Abstract
Background and Objective
CDKL5 deficiency disorder presents as a challenging condition with early-onset refractory seizures, severe developmental delays, and a range of other neurological symptoms. Our study aimed to explore the benefits and side effects of anti-seizure medications (ASMs) in managing seizures among individuals with CDKL5 deficiency disorder, drawing on data from the International CDKL5 Disorder Database.
Methods
Data for this retrospective cohort study were obtained from the International CDKL5 Disorder Database, which contains responses from a baseline questionnaire administered between 2012 and 2022 and a follow-up questionnaire administered between 2018 and 2019. Families of eligible individuals were asked to provide information on ASMs that were previously and currently taken, the dose prescribed, the age at starting the medications, and the age at discontinuation for past medications. The outcome variables of interest were perceived seizure-related benefits for the current and past use of ASMs and caregiver-reported side effects. Rescue medications and infrequently used ASMs were excluded from the analysis. Descriptive statistics were used to summarise the characteristics of the study population.
Results
The study included 399 children and adults with CDKL5 deficiency disorder, descriptively analysing the perceived benefits and side effects of 23 unique ASMs based on caregiver reports. The study identified levetiracetam, topiramate, sodium valproate, vigabatrin, phenobarbital and clobazam as the most used ASMs. Notably, cannabidiol showed highly beneficial outcomes with few side effects, whereas levetiracetam and phenobarbital exhibited less favourable benefit-to-side-effect ratios. Dual therapy involving sodium valproate and levetiracetam was only used a small number (n = 5) of times but appeared effective in reducing seizure activity with relatively few side effects. Compared with monotherapy, polytherapy had a relatively higher likelihood of reported side effects than benefits.
Conclusions
The study, leveraging a large sample size that exceeds that of previous research, emphasises the complex nature of seizure management in CDKL5 deficiency disorder. Our findings underscore the necessity of ongoing research to optimise treatment strategies, considering both the efficacy of seizure control and the potential for adverse effects. The study also points to the need for future investigations into the therapeutic potential of emerging treatments such as ganaxolone and the unresolved efficacy of cannabis products in seizure management.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-024-01105-z.
Key Points
| The study evaluated the effectiveness of anti-seizure medication for CDKL5 deficiency disorder using caregiver experiences. |
| Vigabatrin, sodium valproate and lamotrigine demonstrated a favourable balance between benefits and side effects, and the combination of levetiracetam and sodium valproate also proved effective, although the number of uses was small. |
| The complexity of managing seizures in CDKL5 deficiency disorder underscores the need for ongoing research into better treatments, including new medications. |
Introduction
CDKL5 deficiency disorder (CDD) is a rare X-linked epileptic encephalopathy [1]. Based on four cases identified in a West of Scotland population-based study [2], its birth prevalence has been estimated as one in 40,000–60,000 births, with a male-to-female ratio of between 1:4 [3] and 1:5 [4]. CDD is caused by a pathogenic variant in the CDKL5 gene that encodes for cyclin-dependent kinase-like 5, a serine-threonine protein distributed widely in the body, with the highest expression in the forebrain [5, 6]. CDD plays an important role in regulating and maintaining neuronal synapses and developing and forming neurons [6, 7].
Notwithstanding some clinical variability, common features of CDD include early-onset refractory seizures (less than 3 months in 90% of individuals), sometimes associated with an early ‘honeymoon period’ (often defined as a temporary cessation of seizures that inevitably returns), as well as severe global developmental delay and gross motor impairment [5, 8, 9]. Other features include cortical visual impairment, hypotonia, sleep abnormalities, gastrointestinal disturbance, and some mild dysmorphic features [1, 3, 5, 10]. The cognitive impairment in CDD is severe and is also associated with a significant reduction in everyday motor functioning, with only about a quarter of female individuals and even fewer male individuals able to walk independently [11]. Furthermore, only a quarter of female individuals can communicate using sign or spoken language, and about one third of female individuals cannot grasp small or large objects [11]. Recent research has also shown that in addition to polytherapy, impairment of everyday functional ability is one of the most significant contributors to reduced quality of life in children and adults with this condition [12].
The epilepsy syndrome in CDD is severe and often includes multiple seizure types, the most common being epileptic spasms (with or without hypsarrhythmia), tonic seizures, and generalised tonic-clonic seizures [1]. There is some relationship between CDD variant groups [13] and the frequency of seizures, with individuals with no functional CDKL5 protein being more likely to have frequent seizures [8]. Reduced seizure frequency has been shown to be associated with better functional ability, including independent walking and expressive communication [8]. Treatment of seizures in CDKL5 deficiency disorder is complex, generally requiring multiple therapeutic agents, which often have a short and temporary period of efficacy before a return to baseline of rebound seizure activity [8, 14, 15].
Data from the International CDKL5 Disorder Database (ICDD) identified a mean seizure rate of two per day among individuals with CDD, while 15% experienced more than five daily seizures [8]. In addition to this high frequency of seizure activity, epilepsy is highly refractory, with fewer than half of caregivers reporting a seizure-free period of 2 months or more [8]. A 2022 Delphi study found a lack of consensus among clinicians on first-line and second-line treatment options for CDD [16]. While there is some evidence to suggest a link between improved seizure control and functional ability, some anti-seizure medications (ASMs) can worsen seizure activity [17], and the adverse effects of polytherapy in CDD may negatively impact the quality of life to a similar extent as lack of seizure control [12].
It is, therefore, essential to establish the efficacy of different ASM treatments to minimise seizure activity as well as reduce the psychological and physiological burden associated with frequent medication adjustments and changes for individuals with CDD taking multiple therapeutic agents. A recent US-based study that explored ASM use in individuals with CDD collated data on which medications were most helpful for seizure control [14]. The overall results of this study demonstrated poor response to treatment, which ranged from a 5 to a 36% reduction in frequency for most medications at 3 months [14]. The study also identified that levetiracetam was the most used ASM. Medications most helpful for seizure control were available for 41 individuals and included cannabis-derived medications (36%), clobazam (36%), prednisone/prednisolone (33%), lamotrigine (33%), adrenocorticotropic hormone [ACTH] (29%), valproate (18%), topiramate (17%) and vigabatrin (14%). A subsequent review evaluated the limited available literature worldwide on seizure management [15]. Except for the US study, which included 168 individuals but with seizure response data only available for 86 [14], most studies were relatively small (< 50 patients) case series [17–19]. The review reinforced the need to consider the possible harmful effects of polytherapy [15], which may occur in the course of efforts to control seizures and include the quality of life as an equally important outcome [12].
Ongoing recruitment to the ICDD, which includes the ASM history of individuals with CDD, has resulted in a much larger dataset [20] than the previously published series [15]. We aim to describe both the perceived benefits of ASMs in reducing seizure activity as well as the frequency of side effects of individual and combination therapies as reported by caregivers of individuals with CDD.
Methods
Data Source and Study Population
Data required for the study, including information related to the usage of ASMs, were obtained from the ICDD, which contained responses from the baseline questionnaire administered between 2012 and 2022 and a follow-up questionnaire administered between 2018 and 2019. This retrospective cohort study included 399 individuals with a pathogenic or likely pathogenic CDKL5 variant who have used any ASM.
Exposure Variables
Families of eligible individuals were asked to provide information about the generic/trade name of the ASMs that were previously and currently taken, the dose prescribed, the age at starting the medications and the age at discontinuation for past medications. These data were primarily obtained from the baseline questionnaire and supplemented, where available, from information in the follow-up questionnaires to provide a comprehensive profile of the ASMs used. Diazepam, midazolam and lorazepam were assumed to be used as rescue medications and were excluded from the analysis. Infrequently used (i.e. being used less than ten times among the cohort) medications were also excluded. Oral corticosteroids (steroids) and ACTH, a unique treatment regime that is commonly used for short periods of time for infantile epileptic spasms, were separately reported in the analysis.
