Abstract
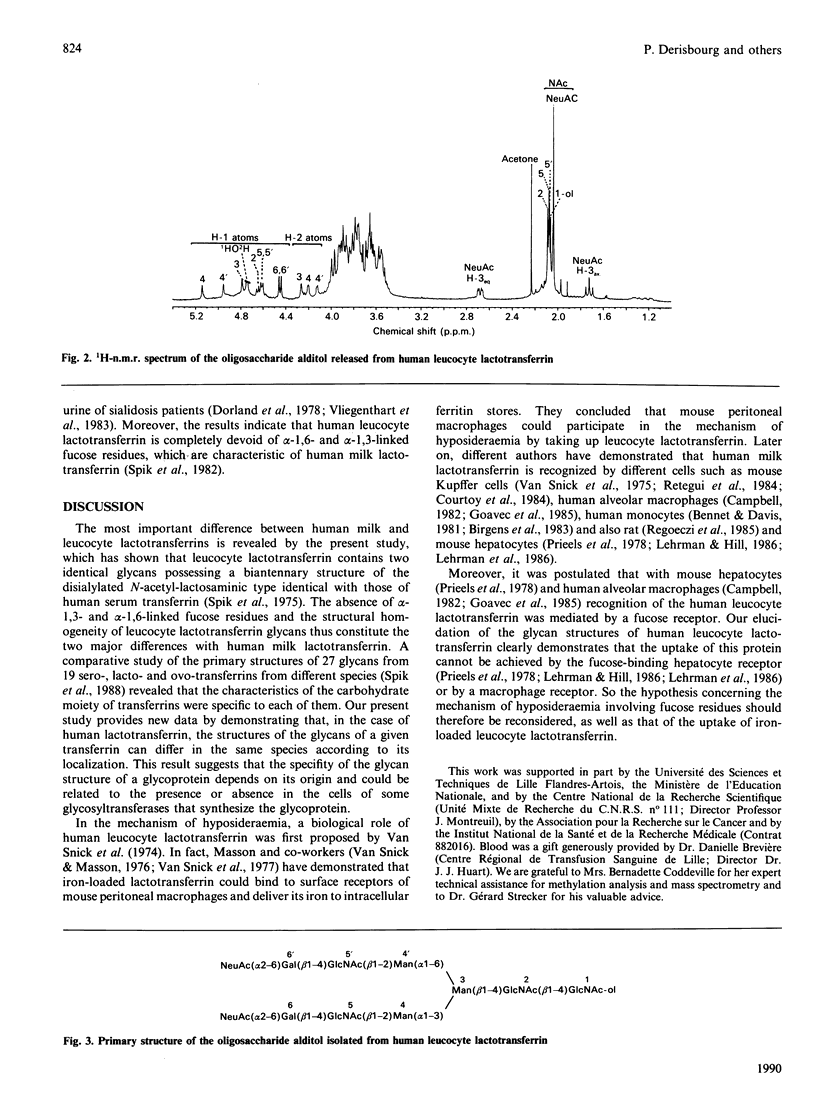
Lactotransferrin was highly purified from lysates of human neutrophilic leucocytes by immuno-affinity chromatography. A comparative analysis of the molar carbohydrate compositions of human leucocyte lactotransferrin and human milk lactotransferrin reveals that the glycans of leucocyte lactotransferrin differ essentially by the absence of fucose residues. Structural analysis combining methylation-mass spectrometry and 400 MHz 1H-n.m.r. spectrometry of oligosaccharide alditols released from human leucocyte lactotransferrin shows the presence of two disialylated and non-fucosylated biantennary glycans of the N-acetyl-lactosaminic type. These results question a previously proposed mechanism for hyposideraemia in which the leucocyte lactotransferrin was involved and in which the fucose residues played a key role.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendiak B., Cumming D. A. Purification of oligosaccharides having a free reducing-end from glycopeptide sources. Carbohydr Res. 1986 Aug 15;151:89–103. doi: 10.1016/s0008-6215(00)90332-x. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Kokocinski T. Lactoferrin content of peripheral blood cells. Br J Haematol. 1978 Aug;39(4):509–521. doi: 10.1111/j.1365-2141.1978.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Birgens H. S., Hansen N. E., Karle H., Kristensen L. O. Receptor binding of lactoferrin by human monocytes. Br J Haematol. 1983 Jul;54(3):383–391. doi: 10.1111/j.1365-2141.1983.tb02113.x. [DOI] [PubMed] [Google Scholar]
- Campbell E. J. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6941–6945. doi: 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtoy P. J., Moguilevsky N., Retegui L. A., Castracane C. E., Masson P. L. Uptake of lactoferrin by the liver. II. Endocytosis by sinusoidal cells. Lab Invest. 1984 Mar;50(3):329–334. [PubMed] [Google Scholar]
- Dorland L., Haverkamp J., Schut B. L., Vliegenthart J. F. The structure of the asialo-carbohydrate units of human serotransferrin as proven by 360 MHz proton magnetic resonance spectroscopy. FEBS Lett. 1977 May 1;77(1):15–20. doi: 10.1016/0014-5793(77)80183-x. [DOI] [PubMed] [Google Scholar]
- Dorland L., Haverkamp J., Viliegenthart J. F., Strecker G., Michalski J. C., Fournet B., Spik G., Montreuil J. 360-MHz 1H nuclear-magnetic-resonance spectroscopy of sialyl-oligosaccharides from patients with sialidosis (mucolipidosis I and II). Eur J Biochem. 1978 Jun 15;87(2):323–329. doi: 10.1111/j.1432-1033.1978.tb12381.x. [DOI] [PubMed] [Google Scholar]
- Fournet B., Strecker G., Leroy Y., Montreuil J. Gas--liquid chromatography and mass spectrometry of methylated and acetylated methyl glycosides. Application to the structural analysis of glycoprotein glycans. Anal Biochem. 1981 Sep 15;116(2):489–502. doi: 10.1016/0003-2697(81)90393-6. