Abstract
Live recombinant vesicular stomatitis viruses (VSVs) expressing foreign antigens are highly effective vaccine vectors. However, these vectors induce high-titer neutralizing antibody directed at the single VSV glycoprotein (G), and this antibody alone can prevent reinfection and boosting with the same vector. To determine if efficient boosting could be achieved by changing the G protein of the vector, we have developed two new recombinant VSV vectors based on the VSV Indiana serotype but with the G protein gene replaced with G genes from two other VSV serotypes, New Jersey and Chandipura. These G protein exchange vectors grew to titers equivalent to wild-type VSV and induced similar neutralizing titers to themselves but no cross-neutralizing antibodies to the other two serotypes. The effectiveness of these recombinant VSV vectors was illustrated in experiments in which sequential boosting of mice with the three vectors, all encoding the same primary human immunodeficiency virus (HIV) envelope protein, gave a fourfold increase in antibody titer to an oligomeric HIV envelope compared with the response in animals receiving the same vector three times. In addition, only the animals boosted with the exchange vectors produced antibodies neutralizing the autologous HIV primary isolate. These VSV envelope exchange vectors have potential as vaccines in immunizations when boosting of immune responses may be essential.
Vesicular stomatitis virus (VSV) is the prototype of the family Rhabdoviridae. The 11-kb, nonsegmented, negative-strand RNA genome of VSV encodes five mRNAs directing synthesis of four internal structural proteins called the nucleocapsid (N), phosphoprotein (P), matrix protein (M), and polymerase (L), as well as one transmembrane glycoprotein (G) exposed on the exterior of the virion (39).
Earlier studies from our laboratory have established that VSV can serve as a highly effective vaccine vector. For example, a single intranasal (i.n.) inoculation with a VSV recombinant expressing influenza virus hemagglutinin (HA) generates serum neutralizing antibody titers to influenza virus of 1:4,000 and completely protects mice from a normally lethal influenza virus (32, 33). Also, inoculation with a VSV recombinant expressing the measles virus H protein induces higher measles virus neutralizing titers than measles virus itself in cotton rats and can immunize cotton rats against measles even in the presence of passively transferred “maternal” antibodies to measles virus (36). Replication of the VSV vectors given i.n. is required to generate an immune response, since UV-inactivated virus is not effective in generating neutralizing antibodies or protecting from a lethal virus challenge (33).
Both antibody and cellular immune responses are likely to be important in the development of immunity to human immunodeficiency virus (HIV) (3, 25). Because of the strong antibody and cellular immune responses induced by VSV vectors (21, 32, 33, 41) we have begun investigating the possibility of using recombinant VSV as an AIDS vaccine. Recombinant VSVs which express functional HIV envelope proteins from primary (patient-derived) and laboratory-adapted HIV strains have been prepared (18). In addition, a single VSV recombinant can be made to express both HIV Gag and Env proteins from separate genes (15). HIV neutralizing antibodies are directed to the HIV envelope protein (29, 40), while cytotoxic T cells recognize epitopes in multiple HIV proteins, including Env and Gag (1, 14).
Initial studies from our laboratory showed that i.n. vaccination of mice with a VSV encoding the envelope protein of the laboratory-adapted HIV IIIb strain induced serum HIV neutralizing antibody titers as high as 1:125 (11a). However, subsequent studies using a VSV encoding the envelope of the primary HIV isolate 89.6 showed induction of antibodies to this envelope protein but no detectable neutralizing antibodies even after multiple inoculations. It is often more difficult to neutralize infection by primary HIV isolates compared with laboratory-adapted HIV strains, although a wide range of neutralization sensitivities exists in both (4, 28). To determine if VSV vectors could be modified to allow boosting of immune responses and generation of neutralizing antibodies to the HIV 89.6 envelope protein, we developed a new vector strategy based on VSV vectors expressing G proteins from different serotypes.
A basic limitation of live viral vector systems is that animals develop neutralizing antibodies to the vector after the first vaccination and these antibodies prevent subsequent boosting (25, 32). Such vector-directed immunity normally dictates an alternate means of boosting, such as using more than one type of vector or boosting with purified protein (25). In the case of VSV, neutralizing antibodies are directed to the single G glycoprotein (20), and these anti-G antibodies are highly effective at preventing reinfection (32). To get around such neutralizing antibodies, we have generated VSV envelope exchange vectors that allow effective boosting even in the presence of neutralizing antibodies directed against the first vector. Our original VSV vectors had the genes encoding the N, P, M, G, and L proteins all derived from the VSV Indiana (I) serotype (23, 37). The G protein exchange vectors described here have the same N, P, M, and L genes but express a G protein from either the New Jersey (NJ) or Chandipura (Ch) serotype of VSV. These G proteins do not generate cross-neutralizing antibodies and allow reinfection and effective boosting of antibody responses to foreign proteins encoded by the vector.
MATERIALS AND METHODS
Plasmid construction.
