Abstract
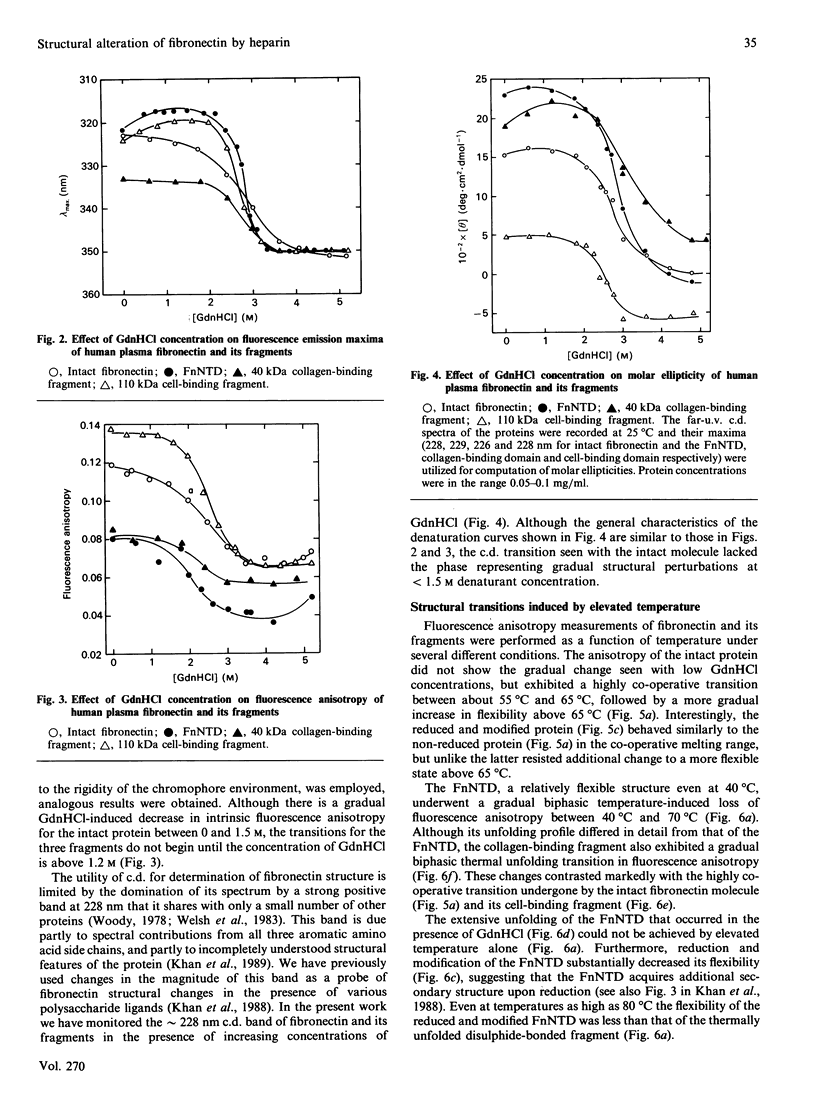
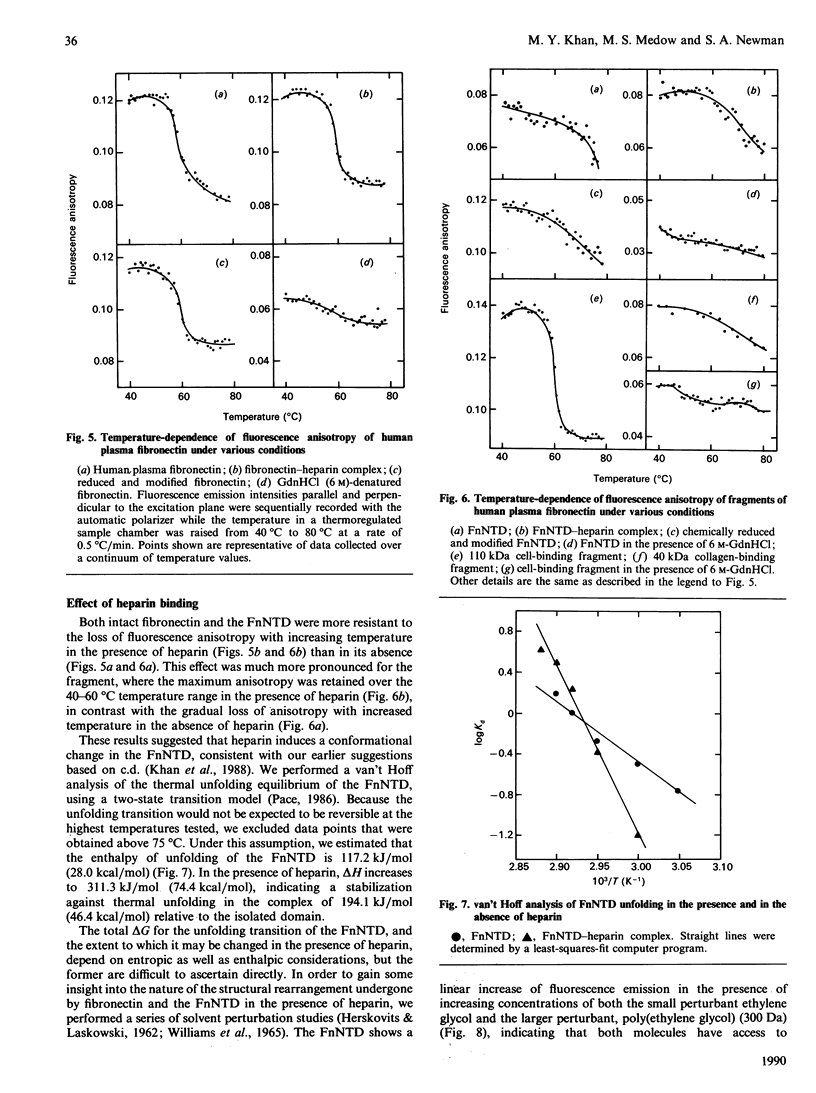
Changes in the conformational state of human plasma fibronectin and several of its fragments were studied by fluorescence emission, intrinsic fluorescence polarization and c.d. spectroscopy under conditions of guanidinium chloride-and temperature-induced unfolding. Fragments were chosen to represent all three types of internal structural homology in the protein. Low concentration (less than 2 M) of guanidinium chloride induced a gradual transition in the intact protein that was not characteristic of any of the isolated domains, suggesting the presence of interdomain interactions within the protein. Intermediate concentrations of guanidinium chloride (2-3 M) and moderately elevated temperatures (55-60 degrees C) induced a highly co-operative structural transition in intact fibronectin that was attributable to the central 110 kDa cell-binding domain. High temperatures (greater than 60 degrees C) produced a gradual unfolding in the intact protein attributable to the 29 kDa N-terminal heparin-binding and 40 kDa collagen-binding domains. Binding of heparin to intact fibronectin and to its N-terminal fragment stabilized the proteins against thermal unfolding. This was reflected in increased delta H for the unfolding transitions of the heparin-bound N-terminal fragment, as well as decreased accessibility to solvent perturbants of internal chromophores in this fragment when bound to heparin. These results help to account for the biological efficacy of the interaction between the fibronectin N-terminal domain and heparin, despite its relatively low affinity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. Fibronectin. Adv Enzymol Relat Areas Mol Biol. 1987;59:1–57. doi: 10.1002/9780470123058.ch1. [DOI] [PubMed] [Google Scholar]
- Alexander S. S., Jr, Colonna G., Edelhoch H. The structure and stability of human plasma cold-insoluble globulin. J Biol Chem. 1979 Mar 10;254(5):1501–1505. [PubMed] [Google Scholar]
- Benecky M. J., Kolvenbach C. G., Amrani D. L., Mosesson M. W. Evidence that binding to the carboxyl-terminal heparin-binding domain (Hep II) dominates the interaction between plasma fibronectin and heparin. Biochemistry. 1988 Sep 20;27(19):7565–7571. doi: 10.1021/bi00419a058. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Colonna G., Alexander S. S., Jr, Yamada K. M., Pastan I., Edelhoch H. The stability of cell surface protein to surfactants and denaturants. J Biol Chem. 1978 Nov 10;253(21):7787–7790. [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P., Carrell N. A. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J Biol Chem. 1983 Dec 10;258(23):14539–14544. [PubMed] [Google Scholar]