The number of unique ASMs used in the first year of life was defined as such if the starting age was available. Lifetime use was defined as the number of unique ASMs used until the time of the last responded questionnaire. The duration of use was determined by calculating the difference between the age at which the medication was commenced and the age at which it was discontinued for previously used ASMs. For ASMs currently being used, the duration was calculated up to the age at questionnaire completion. This endpoint was selected because it reflects ongoing usage and represents the most recent data on medication use. Furthermore, for currently used ASMs, to minimise bias related to records completed at a very young age, results from individuals aged older than 2 years at the time of questionnaire completion were reported. However, this restriction did not apply to steroids/ACTH, as they are typically used for a short period often under the age of 1 year.
Outcome Variables
The outcome variables for this study were perceived seizure-related benefits for the current and past use of ASMs (baseline questionnaire: provided as open text; follow-up questionnaire: provided using the following categories: improved, improved temporarily, no effect, worsened, and unknown) and caregiver-reported side effects provided as open text in both questionnaires. Data quality was optimised before analysis by consolidating and encoding open-ended responses for benefits and side effects into appropriate categories under the guidance of the principal investigators (HL and MJ). The seizure-related benefits for ASMs were classified into five categories: improved, improved temporarily, no effect, worsened and unknown. Meanwhile, the reported side effects of ASM use were coded into distinct groups: initial sleepiness, fatigue, oversedation, irritability, insomnia, reduced muscle tone, reduced appetite/weight loss, constipation, abnormal thermal regulation, Cushingoid manifestations, weight gain, behavioural change, vomiting, non-specific gastrointestinal symptoms, eye problems, skin rash, respiratory problems, oral/dental problems, renal problems, liver problems, cardiovascular problems, haematological manifestations, dependence, dizziness, abnormal hair loss, other side effects, none, and unknown. Initial sleepiness was defined as temporary drowsiness occurring after the introduction of the medication, possibly during up-titration, which subsequently resolved. This was differentiated from fatigue, which was more persistent, with over-sedation being a more severe form of fatigue. For gastrointestinal symptoms, separate coding was used for constipation, vomiting and reduced appetite/weight loss. A small number of caregivers mentioned bloating, reflux, diarrhoea and general “gastrointestinal issues”, which were coded under non-specific gastrointestinal symptoms due to their infrequency. Other side effects that did not fit into any specific category included descriptions such as “very bad”, “affected every aspect of their life”, “lost control”, “hateful” and “fever”.
The two outcomes (i.e. seizure-related benefits and side effects) were then dichotomised into three binary variables for analysis: (1) any seizure-related non-temporary improvement or not (any benefits); (2) any seizure-related worsening or not (any worsening); and (3) any coded side effects or not (any side effects). Seizure frequency at the time of questionnaire completion was included to investigate its relationship with polytherapy, and the variable was classified into four severity levels: (1) seizure free: seizure free for at least 2 months; (2) mild: weekly or less; (3) moderate, one to four per day; and (4) severe, at least five per day.
Statistical Analysis
Descriptive statistics were used to summarise the characteristics of the study population. This included sex, country of residence, age at last questionnaire completion, year at last questionnaire completion, variant group, age at seizure onset and occurrence of any honeymoon period. A single medication analysis was first conducted to examine the profile of individual ASMs that were ever used, including the age at first use, the duration of use and the likelihood of any benefits, worsening or side effects. Next, a polytherapy analysis examined the profile of ASM combinations at the time of questionnaire completion. The analysis explored any benefits, worsening or side effects based on the number of concurrent ASMs (e.g. dual therapy, triple therapy) and the most frequently used combinations (e.g. levetiracetam and topiramate). Spearman’s rank correlation test was used to examine the association between seizure frequency and the number of ASMs concurrently used. Given that some individuals took the same medication on more than one occasion during their lifetime, the number of uses, instead of the number of individuals, was reported in selected analyses: (1) benefits, worsening and side effects (rationale: these events were reported for each occasion of medication use [e.g., previously used, currently used]) and (2) the number of concurrent medications used (rationale: the medications were ascertained from the baseline and/or follow-up questionnaires). For all analyses, missing data were assumed to be missing at random. Stata version 18 (2003; StataCorp LLC, College Station, TX, USA) was used for all data analyses.
Results
The characteristics of the study cohort are presented in Table 1. In total, 399 children and adults with CDD were included in this study, with the majority (83.7%, n = 334) female and almost half (47.9%, n = 191) residing in the USA and Canada. Just over a third (35.6%, n = 142) had a follow-up questionnaire completed in addition to a baseline questionnaire. When the most recent questionnaire was completed, nearly two thirds (62.9%, n = 251) of individuals were between 1 and 9 years of age. The most common variant type was missense/in-frame variants within the catalytic domain (31.1%, n =124), followed by truncating variants between amino acids 172 and 781 (27.6%, n = 110). Over half (56.9%) of the individuals experienced their first seizure at ≤1.5 months of age, and many (58.7%, n = 234) did not experience a seizure honeymoon period.
Table 1.
Characteristics of the 399 children and adults with CDKL5 deficiency disorder
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 334 (83.7) |
| Male | 65 (16.3) |
| Country of residence | |
| USA and Canada | 191 (47.9) |
| Western Europe and UK | 93 (23.3) |
| Australia and New Zealand | 34 (8.5) |
| Othera | 81 (20.3) |
| Age at the last questionnaire, years | |
| Less than 1 | 49 (12.3) |
| 1–3 | 125 (31.3) |
| 4–9 | 126 (31.6) |
| 10–15 | 58 (14.5) |
| 16 or older | 41 (10.3) |
| Year of the last questionnaire | |
| 2012–15 | 66 (16.5) |
| 2016–19 | 235 (58.9) |
| 2020–22 | 98 (24.6) |
| Variant group | |
| No functional protein | 93 (23.3) |
| Missense/in-frame variants within the catalytic domain | 124 (31.1) |
| Truncating variants between aa172 and aa781 | 110 (27.6) |
| Truncating variants after aa781 | 39 (9.8) |
| Other variants | 22 (5.5) |
| Missing | 11 (2.8) |
| Age at seizure onset, months | |
| Less than or equal to 1.5 | 227 (56.9) |
| More than 1.5 | 165 (41.4) |
| Missing | 7 (1.8) |
| Ever honeymoon period | |
| Yes | 157 (39.4) |
| No | 234 (58.7) |
| Missing | 8 (2.0) |
n number of individuals
aArgentina, Belarus, Brazil, Bulgaria, Chile, China, Georgia, Hungary, India, Israel, Japan, Kazakhstan, Mexico, Peru, Poland, Romania, Russia, Saudi Arabia, Turkey, Ukraine, United Arab Emirates
Single Medication Analysis
In the study cohort, the ASMs used included carbamazepine, cannabidiol, clonazepam, clorazepate, clobazam, ethosuximide, felbamate, gabapentin, lacosamide, lamotrigine, levetiracetam, nitrazepam, oxcarbazepine, perampanel, phenobarbital, phenytoin, rufinamide, sodium valproate, steroids/ACTH, sulthiame, topiramate, vigabatrin and zonisamide. Levetiracetam was the most used ASM among the cohort (76.7%, n = 306), followed by topiramate (59.1%, n = 236) and sodium valproate (58.4%, n = 233) (Table 2). Vigabatrin (53.6%, n = 214), phenobarbital (49.6%, n = 198) and clobazam (45.4%, n = 181) were also frequently used. The least used medications (< 10%) were perampanel, felbamate, ethosuximide, nitrazepam, gabapentin, clorazepate and sulthiame.
Table 2.