A plasmid containing the Chandipura glycoprotein [G(Ch)] gene (27) was kindly provided by Amiya Banerjee. To construct the VSV vector containing the G(Ch) gene in place of the Indiana glycoprotein [G(I)] gene, we first eliminated an XhoI site from within the G(Ch) gene using oligonucleotide-directed mutagenesis with the complementary primers 5′-CCCCTAGTGGGATCTCCAGTGATATTTGGAC and 5′-GTCCAAATATCACTGGAGATCCCACTAGGGG and the Stratagene QuikChange mutagenesis kit. The mutation of CTCGAG to CTCCAG eliminated the XhoI site without affecting the amino acid sequence of the G(Ch) protein. The gene was then amplified by PCR using Vent DNA polymerase (New England Biolabs). The forward primer was 5′-GATCGATCGAATTCACGCGTAACATGACTTCTTCAG, containing an MluI site (underlined) upstream of the ATG initiation codon for the G(Ch) protein. The reverse primer was 5′-GGAACGGTCGACGCGCC TCGAGCGTGATATCTGTTAGTTTTTTTCATATCATGTTGTTGGGCTTG AAGATC and contained SalI and XhoI sites (bold), followed by VSV transcription start and stop signals (underlined), followed by the complement of the 3′ coding sequence of G(Ch). The PCR product was digested with MluI and SalI and cloned into the pVSVXN-1 vector (37) that had been digested with MluI and XhoI to remove the VSV G(I) coding sequence (SalI and XhoI leave compatible ends for ligation). The plasmid derived by this method was designated pVSV(GCh)XN-1 and contains an expression site for foreign genes flanked by unique XhoI and NheI sites between the G(Ch) gene and the L gene.
A procedure identical to that described above was used to generate the vector containing the G(NJ) protein gene from plasmid pNJG (13). The forward primer was 5′-GATCGATCGAATTCACGCGTAATATGTTGTCTTATCTAATCTTTGC, and the reverse primer was 5′-GGAACGGTCGACGCGCCTCGAGCGTG ATATCTGTTAGTTTTTTTCATATTAACGGAAATGAGCCATTTCCACG. The sites indicated by bold letters and the underlined sequences are as described for the Chandipura construction above, and the subsequent cloning steps were also as described above. The final vector plasmid derived was designated pVSV(GNJ)XN-1 and contains an expression site for foreign genes flanked by unique XhoI and NheI sites between the G(NJ) gene and the VSV L gene.
To generate the above vectors containing the HIV Env 89.6 G gene, the gene encoding the 89.6 envelope protein with the VSV G cytoplasmic tail was excised with XhoI and NheI from pVSV-89.6gp160G (18) and cloned between the XhoI and NheI sites in vector pVSV(GNJ)XN-1 or pVSV(GCh)XN-1. This gp160G gene encodes all of gp120 and the ecto- and transmembrane domains of gp41 and has four amino acids of the 89.6 Env cytoplasmic domain (N-R-V-R) fused to the 26 C-terminal amino acids of the VSV G cytoplasmic domain, beginning with the sequence I-H-L-C (18).
Recoveries of recombinant viruses.
Recombinant VSVs were recovered using established methods (23, 37). Briefly, baby hamster kidney (BHK) cells were grown to approximately 60% confluency on 10-cm dishes. The cells were then infected at a multiplicity of infection (MOI) of 10 with vTF7-3, a recombinant vaccinia virus that expresses T7 RNA polymerase (12). After 1 h, each dish of cells was transfected with 3 μg of pBS-N, 5 μg of pBS-P, 1 μg of pBS-L, and 10 μg of the plasmid encoding one of the three full-length recombinants described above. Transfections were performed with a cationic liposome reagent containing dimethyldioctadecyl ammonium bromide and dioleyl-phosphatidylethanolamine (34). Cells were then incubated at 37°C for 48 h. Cell supernatants were passed through a 0.2-μm filter to remove the majority of the vaccinia virus and then applied to fresh BHK cells for an additional 48 h at 37°C. For some recoveries, an additional plasmid encoding VSV G (pBS-G), 4 μg/plate, was included with the N, P, and L support plasmids. Recovery of infectious virus was confirmed by scanning BHK cell monolayers for VSV cytopathic effect. Virus plaques were then isolated on BHK cells, and virus stocks from individual plaques were grown by adding virus from a single plaque to a 10-cm-diameter plate of BHK cells. These stocks were then stored at −80°C. The titers obtained for VSV(GI)-89.6G, VSV(GCh)-89.6G, and VSV(GNJ)-89.6G were all in the range of 107 to 108 PFU/ml after freezing and thawing, a procedure that reduces VSV titers approximately threefold.
Metabolic labeling of infected cells.
BHK cells on 35-mm-diameter plates (nearly confluent) were infected with wild-type (wt) VSV or the recombinant viruses at an MOI of approximately 10. Plates were incubated at 37°C for 4 h or until cytopathic effect was seen. The medium was removed, and the cells were washed twice with warmed, methionine-free Dulbecco's modified Eagle's medium (DMEM). Then, 1 ml of methionine-free DMEM containing 100 μCi of [35S]methionine was added to each plate for 1 h at 37°C. To prepare labeled cell extracts, the medium was removed, and the cells were washed twice with phosphate-buffered saline (PBS) and lysed in 0.5 ml of detergent solution (1% Nonidet P-40, 0.4% deoxycholate, 50 mM Tris-HCl [pH 8], 62.5 mM EDTA) on ice for 5 min. The cell lysates were collected into 1.5-ml Eppendorf tubes and centrifuged for 2 min at 14,000 rpm in an Eppendorf microcentrifuge. Labeled lysates were analyzed by electrophoresis on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel.
Inoculation of mice.