- Erickson H. P., Carrell N., McDonagh J. Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J Cell Biol. 1981 Dec;91(3 Pt 1):673–678. doi: 10.1083/jcb.91.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgacs G., Jaikaria N. S., Frisch H. L., Newman S. A. Wetting, percolation and morphogenesis in a model tissue system. J Theor Biol. 1989 Oct 9;140(3):417–430. doi: 10.1016/s0022-5193(89)80096-7. [DOI] [PubMed] [Google Scholar]
- Frenz D. A., Akiyama S. K., Paulsen D. F., Newman S. A. Latex beads as probes of cell surface-extracellular matrix interactions during chondrogenesis: evidence for a role for amino-terminal heparin-binding domain of fibronectin. Dev Biol. 1989 Nov;136(1):87–96. doi: 10.1016/0012-1606(89)90132-2. [DOI] [PubMed] [Google Scholar]
- Frenz D. A., Jaikaria N. S., Newman S. A. The mechanism of precartilage mesenchymal condensation: a major role for interaction of the cell surface with the amino-terminal heparin-binding domain of fibronectin. Dev Biol. 1989 Nov;136(1):97–103. doi: 10.1016/0012-1606(89)90133-4. [DOI] [PubMed] [Google Scholar]
- HERSKOVITS T. T., LASKOWSKI M., Jr Location of chromophoric residues in proteins by solvent perturbation. I. Tyrosyls in serum albumins. J Biol Chem. 1962 Aug;237:2481–2492. [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983 Mar 10;258(5):3332–3340. [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Hörmann H., Richter H. Models for the subunit arrangement in soluble and aggregated plasma fibronectin. Biopolymers. 1986 May;25(5):947–958. doi: 10.1002/bip.360250513. [DOI] [PubMed] [Google Scholar]
- Ingham K. C., Brew S. A., Broekelmann T. J., McDonald J. A. Thermal stability of human plasma fibronectin and its constituent domains. J Biol Chem. 1984 Oct 10;259(19):11901–11907. [PubMed] [Google Scholar]
- Isaacs B. S., Brew S. A., Ingham K. C. Reversible unfolding of the gelatin-binding domain of fibronectin: structural stability in relation to function. Biochemistry. 1989 Jan 24;28(2):842–850. doi: 10.1021/bi00428a065. [DOI] [PubMed] [Google Scholar]
- Khan M. Y., Jaikaria N. S., Frenz D. A., Villanueva G., Newman S. A. Structural changes in the NH2-terminal domain of fibronectin upon interaction with heparin. Relationship to matrix-driven translocation. J Biol Chem. 1988 Aug 15;263(23):11314–11318. [PubMed] [Google Scholar]