Frequency distribution of antiseizure medications used in 399 individuals with CDKL5 deficiency disorder
| Anti-seizure medicationa | Number of individuals (%b) | Number of usesc (%d) |
|---|---|---|
| Levetiracetam | 306 (76.7) | 347 (13.1) |
| Topiramate | 236 (59.1) | 291 (11.0) |
| Sodium valproate | 233 (58.4) | 264 (10.0) |
| Vigabatrin | 214 (53.6) | 243 (9.2) |
| Phenobarbital | 198 (49.6) | 219 (8.3) |
| Clobazam | 181 (45.4) | 213 (8.1) |
| Corticosteroids/ACTH | 120 (30.1) | 135 (5.1) |
| Clonazepam | 119 (29.8) | 135 (5.1) |
| Lamotrigine | 115 (28.8) | 132 (5.0) |
| Zonisamide | 95 (23.8) | 105 (4.0) |
| Oxcarbazepine | 91 (22.8) | 96 (3.6) |
| Carbamazepine | 81 (20.3) | 84 (3.2) |
| Rufinamide | 62 (15.5) | 70 (2.7) |
| Cannabidiol | 61 (15.3) | 69 (2.6) |
| Lacosamide | 42 (10.5) | 49 (1.9) |
| Phenytoin | 42 (10.5) | 42 (1.6) |
| Perampanel | 30 (7.5) | 31 (1.2) |
| Felbamate | 28 (7.0) | 31 (1.2) |
| Ethosuximide | 21 (5.3) | 22 (0.8) |
| Nitrazepam | 20 (5.0) | 21 (0.8) |
| Gabapentin | 16 (4.0) | 17 (0.6) |
| Clorazepate | 10 (2.5) | 13 (0.5) |
| Sulthiame | 10 (2.5) | 10 (0.4) |
ACTH adrenocorticotropic hormone
aDiazepam, midazolam and lorazepam, assumed to be rescue medications, and uncommon (< 10 uses) medications, including methsuximide, piracetam, ganaxolone, stiripentol, tiagabine and pregabalin, were excluded
bPercentage of the total number of individuals (n = 399)
cNumber of uses was reported because some individuals took medication on more than one occasion during their lifetime
dPercentage of the total number of uses (n = 2639)
Approximately half (51.9%, n = 207) of the study cohort took one to three unique ASMs in their first year of life. Comparatively, a smaller fraction of individuals used four to five (19.3%, n = 77) or at least six medications (8.5%, n = 34) (Table 3). In terms of lifetime use, most individuals used two to five unique medications early in life (i.e. before the age of 1 year), and the number gradually increased to six to eight by adolescence.
Table 3.
Number of unique ASMs used, by age at the last questionnaire in 399 individuals with CDKL5 deficiency disorder
| Age at the last questionnaire, years | Overall | |||||
|---|---|---|---|---|---|---|
| < 1 | 1–3 | 4–9 | 10–15 | ≥ 16 | ||
| n | 49 | 125 | 126 | 58 | 41 | 399 |
| Time period/number of ASM | n (column %) | |||||
|---|---|---|---|---|---|---|
| First year of life | ||||||
| None | 0 | 17 (5) | 17 (4) | |||
| 1 | 10 (20) | 62 (18) | 72 (18) | |||
| 2–3 | 19 (39) | 116 (33) | 135 (34) | |||
| 4–5 | 9 (18) | 68 (19) | 77 (19) | |||
| 6 or more | 5 (10) | 29 (8) | 34 (9) | |||
| Missing | 6 (12) | 58 (17) | 64 (16) | |||
| Lifetimea | ||||||
| 1 | 2 (4) | 4 (3) | 2 (2) | 1 (2) | 2 (5) | 11 (3) |
| 2–3 | 27 (55) | 30 (24) | 19 (15) | 3 (5) | 4 (10) | 83 (21) |
| 4–5 | 12 (24) | 43 (34) | 24 (19) | 11 (19) | 9 (22) | 99 (25) |
| 6–8 | 6 (12) | 35 (28) | 50 (40) | 23 (40) | 14 (34) | 128 (32) |
| 9 or more | 2 (4) | 13 (10) | 31 (25) | 20 (34) | 12 (29) | 78 (20) |
ASM antiseizure medication, n number of individuals
aUp to the age at the last questionnaire
The median age at first use and duration of use of the selected ASMs are shown in Table 4. Phenobarbital was used the earliest at a median age of 2 months (interquartile range [IQR] 1–3). Among the most used medications (over 100 uses), levetiracetam (median age: 5 months), clonazepam (median age: 7 months), steroids/ACTH (median age: 8 months), sodium valproate (median age: 8 months), topiramate (median age: 9 months) and vigabatrin (median age: 9 months) were all used before the child’s first birthday. In contrast, lamotrigine, clobazam and rufinamide were used at 21 (IQR 12–39), 22 (IQR 8–63) and 33 (IQR 20–62) months of age, respectively. In general, most used medications with a reported end date (i.e., previously used) were used for 6 months or less, except for lamotrigine (14 months) and sodium valproate (13 months).
Table 4.
Median age at first use and duration of use of anti-seizure medications in 399 individuals with CDKL5 deficiency disorder (in ascending order of median age at first use)
| Anti-seizure medication | n | Age at first use, months | Duration of usea, months | |
|---|---|---|---|---|
| Previously usedb (n = 363) | Currently usedc (n = 283d) | |||
| Median (Q1, Q3), n | ||||
| Phenobarbital | 198 | 2 (1, 3), 159 | 5 (2, 11), 123 | 73 (48, 160), 14 |
| Carbamazepine | 81 | 5 (2, 17), 57 | 4 (1, 8), 41 | 42 (8, 82), 11 |
| Levetiracetam | 306 | 5 (2, 14), 225 | 6 (2, 14), 167 | 31 (15, 66), 43 |
| Phenytoin | 42 | 5 (3, 41), 23 | 3 (1, 5), 15 | 20 (13, 35), 4 |
| Oxcarbazepine | 91 | 6 (3, 20), 67 | 3 (1,12), 50 | 62 (35, 93), 10 |
| Clonazepam | 119 | 7 (4, 22), 87 | 6 (2, 19), 57 | 24 (11, 32), 21 |
| Corticosteroids/ACTH | 120 | 8 (5, 13), 98 | 2 (1, 4), 88 | 8 (3, 19), 4 |
| Sodium valproate | 233 | 8 (3, 21), 182 | 13 (6, 34), 114 | 35 (20, 76), 68 |
| Clorazepate | 10 | 9 (2, 157), 3 | 69 (45, 94), 2 | - |
| Sulthiame | 10 | 9 (2, 21), 7 | 3 (1, 12), 6 | - |
| Topiramate | 236 | 9 (4, 22), 169 | 7 (2, 15), 115 | 31 (22, 62), 37 |
| Vigabatrin | 214 | 9 (5, 18), 165 | 8 (3, 19), 90 | 24 (7, 49), 55 |
| Nitrazepam | 20 | 17 (7, 39), 14 | 12 (6, 23), 8 | 47 (25, 70), 6 |
| Zonisamide | 95 | 20 (9, 41), 65 | 3 (1, 15), 40 | 25 (11, 49), 21 |
| Lamotrigine | 115 | 21 (12, 39), 79 | 14 (6, 41), 40 | 31 (12, 78), 34 |
| Clobazam | 181 | 22 (8, 63), 133 | 6 (2, 21), 82 | 26 (12, 60), 50 |
| Ethosuximide | 21 | 24 (16, 78), 11 | 3 (2, 24), 9 | 22 (1, 41), 3 |
| Cannabidiol | 61 | 26 (10, 54), 46 | 4 (1, 14), 18 | 19 (12, 38), 20 |
| Rufinamide | 62 | 33 (20, 62), 45 | 6 (2, 21), 34 | 16 (2, 48), 11 |
| Lacosamide | 42 | 35 (13, 63), 29 | 6 (1, 20), 20 | 29 (10, 47), 9 |
| Felbamate | 28 | 36 (18, 53), 21 | 4 (4, 8), 13 | 41 (13, 71), 8 |
| Gabapentin | 16 | 49 (30, 121), 8 | 4 (2, 36), 7 | 60 (25, 94), 2 |
| Perampanel | 30 | 67 (28, 110), 19 | 3 (1, 9), 5 | 6 (3, 9), 13 |
ACTH adrenocorticotropic hormone, n number of individuals, Q1 first quartile, Q3 third quartile
aAs medications can be used more than once before the last questionnaire, the duration for previously used ASMs is an aggregate number
bPreviously used relative to the time of the last questionnaire, which can be a baseline or a follow-up questionnaire
cCurrently used at the time of the last questionnaire, which can be a baseline or a follow-up questionnaire
dOnly included individuals aged older than 2 years among the 377 individuals in the currently used group
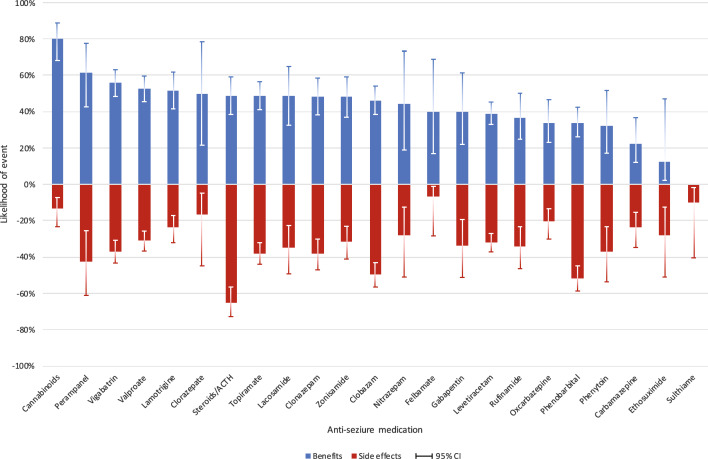
