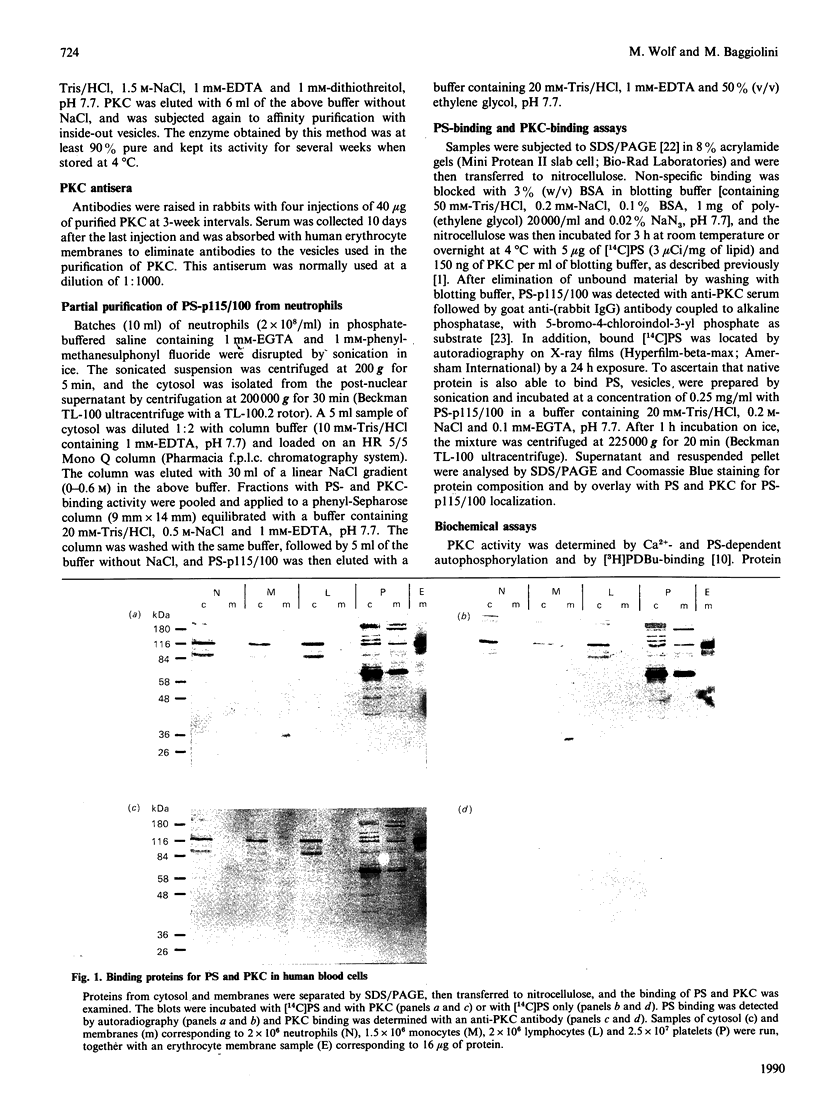
Cytosol and membrane fractions from human neutrophils, monocytes, lymphocytes and platelets were separated by SDS/PAGE, blotted on to nitrocellulose and assayed for selective binding of phosphatidylserine (PS). Two PS-binding proteins with apparent molecular masses of 115 kDa and 100 kDa were identified in the cytosol of neutrophils, monocytes and lymphocytes. Corresponding bands along with other PS-binding proteins were detected in platelets in both cytosol and membrane fractions. These proteins were also found to bind protein kinase C (PKC) provided that PS was present. The 115 kDa and 100 kDa proteins (PS-p115/110) were partially purified from neutrophils and were used for the study of PS and PKC binding. The binding of PS did not require Ca2+ or Mg2+ and was inhibited by phosphatidic acid, by 1-alkyl-2-acetylphosphocholine and, to a lesser extent, by other lipids. The binding of PKC, however, was strictly PS- and Ca2(+)-dependent and seems to occur secondarily to PS binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgener R., Wolf M., Ganz T., Baggiolini M. Purification and characterization of a major phosphatidylserine-binding phosphoprotein from human platelets. Biochem J. 1990 Aug 1;269(3):729–734. doi: 10.1042/bj2690729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., McGregor J. L., McEver R. P., Jacques Y. V., Bainton D. F., Domzig W., Baggiolini M. Absence of platelet membrane glycoproteins IIb/IIIa from monocytes. J Exp Med. 1985 May 1;161(5):972–983. doi: 10.1084/jem.161.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. Phorbol ester- and Ca2+-dependent phosphorylation of human red cell membrane skeletal proteins. J Biol Chem. 1986 Jun 15;261(17):7701–7709. [PubMed] [Google Scholar]
- Creutz C. E., Zaks W. J., Hamman H. C., Crane S., Martin W. H., Gould K. L., Oddie K. M., Parsons S. J. Identification of chromaffin granule-binding proteins. Relationship of the chromobindins to calelectrin, synhibin, and the tyrosine kinase substrates p35 and p36. J Biol Chem. 1987 Feb 5;262(4):1860–1868. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M. Methods for assessing exocytosis by neutrophil leukocytes. Methods Enzymol. 1986;132:267–277. doi: 10.1016/s0076-6879(86)32014-7. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Protein kinase C translocates from cytosol to membrane upon hormone activation: effects of thyrotropin-releasing hormone in GH3 cells. Biochem Biophys Res Commun. 1985 Apr 30;128(2):531–537. doi: 10.1016/0006-291x(85)90079-8. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Thomas T. P., Anderson W. B. Altered cytosol/membrane enzyme redistribution on interleukin-3 activation of protein kinase C. Nature. 1985 May 16;315(6016):235–237. doi: 10.1038/315235a0. [DOI] [PubMed] [Google Scholar]
- Fearon C. W., Tashjian A. H., Jr Thyrotropin-releasing hormone induces redistribution of protein kinase C in GH4C1 rat pituitary cells. J Biol Chem. 1985 Jul 15;260(14):8366–8371. [PubMed] [Google Scholar]
- Gardner K., Bennett V. A new erythrocyte membrane-associated protein with calmodulin binding activity. Identification and purification. J Biol Chem. 1986 Jan 25;261(3):1339–1348. [PubMed] [Google Scholar]
