ABSTRACT
Venous thromboembolism (VTE) is a major cause of morbidity and mortality in the United States. VTE is caused by genetic and acquired conditions, but the genetic variants that increase the risk of VTE are not fully characterized. Recent genome-wide association studies (GWAS) have discovered novel genetic loci linked to VTE. Some of these loci have been characterized, uncovering new pathways that regulate VTE. Functional characterization of candidate genes discovered by GWAS may reveal new therapeutic targets to treat and prevent abnormal thrombosis or bleeding.
INTRODUCTION
VTE includes deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE is the third leading cause of cardiovascular death in the United States (1). About 900,000 people have VTE each year in the United States, and between 60,000 and 100,000 die from VTE (2,3). The goals for VTE therapy include resolution of thrombus and prevention of recurrent thrombosis. Treatment for stable patients with VTE involves balancing risks and benefits of anticoagulation, and if appropriate, immediate treatment with higher dose anticoagulation (such as low molecular weight heparin or Factor X inhibitors), followed by long-term treatment with lower dose anticoagulation (such as a Factor X inhibitor) (4,5). Guidelines for the duration of anticoagulation have changed over time, as we learn more about the risks of recurrent VTE.
Rudolph Virchow explored the pathophysiology of VTE in the late nineteenth century (6). Based on his experiments with dogs in the Charite Hospital in Berlin, he noted that three factors can contribute to the formation of a venous thrombus: stasis of the blood, injury to the vessel, and hypercoagulability of blood components. Approximately 80% of patients with VTE have at least one of these factors.
The pathophysiology of thrombosis differs between type of vessel. The coagulation cascade dominates thrombosis in veins. Thrombosis in veins is thought to begin with injury to the vessel wall, the release of tissue factor (Factor III), activation of the coagulation cascade, and formation of a fibrin mesh which traps erythrocytes and some platelets. In contrast, platelets dominate arterial thrombosis. Injury to the vessel wall exposes vessel wall components, von Willebrand Factor (VWF) and platelets bind to the subendothelial matrix, activated platelets release mediators such as adenosine triphosphate (ATP) that trigger activation of adjacent platelets, and the coagulation cascade is activated forming a fibrin mesh that stabilizes the platelet plug. Histological and electron microscopic data support this distinction: venous thrombi have more fibrin and erythrocytes; arterial thrombi have more platelets.
Risks for VTE are divided into two broad categories, acquired and inherited risks (7). Acquired risk factors include immobility, surgery, malignancy, infection, pregnancy, and drugs. However, it is unlikely that an acquired risk factor alone is sufficient to cause thrombosis; for example, most people have surgery without developing VTE, most pregnancies do not cause VTE, and most people who take oral contraceptives do not develop VTE. Genetic factors are also involved in VTE risk.
The heritability of VTE (variation in thrombosis risk that is due to genetic variation between individuals) is approximately 50% (8,9). The risk of a sibling developing VTE if another sibling has VTE is approximately 2.5-fold (8,9). Until recently, only a few genes were known to be linked to inherited thrombophilia, including Factor V, prothrombin, protein C, protein S, and antithrombin. Mutations in the Factor V locus and the prothrombin locus account for approximately 50% of the risk of VTE, and the identity of other genes contributing to the risk of VTE has been less understood.
In the past decade, genome-wide association studies have identified new candidate genes that may play a role in the risk of VTE. Initially, several small-scale GWAS identified a few susceptibility loci. Subsequently, individual teams of population studies collaborated to amass larger and larger cohorts, increasing the power to detect genetic loci contributing to the risk of thrombosis (Table 1) (10-13). One of the earliest large-scale GWAS for VTE was produced by the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium and the International Network Against Venous Thrombosis (INVENT) consortium. Combining 12 GWAS with over 7,500 VTE cases and 52,000 control subjects, the consortium identified nine genetic loci linked to VTE risk (12). A second genetic study by the INVENT and CHARGE consortia combined 18 studies with about 30,000 VTE cases and 170,000 controls and used GWAS and transcriptome-wide association studies (TWAS) to detect 34 genetic signals for VTE (13). A third genetic study by the INVENT and CHARGE consortia combined 30 population studies including more than 55,000 VTE cases and 1,000,000 controls, and a meta-analysis of GWAS results identified 135 independent genomic loci associated with VTE risk (11).