- Khan M. Y., Villanueva G., Newman S. A. On the origin of the positive band in the far-ultraviolet circular dichroic spectrum of fibronectin. J Biol Chem. 1989 Feb 5;264(4):2139–2142. [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteliansky V. E., Bejanian M. V., Smirnov V. N. Electron microscopy study of fibronectin structure. FEBS Lett. 1980 Nov 3;120(2):283–286. doi: 10.1016/0014-5793(80)80317-6. [DOI] [PubMed] [Google Scholar]
- Koteliansky V. E., Glukhova M. A., Bejanian M. V., Smirnov V. N., Filimonov V. V., Zalite O. M., Venyaminov SYu A study of the structure of fibronectin. Eur J Biochem. 1981 Oct;119(3):619–624. doi: 10.1111/j.1432-1033.1981.tb05652.x. [DOI] [PubMed] [Google Scholar]
- Lai C. S., Tooney N. M., Ankel E. G. Structure and flexibility of plasma fibronectin in solution: electron spin resonance spin-label, circular dichroism, and sedimentation studies. Biochemistry. 1984 Dec 18;23(26):6393–6397. doi: 10.1021/bi00321a017. [DOI] [PubMed] [Google Scholar]
- Marković Z., Lustig A., Engel J., Richter H., Hörmann H. Shape and stability of fibronectin in solutions of different pH and ionic strength. Hoppe Seylers Z Physiol Chem. 1983 Dec;364(12):1795–1804. doi: 10.1515/bchm2.1983.364.2.1795. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Quade B. J., Broekelmann T. J., LaChance R., Forsman K., Hasegawa E., Akiyama S. Fibronectin's cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J Biol Chem. 1987 Mar 5;262(7):2957–2967. [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Newman S. A., Frenz D. A., Hasegawa E., Akiyama S. K. Matrix-driven translocation: dependence on interaction of amino-terminal domain of fibronectin with heparin-like surface components of cells or particles. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4791–4795. doi: 10.1073/pnas.84.14.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. A., Frenz D. A., Tomasek J. J., Rabuzzi D. D. Matrix-driven translocation of cells and nonliving particles. Science. 1985 May 17;228(4701):885–889. doi: 10.1126/science.4001925. [DOI] [PubMed] [Google Scholar]
- Odermatt E., Engle J., Richter H., Hörmann H. Shape, conformation and stability of fibronectin fragments determined by electron microscopy, circular dichroism and ultracentrifugation. J Mol Biol. 1982 Jul 25;159(1):109–123. doi: 10.1016/0022-2836(82)90034-1. [DOI] [PubMed] [Google Scholar]
- Pace C. N. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T. M., Rudee M. L., Pierschbacher M., Ruoslahti E. Structure of fibronectin and its fragments in electron microscopy. Eur J Biochem. 1982 Dec 15;129(2):359–363. doi: 10.1111/j.1432-1033.1982.tb07058.x. [DOI] [PubMed] [Google Scholar]