Female BALB/c mice 5 to 6 weeks old (Charles River Laboratories) (five to seven mice per experimental or control group) were housed in filter/isolette cages upon arrival. Mice were inoculated no earlier than 4 days after arrival. Prior to inoculation (day 0), mice were lightly anesthetized with Metofane (Mallinckrodt Veterinary, Inc., Mundelein, Ill.) and marked by ear punch. Twenty-five microliters of virus inoculum (105 PFU) in DMEM was delivered i.n. by 200-μl pipette to anesthetized mice. Mice were weighed in a plastic beaker to ±0.02 g. Subsequent i.n. boosts were given in an identical manner. Mice were observed and weighed on a daily basis. For intraperitoneal (i.p.) boosting with VSV vectors, 200 μl of DMEM containing 6.25 × 106 PFU of each of the three purified viruses was injected. Vaccinia virus encoding HIV Env 89.6 protein was generously provided by Robert Doms and administered by i.p. injection of 2.0 × 106 PFU in 100 μl of DMEM.
Virus purification for i.p. boost.
BHK cells were infected with VSV recombinants at an MOI of 0.1 for 20 h. The medium from the infected cells was collected and clarified by centrifugation at 2,000 × g for 10 min. Virus in the supernatant was pelleted by centrifugation in a Beckman ultracentrifuge using a 19K rotor at 18,000 rpm for 90 min at 4°C. The virus pellets were resuspended in PBS, loaded onto 10% (wt/vol) sucrose in PBS, and centrifuged at 4°C and 39,000 rpm for 65 min using an SW41 rotor. The virus pellets were resuspended in PBS, aliquoted, and stored at −80°C. The titers of the purified viruses were determined by plaque assays on BHK cells with an overlay of 1% methylcellulose in DMEM supplemented with 5% fetal bovine serum (FBS).
Preparation of sera.
Blood samples from inoculated mice were collected and allowed to clot at 4°C overnight. Clots were removed, and samples were centrifuged in a TOMY MTX-150 centrifuge (TMA-11 fixed-angle rotor) at 4°C for 10 min at 5,500 rpm. Clarified sera were transferred to sterile Eppendorf tubes and heat inactivated at 56°C for 1 h. Blood samples from mice inoculated with the same virus constructs (or with DMEM) were pooled.
Antigen for ELISAs.
The HIV 89.6 gp140 envelope protein for the enzyme-linked immunosorbent assays (ELISAs) was derived from the primary isolate HIV 89.6 (5) and expressed by recombinant vaccinia virus vector vBD1 (R. Doms, unpublished). This virus expresses an oligomeric gp140 protein containing the extracellular domains of gp120 and gp41 but lacking the transmembrane and cytoplasmic domains of gp41. The protein is secreted into the medium of vBD1-infected cells. To produce gp140 protein, 293 cells (human embryonic kidney, epithelial) were infected with vBD1 at an MOI of 4. The infecting virus was replaced with serum-free DMEM at 3 h postinfection. The medium containing gp140 was collected at 24 h postinfection and clarified by centrifugation at 2,000 × g for 5 min. The optimal amount of gp140 added to each well was determined by ELISA, using different amounts of supernatant together with a fixed amount of mouse serum positive for HIV envelope antibodies. In subsequent ELISAs, reported here, 30 μl of the gp140 supernatant was added to each well.
ELISAs.
ELISAs were performed essentially as described previously (31). Costar 96-well plates were first coated with 0.1 mg of concanavalin A (ConA; Sigma) per ml in 20 mM Tris-HCl–1 M NaCl (pH 8.5) for 2 h at room temperature. In the next step, gp140 diluted in PBS was added. After the binding of gp140 to ConA overnight at 4°C, a blocking step with PBS containing 10% calf serum (blocking solution) was carried out for 30 min. Each mouse serum was diluted twofold with blocking solution from 1:100 to 1:800 and added to the wells. Plates were incubated at room temperature for 2 h. The secondary antibody, biotinylated goat anti-mouse immunoglobulin antibody (Pierce), was diluted 1:100,000 in blocking solution. After 0.5 h of incubation with the secondary antibody, horseradish peroxidase-conjugated avidin (Pierce) was added to the incubation at a final concentration of 1:10,000, and the plates were incubated for an additional hour at room temperature. The substrate used for colorimetric analysis was 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt tablets, 10 mg/tablet (Immunopure ABTS; Pierce). Following each incubation step, wells were washed three times with 200 μl of PBS–0.05% Tween 20. Following conjugate incubation, a single 2× PBS–0.05% Tween high-salt wash was performed to lower background binding (22). This wash was followed by three washes with 1× PBS–0.05% Tween 20 wash solution. The volume added to the wells in each step was 100 μl except for the blocking step, where a volume of 200 μl/well was added. All incubations except the binding of gp140 were carried out at room temperature. Optical densities were determined at a wavelength of 405 nm in a BioRad ELISA plate reader.
VSV neutralization assays.