TABLE 1.
Large-Scale Genetic Studies of Genetic Variants Linked to VTE
| Year of Study Publication | VTE Cases | Controls | Genetic Loci Linked to VTE Risk |
|---|---|---|---|
| 2015 | 7,507 | 52,632 | 9 |
| 2019 | 30,234 | 172,122 | 34 |
| 2022 | 55,330 | 1,081,973 | 135 |
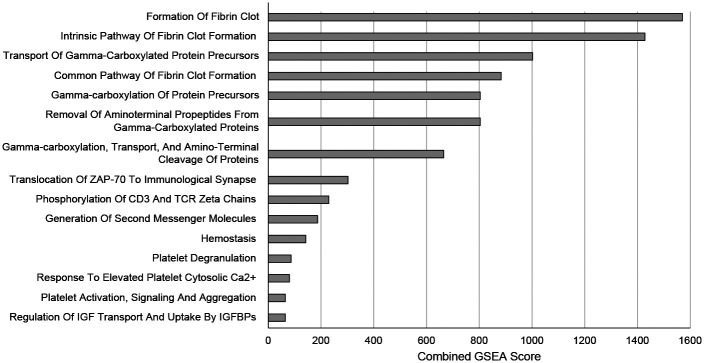
Gene set enrichment analyses of the genetic loci linked to VTE confirms pathways expected to regulate thrombosis (such as formation of a fibrin clot and activation of platelets), but also novel pathways never before linked to thrombosis [such as phosphorylation of CD3, or regulation of insulin-like growth factor (IGF transport)] (Figure 1).
Fig. 1.
Gene Set Enrichment Analysis (GSEA) of 135 Genetic Variants Linked to Risk of VTE Grouped by Reactome Subset of Canonical Pathways. The genetic loci linked to the risk of VTE was analyzed by Gene Ontology for gene set enrichment.
These GWAS identify new variants linked to VTE, and the genes closest to these loci may play a role in VTE. Which ones are true positives, and which are false positives? Functional biology offers an approach to identify candidate genes identified by GWAS that are true positives. Here we summarize our exploration of the role of one such candidate gene, SLC44A2, in the pathophysiology of VTE.
MATERIALS AND METHODS
The scientific approaches to study SLC44A2 are listed in prior publications (14–17). We refer to the human gene as SLC44A2 and the murine gene as Slc44a2.
RESULTS
We proposed that SLC44A2 is a gene that regulates thrombosis. We based our hypothesis on a GWAS that linked genetic variation in the SLC44A2 locus to increased risk of VTE (12). To test this hypothesis, we compared wild-type mice that express Slc44a2 [referred to as Slc44a2(WT)] to transgenic mice that lack Slc44a2 [referred to as Slc44a2(KO)], and we compared platelets from Slc44a2(WT) and Slc44a2(KO) mice (14).
We found that Slc44a2(KO) mice have more bleeding and less thrombosis than Slc44a2(WT) mice (14). The bleeding time is prolonged in mice that lack Slc44a2, the thrombosis time is slower in mice that lack Slc44a2, and the thrombus formation in the inferior vena cave is less in mice lacking Slc44a2. Platelets account for at least part of this defect, because transfusion of platelets from Slc44a2(WT) mice into Slc44a2(KO) mice restores normal bleeding times.
We also believe that platelets in Slc44a2(KO) mice are defective in several ways (14). Compared to platelets from Slc44a2(WT) mice, platelets from Slc44a2(KO) mice have less aggregation, less secretion of alpha-granules, and less activation of GPIIb IIIa (14).
The mitochondria of platelets lacking Slc44a2 have a metabolic defect (14). Slc44a2 is localized to mitochondria in platelets, and Slc44a2 serves as a choline transporter (18). Mitochondria from mice lacking Slc44a2 have a defect in oxygen consumption. Furthermore, the mitochondria copy number is increased in platelets lacking Slc44a2 (14).