- Rocco M., Aresu O., Zardi L. Conformational state of circulating human plasma fibronectin. FEBS Lett. 1984 Dec 10;178(2):327–330. doi: 10.1016/0014-5793(84)80627-4. [DOI] [PubMed] [Google Scholar]
- Rocco M., Infusini E., Daga M. G., Gogioso L., Cuniberti C. Models of fibronectin. EMBO J. 1987 Aug;6(8):2343–2349. doi: 10.1002/j.1460-2075.1987.tb02510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Skorstengaard K., Jensen M. S., Petersen T. E., Magnusson S. Purification and complete primary structures of the heparin-, cell-, and DNA-binding domains of bovine plasma fibronectin. Eur J Biochem. 1986 Jan 2;154(1):15–29. doi: 10.1111/j.1432-1033.1986.tb09353.x. [DOI] [PubMed] [Google Scholar]
- Thompson L. K., Horowitz P. M., Bentley K. L., Thomas D. D., Alderete J. F., Klebe R. J. Localization of the ganglioside-binding site of fibronectin. J Biol Chem. 1986 Apr 15;261(11):5209–5214. [PubMed] [Google Scholar]
- Tooney N. M., Mosesson M. W., Amrani D. L., Hainfeld J. F., Wall J. S. Solution and surface effects on plasma fibronectin structure. J Cell Biol. 1983 Dec;97(6):1686–1692. doi: 10.1083/jcb.97.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venyaminov SYu, Metsis M. L., Chernousov M. A., Koteliansky V. E. Distribution of secondary structure along the fibronectin molecule. Eur J Biochem. 1983 Oct 3;135(3):485–489. doi: 10.1111/j.1432-1033.1983.tb07677.x. [DOI] [PubMed] [Google Scholar]
- Welsh E. J., Frangou S. A., Morris E. R., Rees D. A., Chavin S. I. Tyrosine optical activity as a probe of the conformation and interactions of fibronectin. Biopolymers. 1983 Mar;22(3):821–831. doi: 10.1002/bip.360220305. [DOI] [PubMed] [Google Scholar]
- Williams E. C., Janmey P. A., Ferry J. D., Mosher D. F. Conformational states of fibronectin. Effects of pH, ionic strength, and collagen binding. J Biol Chem. 1982 Dec 25;257(24):14973–14978. [PubMed] [Google Scholar]
- Williams E. J., Herskovits T. T., Laskowski M., Jr Location of chromophoric residues in proteins by solvent perturbation. 3. Tryptophyls in lysozyme and in alpha-chymotrypsinogen and its derivatives. J Biol Chem. 1965 Sep;240(9):3574–3579. [PubMed] [Google Scholar]
- Woods A., Johansson S., Hök M. Fibronectin fibril formation involves cell interactions with two fibronectin domains. Exp Cell Res. 1988 Aug;177(2):272–283. doi: 10.1016/0014-4827(88)90461-2. [DOI] [PubMed] [Google Scholar]