We used VSV Indiana or VSV New Jersey to detect antibodies neutralizing the corresponding G proteins. To detect antibodies neutralizing the G(Ch) protein, a recombinant VSV Indiana expressing the G(Ch) in place of the G(I) was used. Neutralization assays were performed as follows. Mouse sera were diluted with PBS in a volume of 50 μl in serial twofold dilutions in 96-well plates. Fifty microliters of each virus (approximately 100 PFU) in serum-free DMEM was added to the diluted serum in each well. The 96-well plates containing serum and virus were incubated at 37°C for 1 h. Approximately 1,500 BHK cells in 100 μl of DMEM–10% FBS were then added to each well. The plates were incubated at 37°C and 5% CO2 for 2 to 3 days. Each assay was performed in duplicate. Neutralizing titers are given as the highest dilutions which correspond to complete inhibition of VSV cytopathic effect. All duplicate assays agreed within one dilution.
Detection of neutralizing antibody recognizing the HIV-1 envelope.
Assays detecting neutralization of the HIV envelope were performed essentially as described previously (2). Approximately 20,000 HeLa T4 cells were plated per well on 96-well plates on the day before the assay to give confluent monolayers. VSVΔG-89.6G-GFP was diluted in DMEM–10% FBS to a final concentration of approximately 100 infectious units per 25 μl. To neutralize traces of infectivity due to residual G protein (2), the virus mixture was incubated for 10 min at room temperature with the I1 monoclonal (24) antibody against VSV G at a concentration of 1 μl/ml. Mouse sera were diluted with DMEM–10% FBS in serial twofold dilutions from 1:10 to 1:640. Equal volumes of diluted virus and diluted antibody were mixed and incubated for 30 min at 37°C, and 50 μl of each mixture was added to the HeLa T4 cells in duplicate wells containing 150 μl of DMEM–10% FBS. At 18 to 20 h postinfection, green fluorescent protein (GFP)-positive cells and syncytia were counted by fluorescence microscopy, using an Olympus CK40 microscope with a 4× objective.
RESULTS
Recovery of recombinant VSVs expressing alternative G proteins.
To generate recombinant VSV vectors that would not be neutralized by antibodies to the VSV Indiana serotype, we prepared plasmid vector DNA constructs representing the full-length VSV Indiana genome but with the G(I) protein gene replaced with the G protein gene from the VSV Chandipura or VSV New Jersey serotype (see Materials and Methods). The new vectors were designed with unique XhoI and NheI sites for cloning of foreign genes to be expressed downstream of the G gene, just as in the original VSV Indiana vector (37). Because the different VSV serotypes do not generate cross-neutralizing antibodies, we thought that viruses derived from these plasmids might be suitable for boosting immune responses in animals previously infected with the VSV Indiana vector. Because we wanted to use these vectors to examine immune responses to an HIV envelope from primary isolate 89.6 (5), a gene encoding the HIV Env (89.6) protein with its cytoplasmic domain replaced with that of the VSV G protein (18) was cloned into both the Chandipura and New Jersey vectors. This hybrid protein is designated EnvG. The presence of the G cytoplasmic domain allows incorporation of the HIV Env protein into the VSV virions (18).
Recombinant viruses were recovered from the infectious clones using standard procedures that involve expression of the full-length antigenomic recombinant VSV RNA in cells also expressing the VSV N, P, and L proteins (23). The antigenomic RNA is packaged into nucleocapsids containing the N, P, and L proteins. These nucleocapsids are templates for replication by the L-P polymerase complex, forming nucleocapsids containing the negative-strand genomic RNA. After transcription of these nucleocapsids and translation of the viral mRNAs, progeny recombinant VSVs are released from the cells. The VSV vector G(I), encoding the HIV 89.6 EnvG hybrid protein, was described previously (18). The structures of the recombinant viral genomes and the viruses encoded are diagrammed in Fig. 1. The titers obtained for the new recombinants were similar to those obtained for the original VSV recombinant encoding the HIV EnvG envelope, indicating that the foreign glycoproteins are compatible with normal VSV assembly and replication.
FIG. 1.
Diagram of VSV G protein exchange vectors. A schematic representation of the negative-strand recombinant VSV genomic RNAs of the G protein exchange vectors is shown at the top, indicating the gene order 3′ to 5′. The proteins expressed by the recombinants are depicted by the symbols above the RNA genome. A gene encoding the HIV (89.6) envelope with its cytoplasmic tail replaced by the cytoplasmic tail of VSV G was cloned into the three different infectious VSV plasmids downstream from the indicated VSV G protein genes. The different G protein trimers are shown with distinct shading patterns, as indicated. Diagrams of the three recombinant viruses are shown below. The HIV EnvG protein has been shown to be incorporated into the VSV virion, as indicated (18).
Protein expression in cells infected with recombinant VSVs.
To examine the expression of the VSV proteins and the HIV EnvG hybrid proteins encoded by each virus, we infected BHK cells with each recombinant or with wt VSV Indiana. Cells were labeled for 1 h with [35S] methionine at 4 h postinfection, and cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). VSV shuts off host cell protein synthesis after infection, and the proteins encoded by the VSV recombinants can therefore be visualized without immunoprecipitation. Figure 2 shows [35S] methionine-labeled lysates from BHK cells infected with wt VSV (control) or the three recombinants, indicated as G(I), G(Ch), and G(NJ). As expected, all five VSV proteins were expressed by each of the recombinants, and the mobilities of the N, P, M, and L proteins were identical for each vector. The mobilities of the VSV G proteins are different on SDS-PAGE, and these differences are readily apparent in Fig. 2. The G(NJ) protein migrates faster than G(I), and the G(Ch) protein migrates slower than G(I). The HIV EnvG protein was expressed at similar levels by all three recombinants.