Finally, platelets from mice lacking Slc44a2 contain less ATP and release less ATP (14). Since ATP is a messenger molecule secreted from platelets that activates neighboring platelets, we proposed that one cause of the defective platelet activation in Slc44a2(KO) mice is the lack of secreted ATP. To test this idea, we added media or adenosine diphosphate (ADP) to platelets lacking Slc44a2. Thrombin activated platelets from Slc44a2(WT) mice, but thrombin failed to activate platelets from Slc44a2(KO) mice. However, addition of ADP to the media restored the ability of thrombin to activate platelets from Slc44a2(KO) mice (14). These data show that Slc44a2 produces ATP necessary for normal platelet aggregation.
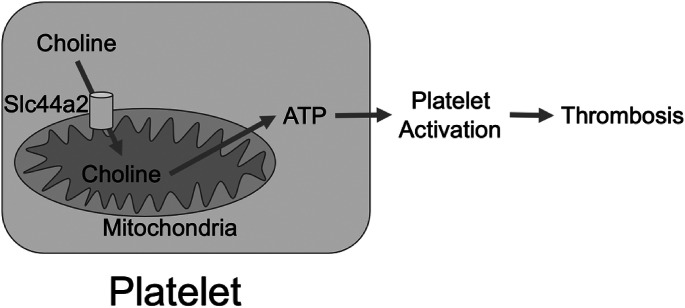
Taken together, our data suggest a novel metabolic pathway of platelet activation (Figure 2). Slc44a2 transports choline into mitochondria which use choline as a fuel to make ATP, and ATP is secreted from platelets to activate adjacent platelets and amplify the process of platelet aggregation.
Fig. 2.
SLC44A2 Regulates Platelet Metabolism and Thrombosis. SLC44A2 transports choline into mitochondria. Mitochondria use choline to produce ATP. Platelets release ATP which activates neighboring platelets, leading to aggregation and thrombosis.
DISCUSSION
Our functional biology studies demonstrate that genetic analyses of disease can reveal new pathways and new targets. The CHARGE and INVENT consortia used human genetic approaches to identify 135 genetic loci linked to the risk of VTE (11). However, the characterization of most of these candidate genes adjacent to these loci is incomplete, and their role in VTE is unclear.
We selected one of these genes linked to risk of VTE, SLC44A2, and used functional biology approaches to test its role in thrombosis. We discovered that SLC44A2 plays a novel role in platelet metabolism and thrombosis. Our findings highlight the importance of using functional biology approaches to validate genetic discovery.
Our findings produce insights into the functions of mitochondria in platelets. Mitochondria play important roles in platelet biology and thrombosis (19). Mitochondria regulate platelet energy metabolism, produce ATP, limit apoptosis of platelets, and control platelet activation. We discovered that platelets may use choline as a metabolic substrate. We also found that choline enables platelets to make ATP which is used to stimulate adjacent platelets.
These studies raise new questions about platelets in thrombosis. What is the effect of SLC44A2 mutations in humans? We studied mice that lack Slc44a2, but some humans have variants in SLC44A2, not complete loss of SLC44A2. Another question is what is the role of platelets in VTE? Pathology and histology of thrombus show that venous thrombosis contains more fibrin and red blood cells, and arterial thrombus contains fibrin and more platelets. Thus, it is surprising that a platelet defect affects venous thrombosis. Finally, SLC44A2 is found in many types of cells throughout the body, but the effect of genetic variants on cell function in most cells is unknown.
In summary, our studies are an example of functional biology characterizing candidate genes identified by genetic studies of humans. The combination of functional and biological approaches may lead to characterization of new disease pathways and ultimately to novel therapeutic targets.
ACKNOWLEDGMENTS AND FINANCIAL SUPPORT
This work was supported by NIH NHLBI R01 HL134894, R61 HL141791, and the Michel Mirowski M.D. Professorship in Cardiology (CJL).