The likelihood of perceived benefits and side effects associated with taking the selected ASMs was investigated, as shown in Fig. 1 and Table 1 of the Electronic Supplementary Material (ESM). Cannabidiol was reported to be highly beneficial (80% of responses reported improved outcomes, n = 45 out of 56 uses) with few worsening (4%, n = 2 out of 56 uses) and side effects (13%, n = 9 out of 68 uses) [the related encoded family provided comments for cannabidiol and perampanel are shown in Table 2 of the ESM]. Similarly, vigabatrin, sodium valproate and lamotrigine were beneficial in more than half of the uses, and fewer than a third reported side effects. Around a third of the responses indicated benefits (37%) and side effects (34%) for rufinamide. Any worsening of seizures was only reported for small numbers but included clorazepate (1/8, 13%), oxcarbazepine (5/59, 8%), lamotrigine (6/89, 7%) and topiramate (9/158, 6%). Despite their widespread use, levetiracetam and phenobarbital had lower likelihoods of benefit (39% and 34%, respectively). The latter medication, phenobarbital, had a much higher probability of side effects (52%). The distribution of individual side effects over 1031 episodes of medication use is shown in Table 5, and the most reported side effect “fatigue” (described by families using words such as “sleepy”, “drowsy”, “lethargic”, “tired” or “dopey”) was present in almost one third (31.2%) of episodes followed by irritability (7.6%), behavioural change (6.7%) and reduced tone (5.3%). Insomnia was reported as a side effect in 3%.
Fig. 1.
Likelihood of any seizure-related benefits and any side effects associated with the use of antiseizure medications in 399 individuals with CDKL5 deficiency disorder. CI confidence interval (constructed using the Wilson method [36])
Table 5.
Frequency distribution of side effects associated with the use of anti-seizure medications in 399 individuals with CDKL5 deficiency disorder
| Side effect | n (%) |
|---|---|
| Fatigue | 322 (31.2) |
| Irritability | 78 (7.6) |
| Behavioural change | 69 (6.7) |
| Reduced tone | 55 (5.3) |
| Reduced appetite and/or weight loss | 54 (5.2) |
| Oversedation | 48 (4.7) |
| Non-specific GI symptomsa | 38 (3.7) |
| Other side effectsb | 38 (3.7) |
| Initial sleepiness | 36 (3.5) |
| Cushingoid | 33 (3.2) |
| Insomnia | 31 (3.0) |
| Oral problems | 29 (2.8) |
| Eye problems | 26 (2.5) |
| Renal problems | 24 (2.3) |
| Vomiting | 22 (2.1) |
| Skin rash | 21 (2.0) |
| Dizziness | 16 (1.6) |
| Abnormal thermal regulation | 15 (1.5) |
| Weight gain | 15 (1.5) |
| Constipation | 14 (1.4) |
| Abnormal hair growth or loss | 14 (1.4) |
| Abnormal bloods | 12 (1.2) |
| Respiratory problems | 8 (0.8) |
| Liver problems | 6 (0.6) |
| CVS problems | 4 (0.4) |
| Dependence | 3 (0.3) |
| Total | 1031 (100) |
CVS cardiovascular system, GI gastrointestinal, n number of uses
aNon-specific GI symptoms encompass infrequent issues such as bloating, reflux, diarrhoea, and general “gastrointestinal problems”
bOther side effects encompass symptoms that did not fit into any specific category and were described with terms such as “very bad”, “affected every aspect of their life”, “lost control”, “hateful” and “fever”
Fatigue was the top reported side effect for individual medications for 16 of the 23 medications (Table 6). Of those 16 ASMs, phenobarbital, clonazepam, cannabidiol and perampanel were those where fatigue was reported in a relatively high percentage (≥ 50%) of uses. For the remaining seven ASMs, the top side effects were reduced appetite/weight loss (topiramate), behavioural change (lamotrigine), irritability (felbamate), insomnia (clorazepate) and respiratory problems (gabapentin and sulthiame). Reduced appetite/weight loss was also reported for rufinamide, phenytoin, clonazepam and zonisamide and behavioural change for levetiracetam, lacosamide and perampanel. Irritability was also specifically reported for levetiracetam, zonisamide, lacosamide, ethosuximide and oxcarbazepine, while insomnia was also reported for lamotrigine.
Table 6.
Top three reported side effects associated with the use of antiseizure medications in 399 individuals with CDKL5 deficiency disorder
| Anti-seizure medication | n | First condition (%) | Second condition (%) | Third condition (%) |
|---|---|---|---|---|
| Clobazam | 119 | Fatigue (42) | Reduced tone (11) | Oral problems (11) |
| Levetiracetam | 116 | Fatigue (35) | Irritability (18) | Behavioural change (16) |
| Phenobarbital | 113 | Fatigue (57) | Oversedation (12) | Reduced tone (6) |
| Corticosteroids/ACTH | 111 | Cushingoid (30) | Irritability (11) | Weight gain (11) |
| Topiramate | 111 | Reduced appetite and/or weight loss (23) | Fatigue (21) | Renal problems (9) |
| Sodium valproate | 96 | Fatigue (27) | Abnormal hair growth or loss (10) | Abnormal bloods (9) |
| Vigabatrin | 91 | Fatigue (25) | Eye problems (20) | Reduced tone (12) |
| Clonazepam | 54 | Fatigue (52) | Reduced tone (15) | Reduced appetite and/or weight loss (9) |
| Zonisamide | 39 | Fatigue (28) | Irritability (15) | Reduced appetite and/or weight loss (13) |
| Lamotrigine | 33 | Behavioural change (18) | Fatigue (15) | Insomnia (15) |
| Rufinamide | 25 | Fatigue (20) | Reduced appetite and/or weight loss (16) | Vomiting (12) |
| Oxcarbazepine | 21 | Fatigue (33) | Renal problems (14) | Irritability (10) |
| Lacosamide | 20 | Fatigue (35) | Irritability (20) | Behavioural change (10) |
| Carbamazepine | 18 | Fatigue (39) | Oversedation (11) | Vomiting (11) |
| Phenytoin | 14 | Fatigue (21) | Reduced appetite and/or weight loss (14) | Eye problems (14) |
| Felbamate | 13 | Irritability (23) | Non-specific GI symptomsa (23) | Fatigue (15) |
| Perampanel | 12 | Fatigue (50) | Initial sleepiness (17) | Behavioural change (17) |
| Cannabidiol | 10 | Fatigue (50) | Vomiting (20) | Initial sleepiness (10) |
| Ethosuximide | 6 | Fatigue (33) | Irritability (17) | Constipation (17) |
| Nitrazepam | 5 | Fatigue (40) | Initial sleepiness (20) | Oversedation (20) |
| Clorazepate | 2 | Insomnia (50) | Other side effectsb (50) | – |
| Gabapentin | 1 | Respiratory problems (100) | – | – |
| Sulthiame | 1 | Respiratory problems (100) | – | – |
ACTH adrenocorticotropic hormone, GI gastrointestinal, n number of uses
aNon-specific GI symptoms encompass infrequent issues such as bloating, reflux, diarrhoea and general “gastrointestinal problems”
bOther side effects encompass symptoms that did not fit into any specific category and were described with terms such as “very bad”, “affected every aspect of their life”, “lost control”, “hateful” and “fever”
Steroids/ACTH were prescribed at a median age of 8 months. The median duration of previous use was 2 months (IQR 1–4 months) and was shorter than traditional ASMs. Benefits were reported at 49% and any worsening at 5%. Side effects were reported for 65%, higher than in any other ASM. Cushingoid symptoms were the top reported side effects, followed by irritability and weight gain.