FIG. 2.
Proteins encoded by VSV vectors. Plates of BHK cells were infected with each of the three recombinant envelope exchange viruses expressing HIV envelope for 4 h and then labeled for 1 h with 100 μCi of [35S] methionine. Cell extracts were prepared and electrophoresed on SDS–10% PAGE. All five VSV proteins (N, P, M, G, and L) are expressed by each recombinant virus. The different mobilities of each of the three VSV glycoproteins, indicated as G(I), G(Ch), and G(NJ), can be seen, and all three constructs express the Env G protein. Recombinant wt VSV (rwt) was used as a control.
Pathogenesis of recombinant VSVs in mice.
Infection of 6-week-old BALB/c mice with VSV recombinants results in significant weight loss and is a good measure of vector-associated pathogenesis (33). To examine the pathogenesis of the three recombinants expressing HIV EnvG, mice were weighed daily for 16 days after the initial i.n. inoculation with 105 PFU of each recombinant.
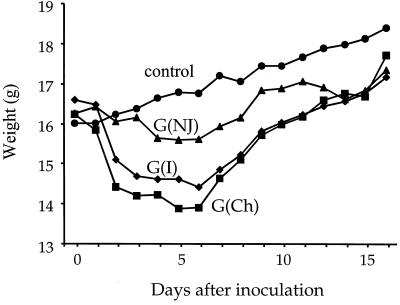
As shown in Fig. 3, the weight loss (average of five to seven mice in each group) was similar after inoculation of naive mice with Indiana or Chandipura vectors, while weight loss following inoculation with the New Jersey vector was less dramatic. The average maximum loss of initial body weight after inoculation with VSV (GI), VSV (GCh), and VSV (GNJ) was 15.2, 15.7, and 5%, respectively. However, an additional group of naive mice inoculated with VSV (GNJ) lost 10% of their initial body weight (not shown), and thus the pathogenesis with this vector may be similar to that with the other two. The mice inoculated with recombinant VSVs started regaining weight at 6 to 7 days after inoculation. A control group of mice that was inoculated with DMEM showed no weight loss.
FIG. 3.
Pathogenesis (weight loss) caused by VSV vectors in mice. Four groups of five to seven mice were inoculated i.n. on day 0 with DMEM (weight control) or with 105 PFU of each of the VSV G protein exchange vectors diagrammed in Fig. 1. After the inoculation, the mice were weighed daily, and average weights are presented.
Induction of neutralizing antibodies to the VSV G proteins after inoculation with each vector separately.
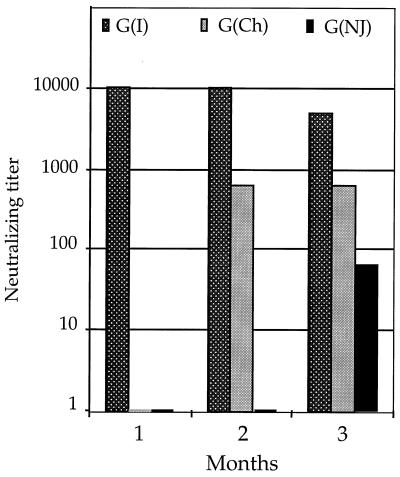
To examine the titers of neutralizing antibodies induced by each vector, mice that had been inoculated i.n. with 105 PFU of each recombinant were bled after 1 month. Pooled serum samples were used to perform virus neutralization assays by endpoint dilution. Serial dilutions of sera from inoculated mice were incubated in duplicate on 96-well plates with appropriate viruses, and BHK cells were then added to this mixture. Plates were incubated at 37°C for 2 to 3 days. Neutralization was scored as lack of cell killing by VSV and was also readily evident from the color (pH) of the medium. As shown in Fig. 4, the titers induced by the three different vectors at 1 month after inoculation (bar labeled 1) were similar, 1:10,240 for VSV (GI) and 1:5,120 for VSV (GCh) or VSV (GNJ). We also attempted to boost these animals with an identical i.n. inoculation of the same virus given 1 month after the initial inoculation. Neutralizing titers obtained 1 month after boosting with the identical vector (Fig. 4, bar labeled 2) showed no increase compared with the neutralizing titers obtained after the first inoculation. Furthermore, there was no weight loss associated with boosting. These results suggest that the mice were immune to reinfection with each vector and are consistent with earlier studies showing that i.n. boosting of immune responses with the VSV (GI) vector has no effect (33).
FIG. 4.
Neutralizing titers to VSV G proteins induced by VSV vectors after initial inoculation and boost with the same vector. Sera from mice inoculated i.n. with the three G protein exchange vectors expressing EnvG 89.6 (Fig. 1) were assayed for neutralizing titer to the homologous G protein at 1 month after inoculation. The mice were boosted with the identical vector at 1 month, and sera were assayed again at 2 months. Neutralizing titers induced to the VSV G proteins at 1 month after inoculation (bars numbered 1) were 1:10,240 to VSV G(I) and 1:5,120 to VSV G(Ch) or VSV G(NJ) and were unchanged 1 month after boost (bars numbered 2). There was no detectable cross-neutralization by heterologous sera (titer less than 1:8).
Sequential boosting with different vectors.