DISCUSSION
Cushman, Vermont: You knew I would get up to ask you something. As a person who is involved in these epidemiology studies in the CHARGE consortium and in other consortia and who has studied the epidemiology of VTE for 25 years, it’s great to see people utilizing the results that we published in these GWAS and other multi-omics data. In our papers, we note the need to learn more about the mechanism for some of these unexpected hits. I want to thank you for doing that work, because those of us who generate that data don’t always see the follow-up research on the functional biology. The GWAS data of VTE have been fairly disappointing because the biggest hits we get are the things we already knew about. You showed that this particular single nucleotide polymorphism (SNP) affects platelet activation, but we think VTE platelets are less important (e.g., the anti-platelet drug aspirin is not a very good drug to prevent VTE). Have you or others looked at this SNP in atherothrombotic events in myocardial infarction (MI) and stroke?
Lowenstein, Baltimore: This is such a great question. The idea is that venous thromboembolism is different from arterial embolism, arterial thromboembolism is more platelet driven, and venous thromboembolism is more coagulation factor driven. The GWAS data support this concept. In our studies, we have shown that this particular SNP affects both arterial thrombosis and venous thrombosis. Since this was a GWAS VTE, we focused on venous thromboembolism, but as you’re pointing out, platelets are key in arterial emboli.
Cushman, Vermont: Thank you.
Garry, Minneapolis: Nice talk, Charlie. Regarding your Slc44a2 gene disruption model, did you see increased bleeding?
Lowenstein, Baltimore: Yes, when we do the tail transection, there’s an increase in bleeding.
Sharma, San Antonio: Great talk. I really love the mechanism with mitochondria. Have you started thinking about doing these studies with the patients’ mitochondria, isolating platelets, and looking at their mitochondria function—potentially with choline? Also, can you think of any environmental or dietary intervention?
Lowenstein, Baltimore: The idea that mitochondria play a major role in platelet activation and thrombosis is such a great topic. Choline is a key metabolite, and there are lots of studies on choline and trimethylamine-N-oxide (TMAO). We have looked at human platelets that are wild-type from graduate students and principal investigators (PIs) in the lab. When you give choline to the platelets, you actually see this burst of oxygen consumption in ATP, so clearly the same pathway is active in humans. I’m not sure if we could ever propose giving choline to patients that have a bleeding deficit, but that is a great question.
Laposata, Galveston: That was great, Charlie. If somebody has breast cancer, we don’t think twice about doing a whole bunch of mutational analysis. I have seen many patients with thrombosis who always ask: why didn’t they check me for mutations, and why did I have to wait until I had my clot to be treated? I think this pushes thrombosis into the same zone as breast cancer. We must know far more about what mutations we’ve inherited so that we can appropriately prevent thrombosis with anti-platelet agents and anti-thrombotic agents. Do you think that we could get to the point where an exome analysis is the answer, because then we can look at your 135 genetic variants along with all the ones we already know about?
Lowenstein, Baltimore: Okay, this is a great question, so just think about the traditional risk factors for thrombosis. We call surgery a risk factor for thrombosis; we call airplane flights a risk for thrombosis. However, many of us take airplane flights, and some of us have surgery, but most of us do not get blood clots. So, there must be a personalized risk for VTE that involves those 135 genetic variants. When we see patients with thrombosis in the emergency room or in the critical care unit (CCU), we treat some of them with powerful anticoagulants, often forever. I think gradually the field is going to change, and someday we will be ordering genetic tests on our patients. Once they have thrombotic events, we will be tailoring therapy to them so I think you’re absolutely right.
Laposata, Galveston: Thanks, Charlie.
Silverstein, Milwaukee: Thanks, Charlie, that was terrific. I wanted to ask the other side of Mary’s question. If you look at a reverse GWAS and the phenotypes of patients that have the mutation in the gene to see if there’s an increased risk of, say, heart attack after secondary prevention of heart attack, you know which is a much easier phenotype than primary prevention.
Lowenstein, Baltimore: Roy, that’s a great question. There have been three large GWAS for VTE. The last one included a Phe-WAS, but I don’t remember what the answer is for SLC44A2. If you have a genetic risk for VTE that includes mutations for the gene SLC44A2, you’re probably at risk for other diseases. We found that cells lacking SLC44A2 have defects throughout the body. We looked at mitochondria in the liver, heart, and platelets, and they all have a defect in producing ATP.