Polytherapy Analysis
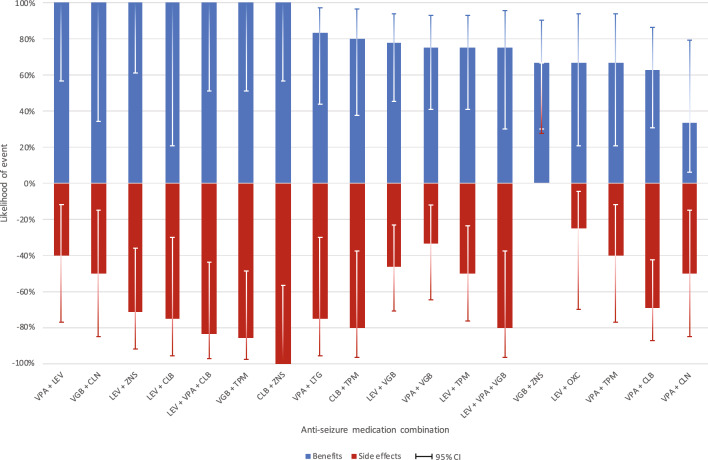
In terms of concurrent medication usage, sodium valproate was the most used ASM (13.7% of all polytherapy uses, n = 152), followed by levetiracetam (13.0%, n = 144) and clobazam (11.7%, n = 130) [Table 3 of the ESM]. Most uses were related to dual therapy (40%) or triple therapy (31%), and this did not seem to vary by age (Table 7). Compared with monotherapy, polytherapy had a higher likelihood of reported side effects (e.g. 61% for triple therapy versus 29% for monotherapy). Benefits were also higher with more medications, but the differences were relatively small (e.g. 88% for triple therapy as opposed to 77% for monotherapy) (Table 8). Increased seizure frequency was associated with more ASM concurrently used (Spearman’s rank correlation test, P < 0.001). An overview of the likelihood of any side effects and benefits of selected dual and triple therapies can be found in Fig. 2 and Table 4 of the ESM. The ASM combination with the largest variance between benefits and side effects was sodium valproate and levetiracetam (100% [5/5] vs 40% [2/5]).
Table 7.
Frequency distribution of concurrent ASMs currently useda, by age at questionnaire, in 379 individuals with CDKL5 deficiency disorder
| Age at questionnaire, years | Overall | |||||
|---|---|---|---|---|---|---|
| < 1 | 1–3 | 4–9 | 10–15 | ≥ 16 | ||
| n | 50 | 146 | 168 | 84 | 61 | 509 |
| Number of concurrent ASMs | n (column %) | |||||
|---|---|---|---|---|---|---|
| 1, monotherapy | 7 (14) | 27 (18) | 34 (20) | 13 (15) | 14 (23) | 95 (19) |
| 2 | 20 (40) | 59 (40) | 70 (42) | 36 (43) | 18 (30) | 203 (40) |
| 3 | 20 (40) | 41 (28) | 48 (29) | 26 (31) | 21 (34) | 156 (31) |
| 4 | 2 (4) | 14 (10) | 13 (8) | 7 (8) | 7 (11) | 43 (8) |
| 5 | 0 | 2 (1) | 3 (2) | 2 (2) | 1 (2) | 8 (2) |
| 6 | 0 | 3 (2) | 0 | 0 | 0 | 3 (1) |
| 7 | 1 (2) | 0 | 0 | 0 | 0 | 1 (0.3) |
ASM anti-seizure medication, n number of uses
aCurrently used at the time of the baseline and/or the follow-up questionnaire
Table 8.
Likelihood of any seizure-related benefits and worsening and any side effects associated with concurrent ASMs currently useda in 379 individuals with CDKL5 deficiency disorder
| Number of concurrent ASMs | n | Any benefits | Any worsening | Any side effects | n | Seizure frequencyb | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seizure free | Mild | Moderate | Severe | P valuec | ||||||
| %, Number of events/n | n (%) | |||||||||
| 1, monotherapy | 95 | 77, 55/71 | 0, 0/71 | 29, 26/89 | 81 | 8 (10) | 34 (42) | 27 (33) | 12 (15) | < 0.001 |
| 2 | 203 | 85, 124/146 | 2, 3/146 | 54, 100/184 | 190 | 11 (6) | 56 (29) | 79 (42) | 44 (23) | |
| 3 | 156 | 88, 82/93 | 1, 1/93 | 61, 90/148 | 149 | 6 (4) | 37 (25) | 61 (41) | 45 (30) | |
| 4 or more | 55 | 81, 22/27 | 4, 1/27 | 57, 26/46 | 52 | 1 (2) | 5 (10) | 22 (42) | 24 (46) | |
| Overall | 509 | 83, 283/337 | 1, 5/337 | 52, 242/467 | 472 | 26 (6) | 132 (28) | 189 (40) | 125 (26) | |
ASM anti-seizure medication, n number of uses
aCurrently used at the time of the baseline and/or the follow-up questionnaire
bSeizure frequency: seizure free, seizure free for at least 2 months; mild, weekly or less; moderate, one to four per day; severe, at least five per day
cP value was determined using the Spearman’s rank correlation test
Fig. 2.
Likelihood of any seizure-related benefits and any side effects associated with selected* concurrent antiseizure medications currently used^ in 379 individuals with CDKL5 deficiency disorder. *Limited to dual or triple therapy used by at least four uses; ^currently used at the time of the baseline and/or the follow-up questionnaire. CI confidence interval (constructed using the Wilson method [36]), CLB clobazam, CLN clonazepam, LEV levetiracetam, LTG lamotrigine, OXC oxcarbazepine, TPM topiramate, VGB vigabatrin, VPA sodium valproate, ZNS zonisamide
Discussion
This international study investigated caregiver perspectives on ASMs used by 399 children and adults with CDD registered in the ICDD by 2022. The most common of the 23 unique ASMs used were levetiracetam, topiramate, sodium valproate, vigabatrin, phenobarbital and clobazam. The median age of first use for medications varied, with phenobarbital, levetiracetam and steroids/ACTH being used earliest, and lamotrigine and clobazam later. Cannabidiol was reported as highly beneficial with few side effects, while vigabatrin, sodium valproate, and lamotrigine were also beneficial but had some side effects. Polytherapy, such as sodium valproate and levetiracetam, showed potential for reduced seizure activity with fewer side effects, though other combinations had more frequent side effects. Overall, polytherapy was associated with a greater likelihood of reported side effects and only a slight improvement in benefits compared with monotherapy.