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Goavec M., Mazurier J., Montreuil J., Spik G. Rôle des glycannes dans la fixation de la sérotransferrine et de la lactotransferrine humaines sur les macrophages alvéolaires humains. C R Acad Sci III. 1985;301(16):689–694. [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Helyar L., Sherman A. R. Iron deficiency and interleukin 1 production by rat leukocytes. Am J Clin Nutr. 1987 Aug;46(2):346–352. doi: 10.1093/ajcn/46.2.346. [DOI] [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Pizzo S. V., Imber M. J., Hill R. L. The binding of fucose-containing glycoproteins by hepatic lectins. Re-examination of the clearance from blood and the binding to membrane receptors and pure lectins. J Biol Chem. 1986 Jun 5;261(16):7412–7418. [PubMed] [Google Scholar]
- MONTREUIL J., MULLET S. [Isolation of lactosiderophilin from human milk]. C R Hebd Seances Acad Sci. 1960 Feb 29;250:1736–1737. [PubMed] [Google Scholar]
- MONTREUIL J., TONNELAT J., MULLET S. [Preparation and properties of lactosiderophilin (lactotransferrin) of human milk]. Biochim Biophys Acta. 1960 Dec 18;45:413–421. doi: 10.1016/0006-3002(60)91478-5. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguilevsky N., Retegui L. A., Masson P. L. Comparison of human lactoferrins from milk and neutrophilic leucocytes. Relative molecular mass, isoelectric point, iron-binding properties and uptake by the liver. Biochem J. 1985 Jul 15;229(2):353–359. doi: 10.1042/bj2290353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente J. P., Cardon P., Leroy Y., Montreuil J., Fournet B., Ricart G. A convenient method for methylation of glycoprotein glycans in small amounts by using lithium methylsulfinyl carbanion. Carbohydr Res. 1985 Aug 15;141(1):41–47. doi: 10.1016/s0008-6215(00)90753-5. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Retegui L. A., Moguilevsky N., Castracane C. F., Masson P. L. Uptake of lactoferrin by the liver. I. Role of the reticuloendothelial system as indicated by blockade experiments. Lab Invest. 1984 Mar;50(3):323–328. [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Spik G., Bayard B., Fournet B., Strecker G., Bouquelet S., Montreuil J. Studies on glycoconjugates. LXIV. Complete structure of two carbohydrate units of human serotransferrin. FEBS Lett. 1975 Feb 15;50(3):296–299. doi: 10.1016/0014-5793(75)80513-8. [DOI] [PubMed] [Google Scholar]
- Spik G., Coddeville B., Montreuil J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie. 1988 Nov;70(11):1459–1469. doi: 10.1016/0300-9084(88)90283-0. [DOI] [PubMed] [Google Scholar]
- Spik G., Strecker G., Fournet B., Bouquelet S., Montreuil J., Dorland L., van Halbeek H., Vliegenthart J. F. Primary structure of the glycans from human lactotransferrin. Eur J Biochem. 1982 Jan;121(2):413–419. doi: 10.1111/j.1432-1033.1982.tb05803.x. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L. The binding of human lactoferrin to mouse peritoneal cells. J Exp Med. 1976 Dec 1;144(6):1568–1580. doi: 10.1084/jem.144.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Snick J. L., Markowetz B., Masson P. L. The ingestion and digestion of human lactoferrin by mouse peritoneal macrophages and the transfer of its iron into ferritin. J Exp Med. 1977 Sep 1;146(3):817–827. doi: 10.1084/jem.146.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]