Brock, Baltimore: Charlie was actually my mentor during residency; I was in his lab for several years! Charlie, a quick comment, not a real question: guess what dietary food contains choline? Eggs! So that might be something to think about in terms of giving your graduate students extra choline.
Prabhu, Birmingham: Charlie, nice presentation. Platelets are obviously derived from megakaryocytes so some of these acquired conditions are an intersection between the hematopoietic system and these new SNP that you’re seeing in your GWAS. Is there a change in the hematopoietic environment that’s leading to a change in platelet function? Is that something we should be looking at as the intersection between inflammation and thrombosis?
Lowenstein, Baltimore: Okay, another great question. The CHARGE hemostasis working group looks not only at VTE but also at hemostatic variables. It turns out that many of these genetic hits including SLC44A2 are linked to size and number of platelets, so there must be something going on in the hematopoietic stem cells (HSC) in the bone marrow. I think you’re absolutely right.
Footnotes
Potential Conflicts of Interest: Dr. Lowenstein received research funding from Novartis to study the effect of P-selectin inhibitors in COVID-19.
REFERENCES
- 1.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol . 2014;34(11):2363–71. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 2.Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med . 2010;38(4 Suppl):S502–9. doi: 10.1016/j.amepre.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol . 2015;12(8):464–74. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becattini C, Agnelli G. Acute treatment of venous thromboembolism. Blood . 2020;135(5):305–16. doi: 10.1182/blood.2019001881. [DOI] [PubMed] [Google Scholar]
- 5.Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood . 2020;135(5):317–25. doi: 10.1182/blood.2019002364. [DOI] [PubMed] [Google Scholar]
- 6.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol . 2008;143(2):180–90. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 7.Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA . 2020;324(17):1765–76. doi: 10.1001/jama.2020.17272. [DOI] [PubMed] [Google Scholar]
- 8.Cohoon KP, Heit JA. Inherited and secondary thrombophilia. Circulation . 2014;129(2):254–7. doi: 10.1161/CIRCULATIONAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen TB, Sorensen HT, Skytthe A, Johnsen SP, Vaupel JW, Christensen K. Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiol . 2003;14(3):328–32. [PubMed] [Google Scholar]
- 10.Lindstrom S, Wang L, Smith EN, et al. Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood . 2019;134(19):1645–57. doi: 10.1182/blood.2019000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thibord F, Klarin D, Brody JA, et al. Cross-ancestry investigation of venous thromboembolism genomic predictors. Circulation . 2022;146(16):1225–42. doi: 10.1161/CIRCULATIONAHA.122.059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabater-Lleal M, Huffman JE, de Vries PS, et al. Genome-wide association transethnic meta-analyses identifies novel associations regulating coagulation Factor VIII and von Willebrand factor plasma levels. Circulation . 2019;139(5):620–35. doi: 10.1161/CIRCULATIONAHA.118.034532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klarin D, Busenkell E, Judy R, et al. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat Genet . 2019;51(11):1574–9. doi: 10.1038/s41588-019-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett JA, Mastrangelo MA, Ture SK, et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat Commun . 2020;11(1):3479. doi: 10.1038/s41467-020-17254-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett JA, Ture SK, Schmidt RA, et al. Acetylcholine inhibits platelet activation. J Pharmacol Exp Ther . 2019;369(2):182–7. doi: 10.1124/jpet.118.253583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu QM, Ko KA, Ture S, et al. Novel thrombotic function of a human SNP in STXBP5 revealed by CRISPR/Cas9 gene editing in mice. Arterioscler Thromb Vasc Biol . 2017;37(2):264–70. doi: 10.1161/ATVBAHA.116.308614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Q, Yamakuchi M, Ture S, et al. Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. J Clin Invest . 2014;124(10):4503–16. doi: 10.1172/JCI71245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair TS, Kozma KE, Hoefling NL, et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci . 2004;24(7):1772–9. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajanel A, Campbell RA, Denorme F. Platelet mitochondria: the mighty few. Curr Opin Hematol . 2023;30(5):167–74. doi: 10.1097/MOH.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]