In the face of the refractory epilepsy occurring in CDD, it is difficult to provide clinicians with clear recommendations as to what best to prescribe and, as we acknowledge, prescribing may have to be tailored to the types of epilepsy, information not available to us in our study. The findings we report represent a caregiver perspective, but when we compare side-effect profiles with those identified in the few meta-analyses in the literature [21–23] our findings are similar, providing some validity to our results. In an attempt to present any guidance on the efficacy of different ASMs, we considered the frequency of use, as well as the likelihood of benefits, side effects and duration of use. Of those ASMs commonly used (> 100), valproate and lamotrigine would appear to be those with the greatest benefits and fewest side effects with a current duration of use (> 30 months), suggesting good tolerability and acceptance. Topiramate and levetiracetam were slightly less beneficial although they had similar durations of use. Of the third-generation ASMs, as would be expected, both perampanel and lacosamide had been used by fewer individuals, but perampanel was rated as particularly beneficial although side effects were more common. More time and data are probably required for the assessment of this group of newer ASMs. Caregivers also rated cannabidiol very highly, but it was not commonly used (n = 61), and there is likely significant variation in the types of products available, which range from pharmaceutically produced options such as Epidiolex® in the USA to those purchased from licensed dispensaries.
This study has many strengths, including the large sample size provided by the ICDD, which has been collecting data for over 10 years. Importantly, all individuals have a pathogenic or likely pathogenic variant, and the benefits and side effects are provided directly by the caregivers. Another strength is that our results demonstrate that CDD remains one of the most medically refractory epilepsies worldwide, regardless of whether certain ASMs are available in different countries and throughout different periods. A unique aspect of our study was reporting the prevalence of side effects, as presented graphically in our figures, as an important contributing factor to the acceptability of an ASM. We also reported on the duration of ASM use. Perhaps surprisingly, phenobarbital had been used the longest despite its high side-effect profile, possibly because it was the first to be used and likely to be continued because of concerns about withdrawal symptoms, including seizure recurrence. The side-effect profiles we identified were in keeping with a 2014 review suggesting that phenobarbital, valproate and levetiracetam were particularly likely to aggravate daytime sleepiness [21]. In contrast, a more recent review focussing on developmental epileptic encephalopathies [23] reported that the effects of valproate on sleep were mixed, and evidence was limited by studies with small sample sizes (n = 20–40) in comparison to ours (n = 218 who provided side-effect data). Nevertheless, the findings of their review with respect to the sedating effects of ASMs (what we described as fatigue) were very consistent [23]. Relationships with irritability were a little less consistent, although for both of the studies, irritability was associated with levetiracetam and zonisamide and insomnia with lamotrigine. It is clear from this recent review [23] and the one undertaken in 2014 [21] that the relationship between epilepsy, the side effects of ASMs, night-time sleep disturbances and daytime sleepiness is highly complex and challenging to unravel. Moreover, we have previously shown that daytime sleepiness is associated with a poorer quality of life [24].
Regarding weaknesses, our study lacked information about which specific seizure type was targeted when an ASM was commenced. We do know that the most typical types of seizures at first initiation of treatment were tonic seizures, followed by epileptic spasms, generalised tonic-clonic seizures and finally focal seizures, and, overall, the most common seizure types were epileptic spasms, followed by tonic, myoclonic and then generalised tonic-clonic seizures [1]. Another limitation is that we did not have information about the exact seizure frequency before introducing a new medication and the subsequent change in frequency other than the caregiver’s assessment of the new medication. Notably, some but not all caregivers may have referred to seizure diaries to provide their information. While also a benefit, the multinational nature of this study has the shortcoming of the data representing the availability of ASMs in different regions of the world and at different periods, as well as the influence of the prescription preferences of different treating physicians given the absence of treatment guidelines for the management of seizures in CDD. For example, perampanel was only US Food and Drug Administration approved for the management of seizures in 2014, which would account for the relatively low number of uses. Similarly, cannabidiol was only approved in 2018, while felbamate has not yet been approved in Canada. Limited use of ganaxolone in the USA during the study period prevented us from including this potentially effective ASM in our analysis [25]. A recently completed clinical trial has demonstrated that the 28-day major motor seizure frequency was reduced in those taking ganaxolone compared with placebo [25]. The latest results from an open-label extension study of 88 patients showed reductions at 22–24 months from baseline in major motor seizure frequency of ≥ 25%, ≥ 50%, ≥ 75% and 100% to be 66% (33/50), 46% (23/50), 24% (12/50) and 6% (3/50), respectively [26]. After including those for which medication was not continued (n = 38) for various reasons, including a lack of efficacy, withdrawal by caregivers and adverse events, the corresponding percentages were 38%, 26%, 16% and 3%, respectively. With the increasing availability of ganaxolone in the USA and elsewhere, subsequent to its being the first US Food and Drug Administration-approved ASM for CDD, it will be important to continue monitoring its ongoing use through the ICDD.
Few other studies have investigated responses to specific ASMs, and the case numbers have been generally small. In 2012, a group from the Mayo Clinic reported a reduction in seizure frequency in six patients with CDD treated with topiramate, vigabatrin and a ketogenic diet [27]. A second study from Germany with aggregated individual responses from 39 patients showed that a small number of commonly prescribed medications (felbamate, vigabatrin, clobazam and valproate) initially reduced seizure frequency by 50% or more [17]. However, this improvement was generally unsustained. In our much larger study, vigabatrin and sodium valproate were ASMs that appeared to provide a more permanent benefit, but the benefit-to-side-effect ratio was less favourable for felbamate and clobazam. In a Georgian case series (n = 8), five patients with CDD responded positively to a combination of vigabatrin and zonisamide, providing the best outcome in reducing seizure frequency [28]. We were unable to report the latter combination because of insufficient cases. However, we found that zonisamide and levetiracetam provided reasonable seizure control but with side effects. After the Georgian case series, a Spanish study reported a reduction in seizure frequency of more than 50% among six of the 19 patients with CDD treated with sodium channel blockers, of which the most frequent were oxcarbazepine, carbamazepine and lacosamide [29]. In the German-based study, seizures were aggravated by carbamazepine in four patients, although one patient had a long period of seizure freedom [17]. Although lacosamide appeared relatively beneficial in our study, oxcarbazepine and carbamazepine appeared less so. Thus, there are contraindicatory data on whether sodium channel blockade ASMs are beneficial or detrimental for seizure control, also considering oxcarbazepine is primarily of value for focal seizures.
In the most recent US study (n = 168), information on a 2-week response to medications was available for 86 patients [14]. Similar to our observations, levetiracetam, topiramate, clobazam, and phenobarbital were frequently used, as were vigabatrin and sodium valproate. In both studies, the responses to these two latter medications were fair to good, as was the response to lamotrigine. In contrast, the response to levetiracetam, as in our study, was considerably poorer. This may be of concern given that levetiracetam was the most frequently used ASM in both studies. In a recently published US study, the response of epileptic spasms to first-line treatments (ACTH, oral corticosteroids and vigabatrin) was 26% at 14 days and sustained response only 4% at 3 months, both significantly worse compared with individuals with spasms of other aetiology from the National Infantile Spasms Consortium [30]. However, in our study, despite apparent benefits from steroids/ACTH, there was a high prevalence of reported side effects. The side-effect profile we saw, predominantly Cushingoid features and obesity, was very similar to that found in a systematic review of the use of steroids/ACTH in epilepsies other than infantile spasms where there was an overall 50% reduction in seizures but which tended not to be universally sustained [31]. Both ours and the earlier US study [14] reported on responses to cannabis derivatives, but in our study, we were unable to separate CBD only (Epidiolex®) from other cannabis derivatives. The 2-week response in the US study was 29%, but it was rated highly beneficial in our study with the least side effects. This was consistent with results from our previous study on the use of medical cannabis in CDD, where, despite favourable reporting, we were unable to identify any definite improvement in seizure frequency [32]. It is gratifying to see the similarities between the findings in our and the US study despite data in the latter being sourced from physician chart reviews. Although our study was international and had mostly non-US participants, some US participants may have also contributed to the published US study [14].