The effects of boosting with vectors expressing different VSV G proteins are shown in Fig. 5. Boosting with VSV (GCh) after an initial inoculation with VSV (GI) gave a neutralizing titer of 1:640 to VSV (GCh) at 1 month after the boost. This response was reduced about eightfold compared to what was obtained in naive mice with the same vector, but was still quite substantial. The second boost with the VSV (GNJ) vector following VSV (GI) and VSV (GCh) gave a titer of 1:64 to VSV New Jersey. This response was reduced 80-fold compared to what was obtained when the VSV (GNJ) vector was used in a naive mouse. Neither boost resulted in detectable weight loss or pathogenesis, consistent with reduced viral replication. Because we have detected no cross-neutralizing antibodies to our vectors in mouse sera, it is likely that the cell-mediated response is limiting replication of the vectors upon boosting and reduces the antibody response to the glycoproteins in sequential inoculations. The internal proteins encoded by each vector (N, P, M, and L) are identical, and it is likely that the cell-mediated response to amino acid sequence in one or more of these proteins or in the HIV EnvG protein may reduce viral replication in the sequential boosts.
FIG. 5.
Neutralizing titers induced to VSV vectors following sequential boosting with G protein exchange vectors. Groups of five to seven mice were inoculated sequentially i.n. with the G(I) vector, followed by boosting with the G(Ch) vector at 1 month and boosting with the G(NJ) vector at 2 months. Neutralizing antibody titers to all three VSV G proteins were determined in sera taken at 1, 2, and 3 months, as indicated. Bars at the baseline indicate undetectable cross-neutralization (titer of less than 1:8).
Induction of antibody to oligomeric HIV Env.
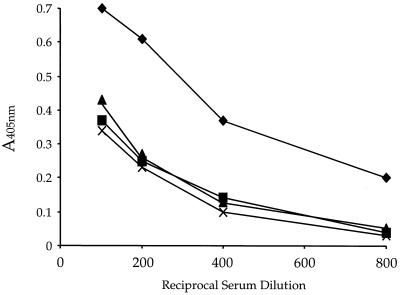
To follow the induction of antibodies to HIV EnvG protein encoded by each vector, we used a published ELISA to oligomeric gp140 (31). The quaternary structure of the oligomeric form of the HIV Env protein has been shown to be important for its antigenic integrity and consequently for binding of antibodies generated against it (9, 35). The lectin ConA, which binds to the terminal α-d-mannosyl and α-d-glucosyl residues of glycans (8), was used to bind gp140 to the plastic surface in order to have gp140 molecules bound in multiple orientations with multiple antibody-binding sites exposed. We first coated 96-well plates with ConA and then added oligomeric HIV gp140 envelope protein derived from a primary HIV 89.6 isolate and expressed from the vaccinia virus recombinant vBD1 (R. Doms, University of Pennsylvania, unpublished). Serial dilutions of sera from mice inoculated and boosted with the recombinant VSVs were added to the wells, and ELISA titers were detected as described in Materials and Methods. The results of the ELISAs are shown in Fig. 6. The mice gave identical antibody responses to HIV Env after three i.n. inoculations with each of the recombinant viruses given 1 month apart. Control experiments performed with only a single inoculation of the VSV (GI) EnvG vector showed that the same ELISA titers to gp140 were obtained after 3 months with only a single inoculation and no boosting and that titers continued to rise for up to 3 months after the initial inoculation (not shown). This result is consistent with immunity to the vector after a single inoculation and consequent lack of boosting of the humoral immune response to HIV Env. In contrast, sequential boosting with the three different vectors resulted in ELISA titers to HIV Env that were fourfold higher at an arbitrary cutoff of 0.25 absorbance units (Fig. 6). Other ELISAS have shown that the majority of the boost to HIV Env occurred after the boost with the VSV (GCh) vector.
FIG. 6.
Antibody titers to oligomeric HIV Env 89.6 determined by ELISA. Oligomeric HIV 89.6 gp140 envelope protein was bound to plates coated with ConA. Serial dilutions of sera from mice inoculated and boosted with the recombinant VSVs were added to the wells, and antibodies were detected using the ELISA method described in Materials and Methods. Antibody responses to HIV Env after three inoculations given 1 month apart with each of the recombinant viruses [×, G(I); ▴, G(Ch); and ▪, G(NJ)] were virtually identical. Sequential boosting with the three different vectors showed antibody titers to HIV Env that were fourfold higher at an absorbance of 0.25 (♦).
Neutralizing antibody titers to HIV Env after boosting.
To examine the induction of neutralizing antibodies to the HIV Env protein, we used an assay based on neutralization of a VSV recombinant designated VSVΔG-89.6G-GFP, lacking VSV G but encoding HIV EnvG (89.6) and GFP (2). This recombinant lacks VSV G and instead encodes a functional HIV Env protein with the same dual tropism as HIV 89.6 (2). The assay gives neutralization titers comparable to those of other more traditional and cumbersome assays for HIV neutralization without the risks associated with exposure to HIV-1 (2). Diluted mouse sera were mixed and incubated in duplicate on 96-well plates with approximately 100 infectious units of the virus VSVΔG-89.6G-GFP. This mixture of serum and virus was then added to HeLa T4 cells (26), which express the HIV receptor CD4 and the CXCR4 coreceptor (11). It should be noted that these assays therefore measure neutralization of virus using the CXCR4 coreceptor and not CCR5. From 18 to 20 h later, individual infected green fluorescent cells or green fluorescent syncytia were observed using a fluorescent microscope and counted. The titers are expressed as the maximal serum dilution that gave greater than 50% reduction of the number of GFP-positive cells or syncytia per well compared to a negative control serum from mice vaccinated with a recombinant VSV encoding influenza virus HA protein. Neutralizing antibodies were not detected at 1 month following the first inoculation and were not detected in the serum of mice boosted twice with the same vector even after 3 months (not shown). However, a positive neutralizing titer to HIV Env 89.6 G was detected at a 1:10 serum dilution after inoculation with the VSV (GI) vector followed by boosting with the VSV (GCh) vector (Fig. 7). A second boost with the VSV (GNJ) vector resulted in an increase in the neutralization titer to 1:20. A further boost, given i.p. using a mixture of equal titers of each of the three purified recombinant viruses, increased neutralizing antibody titers to 1:80 (Fig. 7) and increased ELISA titers to gp140 twofold (not shown). Furthermore, at a low (1:10) serum dilution, these sera even exhibited 100% virus neutralization.