- Gardner K., Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature. 1987 Jul 23;328(6128):359–362. doi: 10.1038/328359a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. Two related but distinct forms of the Mr 36,000 tyrosine kinase substrate (calpactin) that interact with phospholipid and actin in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4258–4262. doi: 10.1073/pnas.83.12.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Hirota T., Aguilera G., Catt K. J. Hormone-induced redistribution of calcium-activated phospholipid-dependent protein kinase in pituitary gonadotrophs. J Biol Chem. 1985 Mar 25;260(6):3243–3246. [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. Ca2+-activated, phospholipid-dependent protein kinase catalyzes the phosphorylation of actin-binding proteins. Biochem Biophys Res Commun. 1984 Feb 14;118(3):736–742. doi: 10.1016/0006-291x(84)91456-6. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling E., Gardner K., Bennett V. Protein kinase C phosphorylates a recently identified membrane skeleton-associated calmodulin-binding protein in human erythrocytes. J Biol Chem. 1986 Oct 25;261(30):13875–13878. [PubMed] [Google Scholar]
- Litchfield D. W., Ball E. H. Phosphorylation of caldesmon77 by protein kinase C in vitro and in intact human platelets. J Biol Chem. 1987 Jun 15;262(17):8056–8060. [PubMed] [Google Scholar]
- Litchfield D. W., Ball E. H. Phosphorylation of the cytoskeletal protein talin by protein kinase C. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1276–1283. doi: 10.1016/0006-291x(86)90388-8. [DOI] [PubMed] [Google Scholar]
- Martin W. H., Creutz C. E. Chromobindin A. A Ca2+ and ATP regulated chromaffin granule binding protein. J Biol Chem. 1987 Feb 25;262(6):2803–2810. [PubMed] [Google Scholar]
- Meers P., Ernst J. D., Düzgünes N., Hong K. L., Fedor J., Goldstein I. M., Papahadjopoulos D. Synexin-like proteins from human polymorphonuclear leukocytes. Identification and characterization of granule-aggregating and membrane-fusing activities. J Biol Chem. 1987 Jun 5;262(16):7850–7858. [PubMed] [Google Scholar]
- Mische S. M., Mooseker M. S., Morrow J. S. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987 Dec;105(6 Pt 1):2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S., Nagata K., Kohmura Y., Ishizuka T., Nozawa Y. Redistribution of phospholipid/Ca2+-dependent protein kinase in mast cells activated by various agonists. Biochem Biophys Res Commun. 1987 Feb 13;142(3):645–653. doi: 10.1016/0006-291x(87)91463-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Modulation of Ca2+-activated, phospholipid-dependent protein kinase in platelets treated with a tumor-promoting phorbol ester. Biochem Biophys Res Commun. 1984 Jul 18;122(1):158–164. doi: 10.1016/0006-291x(84)90453-4. [DOI] [PubMed] [Google Scholar]
- Werth D. K., Pastan I. Vinculin phosphorylation in response to calcium and phorbol esters in intact cells. J Biol Chem. 1984 Apr 25;259(8):5264–5270. [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]
- Wolf M., Sahyoun N. Protein kinase C and phosphatidylserine bind to Mr 110,000/115,000 polypeptides enriched in cytoskeletal and postsynaptic density preparations. J Biol Chem. 1986 Oct 5;261(28):13327–13332. [PubMed] [Google Scholar]