Unlike the US study [14], our analysis was limited to ASM therapy, and data on the use of the ketogenic diet and vagus nerve stimulation were not presented, as we have previously published separately on these [33, 34]. However, we did present data on the number of ASMs currently being used, finding that just over 40% were on three or more. This is of concern given that we had previously found that quality of life was adversely affected by polytherapy [12]. We also presented data on the outcomes of different ASM combinations. The combination with the greatest benefit and fewest side effects appeared to be levetiracetam and sodium valproate despite the less favourable findings for levetiracetam in the single medication analysis. Although the numbers were necessarily small, we consider this an important contribution.
Conclusions and Future Directions
Findings from our study further contribute to the understanding of the refractory nature of epilepsy in CDD and the poor response to ASMs. However, we have confirmed that the individual responses to sodium valproate, lamotrigine and vigabatrin may be more generally favourable and that the combination of levetiracetam and sodium valproate may be suitable for some patients. The increasing availability of ganaxolone in the USA and elsewhere will now be important to monitor through the ICDD. The question about the therapeutic effect of cannabis products for seizures in CDD remains unresolved despite an earlier open-label trial suggesting a positive benefit [35]. Our caregiver-reported findings suggest that a further placebo-controlled trial could be warranted. For individuals with CDD and their families, identifying the optimal treatment approaches for seizure control continues to be a high priority for further research. Our data underscores the unmet need for the successful management of seizures in CDD and the reduced efficacy of existing ASMs to reduce the seizure burden in one of the most refractory epileptic syndromes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the families in the International CDKL5 Disorder Database for participating in this study.
Declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The International CDKL5 Disorder Database was funded by the International Foundation for CDKL5 Research, along with the Orphan Disease Centre at the University of Pennsylvania (CDKL5-19-D-101-3) for analytical support for this study.
Conflict of interest
Heather E. Olson received consulting fees from Takeda Pharmaceuticals and Zogenix regarding the clinical trial design, Ovid Therapeutics regarding clinical trial results and Marinus Pharmaceuticals regarding CDKL5 deficiency disorder, and has done consulting for the FOXG1 Research Foundation. She is the site Principal Investigator for a trial with UCB Pharmaceuticals. Elia M. Pestana-Knight was a speaker and scientific advisory board member for Marinus Pharmaceuticals, for which she has received compensation. Rajsekar R. Rajaraman was a speaker/consultant for Marinus Pharmaceuticals. He was also a consultant for UCB Pharmaceuticals and received funding from the International Foundation for CDKL5 Research. Jenny Downs provided consultancy for Marinus Pharmaceuticals, Neurogene, Ultragenyx Pharmaceutical, Acadia Pharmaceuticals, Novartis Gene Therapies, Orion Corporation and Taysha Gene Therapies; and for clinical trials with Anavex Life Science and Newron Pharmaceuticals. All consultancies are unrelated to this work, and all remuneration has been made to her department. Helen Leonard provided consultancy for Marinus Pharmaceuticals, Acadia Pharmaceuticals, Novartis Gene Therapies and Orion Corporation; and for clinical trials with Anavex Life Science and Newron Pharmaceuticals. All consultancies are unrelated to this work, and all remuneration has been made to her department. Kingsley Wong, Mohammed Junaid and Solomon Alexander have no conflicts of interest that are directly relevant to the content of this article.
Ethical approval
The Human Research Ethics Committee of the University of Western Australia provided approval for this study.
Consent to participate
Written informed consent was obtained from the caregivers of individuals who participated in the study.
Consent for publication
Not applicable.
Author contributions
All authors contributed to the study’s conception and design. M.J., K.W. and S.A. performed material preparation, data collection and analysis. H.L., M.J. and K.W. wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Data availability
The dataset analysed during the current study are not publicly available but may be available from the corresponding author on reasonable request following an application to and with approval from the local ethics committee.
Code availability
Not applicable.
References
- 1.Leonard H, Downs J, Benke TA, Swanson L, Olson H, Demarest S. CDKL5 deficiency disorder: clinical features, diagnosis, and management. Lancet Neurol. 2022;21(6):563–76. 10.1016/S1474-4422(22)00035-7. 10.1016/S1474-4422(22)00035-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symonds JD, Zuberi SM, Stewart K, McLellan A, O’Regan M, MacLeod S, et al. Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019;142(8):2303–18. 10.1093/brain/awz195. 10.1093/brain/awz195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson HE, Demarest ST, Pestana-Knight EM, Swanson LC, Iqbal S, Lal D, et al. Cyclin-dependent kinase-like 5 deficiency disorder: clinical review. Pediatr Neurol. 2019;97:18–25. 10.1016/j.pediatrneurol.2019.02.015. 10.1016/j.pediatrneurol.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKay CI, Wong K, Demarest ST, Benke TA, Downs J, Leonard H. Exploring genotype-phenotype relationships in the CDKL5 deficiency disorder using an international dataset. Clin Genet. 2021;99(1):157–65. 10.1111/cge.13862. 10.1111/cge.13862 [DOI] [PubMed] [Google Scholar]
- 5.Fehr S, Wilson M, Downs J, Williams S, Murgia A, Sartori S, et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur J Hum Genet. 2013;21(3):266–73. 10.1038/ejhg.2012.156. 10.1038/ejhg.2012.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbiero I, De Rosa R, Kilstrup-Nielsen C. Microtubules: a key to understand and correct neuronal defects in CDKL5 deficiency disorder? Int J Mol Sci. 2019;20(17):4075. 10.3390/ijms20174075. 10.3390/ijms20174075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzic B, Cui Y, Edmondson AC, Tang S, Sarmiento N, Zaitseva D, et al. X-linked cellular mosaicism underlies age-dependent occurrence of seizure-like events in mouse models of CDKL5 deficiency disorder. Neurobiol Dis. 2021;148: 105176. 10.1016/j.nbd.2020.105176. 10.1016/j.nbd.2020.105176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehr S, Wong K, Chin R, Williams S, de Klerk N, Forbes D, et al. Seizure variables and their relationship to genotype and functional abilities in the CDKL5 disorder. Neurology. 2016;87(21):2206–13. 10.1212/wnl.0000000000003352. 10.1212/wnl.0000000000003352 [DOI] [PubMed] [Google Scholar]
- 9.Demarest ST, Olson HE, Moss A, Pestana-Knight E, Zhang X, Parikh S, et al. CDKL5 deficiency disorder: relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia. 2019;60(8):1733–42. 10.1111/epi.16285. 10.1111/epi.16285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangatt M, Wong K, Anderson B, Epstein A, Hodgetts S, Leonard H, et al. Prevalence and onset of comorbidities in the CDKL5 disorder differ from Rett syndrome. Orphanet J Rare Dis. 2016;14(11):39. 10.1186/s13023-016-0418-y. 10.1186/s13023-016-0418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr S, Downs J, Ho G, de Klerk N, Forbes D, Christodoulou J, et al. Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A. 2016;170(11):2860–9. 10.1002/ajmg.a.37851. 10.1002/ajmg.a.37851 [DOI] [PubMed] [Google Scholar]