FIG. 7.
Neutralizing antibody titers to HIV envelope protein. Mice were inoculated sequentially with all three VSV G protein exchange vectors encoding HIV EnvG 89.6 and then given a boost (i.p.) with a combination of all three vectors according to the time line shown in panel A. Panel B shows the titers of neutralizing antibody to HIV Env 89.6 measured using an assay based on neutralization of VSVΔG-89.6G-GFP, a virus which lacks the VSV G protein but expresses HIV EnvG 89.6 and GFP (2). Diluted mouse sera were mixed and incubated in duplicate 96-well plates with approximately 100 infectious units of VSVΔG-89.6G-GFP. Then this mixture of serum and virus was added to HeLa T4 cells. A reduction of >50% in the number of GFP-positive cells compared to control mouse serum was scored as positive neutralization.
Subsequent boosting with a vaccinia virus vector.
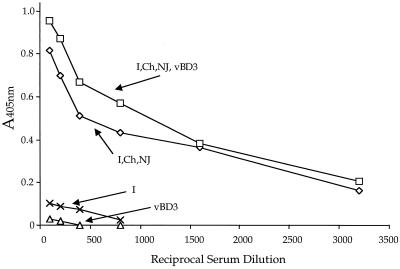
We next determined if the immune response to EnvG 89.6 protein raised by VSV vectors might be boosted further by a subsequent boost (i.p.) using a vaccinia virus vector encoding the same Env 89.6 protein. We boosted mice that had already received the three VSV vectors encoding EnvG 89.6 protein sequentially as well as the i.p. boost with the three VSV vectors combined as described in the legend to Fig. 7. These animals received an additional boost of 2 × 106 PFU of a vaccinia virus recombinant, vBD3, which encodes the HIV 89.6 Env protein (7). The serum antibody response was measured 1 month later by ELISA to HIV Env 89.6 gp140 (Fig. 8). There appeared to be a small increase (less than 20%) in antibody titer after boosting compared to serum from unboosted animals, but it was not evident at all antibody dilutions and may not be significant. Also, we observed no increase in the neutralizing antibody titer to HIV Env after boosting with vBD3. The ELISA antibody titer to Env 89.6 gp140 induced at 1 month by the vBD3 vector alone given i.p. in naive mice was barely detectable (Fig. 8). For comparison, the antibody response induced at 1 month by i.n. inoculation with the VSV vector encoding 89.6 EnvG is also shown and was low but readily detectable.
FIG. 8.
ELISA showing minimal effect of boosting with a vaccinia virus recombinant encoding 89.6 Env protein. Mice were inoculated sequentially with all three VSV G protein exchange vectors encoding HIV EnvG 89.6 and then boosted i.p. with a mixture of all three vectors as in Fig. 7. Mice were rested for 1 month and then boosted i.p. with 2 × 106 PFU of vaccina virus recombinant vBD3 encoding the HIV Env 89.6 protein. One month later, sera were collected from boosted and unboosted animals and assayed by ELISA for antibodies to oligomeric HIV Env 89.6 gp140. Results for serum from control mice vaccinated with vBD3 alone or VSV (GI) EnvG alone and assayed after 1 month are included for comparison.
DISCUSSION
Recombinant VSVs derived from the Indiana serotype and expressing foreign viral proteins have been established as highly effective vaccine vectors when used for i.n. vaccination in animal models (32, 33, 36). For expression of some antigens, a single vaccination with a recombinant VSV generates strong and long-lived protective immunity (32). When it is difficult to generate immunity to the foreign protein, boosting may be desirable. However, boosting of immune responses with the same recombinant VSV is not effective because the strong neutralizing antibody response to the VSV G protein prevents productive reinfection. Here we have described development of new VSV vectors that evade these antibodies and allow boosting of the immune response.
The vectors described here, VSV (GI), VSV (GCh) and VSV (GNJ), do not elicit cross-neutralizing antibodies to their divergent G proteins. Each vector was engineered to encode an HIV envelope protein from the primary isolate 89.6. When inoculated i.n. in mice, the vectors all induced similar neutralizing titers to the parent virus G protein but no cross-neutralizing antibody. They also generated antibodies to the HIV Env protein, as measured in an ELISA, but they did not generate detectable neutralizing antibody to the HIV Env protein. Sequential boosting with the same vector generated no detectable increase in antibody titers to VSV or HIV, indicating immunity to the vector. However, inoculation with the Indiana vector followed by boosting with the Chandipura and New Jersey vectors generated a fourfold increase in antibody titers to HIV Env protein when measured by ELISA. More importantly, neutralizing antibody to the HIV Env protein was readily detectable after heterologous boosting but was not observed without boosting or following a homologous boost.