- 12.Leonard H, Junaid M, Wong K, Demarest S, Downs J. Exploring quality of life in individuals with a severe developmental and epileptic encephalopathy, CDKL5 deficiency disorder. Epilepsy Res. 2021;169: 106521. 10.1016/j.eplepsyres.2020.106521. 10.1016/j.eplepsyres.2020.106521 [DOI] [PubMed] [Google Scholar]
- 13.Bertani I, Rusconi L, Bolognese F, Forlani G, Conca B, De Monte L, et al. Functional consequences of mutations in CDKL5, an X-linked gene involved in infantile spasms and mental retardation. J Biol Chem. 2006;281(42):32048–56. 10.1074/jbc.M606325200. 10.1074/jbc.M606325200 [DOI] [PubMed] [Google Scholar]
- 14.Olson HE, Daniels CI, Haviland I, Swanson LC, Greene CA, Denny AMM, et al. Current neurologic treatment and emerging therapies in CDKL5 deficiency disorder. J Neurodev Disord. 2021;13(1):40. 10.1186/s11689-021-09384-z. 10.1186/s11689-021-09384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong W, Haviland I, Pestana-Knight E, Weisenberg JL, Demarest S, Marsh ED, et al. CDKL5 deficiency disorder-related epilepsy: a review of current and emerging treatment. CNS Drugs. 2022;36(6):591–604. 10.1007/s40263-022-00921-5. 10.1007/s40263-022-00921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin S, Monaghan M, Aledo-Serrano A, Bahi-Buisson N, Chin RF, Clarke AJ, et al. International consensus recommendations for the assessment and management of individuals with CDKL5 deficiency disorder. Front Neurol. 2022;13: 874695. 10.3389/fneur.2022.874695. 10.3389/fneur.2022.874695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller A, Helbig I, Jansen C, Bast T, Guerrini R, Jahn J, et al. Retrospective evaluation of low long-term efficacy of antiepileptic drugs and ketogenic diet in 39 patients with CDKL5-related epilepsy. Eur J Paediatr Neurol. 2016;20(1):147–51. 10.1016/j.ejpn.2015.09.001. 10.1016/j.ejpn.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Amin S, Majumdar A, Mallick AA, Patel J, Scatchard R, Partridge CA, et al. Caregiver’s perception of epilepsy treatment, quality of life and comorbidities in an international cohort of CDKL5 patients. Hippokratia. 2017;21(3):130–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Tohyama J, Takahashi Y, Goto T, Haginoya K, Inoue T, et al. Clinical manifestations and epilepsy treatment in Japanese patients with pathogenic CDKL5 variants. Brain Dev. 2021;43(4):505–14. 10.1016/j.braindev.2020.12.006. 10.1016/j.braindev.2020.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Wong K, Junaid M, Demarest S, Saldaris J, Benke TA, Marsh ED, et al. Factors influencing the attainment of major motor milestones in CDKL5 deficiency disorder. Eur J Hum Genet. 2023;31(2):169–78. 10.1038/s41431-022-01163-1. 10.1038/s41431-022-01163-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain SV, Glauser TA. Effects of epilepsy treatments on sleep architecture and daytime sleepiness: an evidence-based review of objective sleep metrics. Epilepsia. 2013;55(1):26–37. 10.1111/epi.12478. 10.1111/epi.12478 [DOI] [PubMed] [Google Scholar]
- 22.Liguori C, Toledo M, Kothare S. Effects of anti-seizure medications on sleep architecture and daytime sleepiness in patients with epilepsy: a literature review. Sleep Med Rev. 2021;60: 101559. 10.1016/j.smrv.2021.101559. 10.1016/j.smrv.2021.101559 [DOI] [PubMed] [Google Scholar]
- 23.Strzelczyk A, Schubert-Bast S. Psychobehavioural and cognitive adverse events of anti-seizure medications for the treatment of developmental and epileptic encephalopathies. CNS Drugs. 2022;36(10):1079–111. 10.1007/s40263-022-00955-9. 10.1007/s40263-022-00955-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downs J, Jacoby P, Saldaris J, Leonard H, Benke T, Marsh E, et al. Negative impact of insomnia and daytime sleepiness on quality of life in individuals with the cyclin-dependent kinase-like 5 deficiency disorder. J Sleep Res. 2022;31(5): e13600. 10.1111/jsr.13600. 10.1111/jsr.13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pestana-Knight EM, Amin S, Bahi-Buisson N, Benke TA, Cross JH, Demarest ST, et al. Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2022;21(5):417–27. 10.1016/S1474-4422(22)00077-1. 10.1016/S1474-4422(22)00077-1 [DOI] [PubMed] [Google Scholar]
- 26.Olson HE, Amin S, Bahi-Buisson N, Devinsky O, Marsh ED, Pestana-Knight E, et al. Long-term treatment with ganaxolone for seizures associated with cyclin-dependent kinase-like 5 deficiency disorder: two-year open-label extension follow-up. Epilepsia. 2024;65(1):37–45. 10.1111/epi.17826. 10.1111/epi.17826 [DOI] [PubMed] [Google Scholar]
- 27.Moseley BD, Dhamija R, Wirrell EC, Nickels KC. Historic, clinical, and prognostic features of epileptic encephalopathies caused by CDKL5 mutations. Pediatr Neurol. 2012;46(2):101–5. 10.1016/j.pediatrneurol.2011.11.007. 10.1016/j.pediatrneurol.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 28.Melikishvili G, Epitashvili N, Tabatadze N, Chikvinidze G, Dulac O, Bienvenu T, et al. New insights in phenomenology and treatment of epilepsy in CDKL5 encephalopathy. Epilepsy Behav. 2019;94:308–11. 10.1016/j.yebeh.2019.02.013. 10.1016/j.yebeh.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 29.Aledo-Serrano A, Gomez-Iglesias P, Toledano R, Garcia-Penas JJ, Garcia-Morales I, Anciones C, et al. Sodium channel blockers for the treatment of epilepsy in CDKL5 deficiency disorder: findings from a multicenter cohort. Epilepsy Behav. 2021;118: 107946. 10.1016/j.yebeh.2021.107946. 10.1016/j.yebeh.2021.107946 [DOI] [PubMed] [Google Scholar]
- 30.Olson HE, Demarest S, Pestana-Knight E, Moosa AN, Zhang X, Perez-Perez JR, et al. Epileptic spasms in CDKL5 deficiency disorder: delayed treatment and poor response to first-line therapies. Epilepsia. 2023;64(7):1821–32. 10.1111/epi.17630. 10.1111/epi.17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korinthenberg R, Bast T, Haberlandt E, Stephani U, Strzelczyk A, Rucker G. Efficacy and safety of corticosteroids and ACTH in epileptic syndromes beyond infantile epileptic spasms syndrome (IESS): a systematic review and meta-analysis. Epilepsia. 2024;65(5):1155–75. 10.1111/epi.17918. 10.1111/epi.17918 [DOI] [PubMed] [Google Scholar]
- 32.Dale T, Downs J, Wong K, Leonard H. The perceived effects of cannabis products in the management of seizures in CDKL5 deficiency disorder. Epilepsy Behav. 2021;122: 108152. 10.1016/j.yebeh.2021.108152. 10.1016/j.yebeh.2021.108152 [DOI] [PubMed] [Google Scholar]
- 33.Lim Z, Wong K, Downs J, Bebbington K, Demarest S, Leonard H. Vagus nerve stimulation for the treatment of refractory epilepsy in the CDKL5 deficiency disorder. Epilepsy Res. 2018;146:36–40. 10.1016/j.eplepsyres.2018.07.013. 10.1016/j.eplepsyres.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 34.Lim Z, Wong K, Olson HE, Bergin AM, Downs J, Leonard H. Use of the ketogenic diet to manage refractory epilepsy in CDKL5 disorder: experience of >100 patients. Epilepsia. 2017;58(8):1415–22. 10.1111/epi.13813. 10.1111/epi.13813 [DOI] [PubMed] [Google Scholar]
- 35.Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, et al. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7. 10.1016/j.yebeh.2018.05.013. 10.1016/j.yebeh.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 36.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–12. 10.1080/01621459.1927.10502953. 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed during the current study are not publicly available but may be available from the corresponding author on reasonable request following an application to and with approval from the local ethics committee.