The amino acid sequences of the G(I) and G(NJ) proteins are about 50% identical (13). These G proteins are slightly less related to the G(Ch) protein than they are to each other (27), with both showing approximately 40% sequence identity with G(Ch). The humoral immune response to the G(Ch) vector obtained after inoculation into a mouse that had previously been vaccinated with the G(I) vector was reduced eightfold compared to the response to the Chandipura vector seen in a naive mouse. A similar result was obtained when the G(I) vector was followed by the G(NJ) vector, indicating that the extent of relatedness of the glycoproteins is not a major factor in reducing the humoral immune response to the second vector. We think it most likely that the reduced responses to the second glycoprotein are due to cytotoxic T lymphocyte (CTL) responses to the four internal VSV proteins and perhaps also to the HIV Env protein encoded by all three vectors. These responses, although unable to block reinfection directly, would likely inhibit replication of the vector by killing infected cells and limiting virus spread. Such responses would be expected to increase after the second boost and could explain the 64-fold reduction in immune response to the G(NJ) protein seen when the VSV(GNJ) vector is used as the second boost following the Indiana and Chandipura vectors. Another possibility is that nonneutralizing cross-reactive antibodies to the different VSV G proteins are playing a role in the decreasing immune responses in sequential boosts. Such nonneutralizing antibodies have been shown to be protective against VSV infection in mouse models (24).
Concurrent studies of VSV vectors encoding HIV Env protein in rhesus macaques have confirmed the usefulness of the G protein exchange vectors in primates. In this system, 10- to 100-fold increases in antibody responses to HIV Env and neutralizing antibody were seen after a boost with the G(Ch) vector encoding the HIV EnvG 89.6 protein in animals initially vaccinated with the G(I) vector (N. Rose, P. Marx, and J. Rose, unpublished data). The greater boosting efficiency could be due to species differences or to the fact that the boosts have been spaced farther apart (2 months instead of 1), allowing greater decrease in CTL activity prior to the boost.
Neutralizing antibodies to primary isolates often recognize complex epitopes specific to the oligomeric form of the envelope protein, and the ability of VSV vectors to encode correctly folded and functional HIV Env proteins that are incorporated into VSV particles (18) may contribute to their ability to elicit the neutralizing antibody response. This feature, combined with the strong boosting achieved with the G protein exchange vectors, may mimic the long-term immune stimulation and effective immunization achieved with live attenuated simian immunodeficiency virus (SIV) Δnef viruses (6) which replicate at low levels and eventually induce immunity to SIV.
Although we have not studied cell-mediated immune responses here, a previous study has established that VSV vectors induce excellent cytotoxic T-cell responses to encoded ovalbumin (21). Also, preliminary studies using major histocompatibility complex class I tetramers have established that VSV vectors encoding HIV-1 Gag and Env proteins induce a strong primary response to immunodominant Gag and Env peptides in mice (K. Haglund, E. Pamer, and J. Rose, unpublished results).
The immunization studies in mice using sequential inoculation with recombinant VSVs expressing HIV Env and different VSV glycoproteins are promising. Ongoing studies of the same vectors encoding both HIV Env and SIV Gag in rhesus macaques will allow challenge with a pathogenic SHIV89.6P, a hybrid containing the HIV 89.6 env gene and the remaining genes from SIV, which became pathogenic after several passages in monkeys (30). Challenge studies are not possible in mice because primate lentiviruses do not replicate in mice. Successful outcomes of challenge studies in monkeys would warrant preliminary studies of recombinant VSV vectors in humans.
VSV is a natural pathogen of cattle, in which it causes a self-limited disease most often characterized by vesicular lesions of the mouth and tongue. In cattle-growing regions of Panama where many animals are infected with VSV, human seropositivity to VSV is as high as 94%, but VSV infection is not associated with serious human disease in this area (38). Febrile, flu-like illness associated with VSV has been reported in animal handlers and laboratory workers (10, 16, 17, 19). Recombinant VSVs derived from plasmid DNA are attenuated relative to the wt virus in mice, and we have not observed any pathogenesis or weight loss associated with inoculation of rhesus macaques by oral, i.n., or intramuscular routes with up to 5 × 107 PFU of live VSV recombinants (P. Marx, N. Rose, and J. Rose, unpublished results). The low seroprevalence of VSV antibodies in the general human population, the strong immune responses induced, and the lack of pathogenesis suggest that VSV vectors should be given serious consideration for use in humans, especially against pathogens as formidable as HIV-1.
ACKNOWLEDGMENTS
We thank all members of the Rose laboratory for helpful support and suggestions during the course of this work. We thank Robert Doms for advice on setting up the ELISA, for performing some preliminary assays, and for providing the vaccinia virus recombinants encoding soluble oligomeric HIV Env gp140 (89.6) and gp160 (89.6).
This work was supported by grants from the National Institutes of Health to J.K.R. A.R. was supported by a fellowship from the Cancer Research Institute.
REFERENCES
- 1.Berzofsky J A, Ahlers J D, Derby M A, Pendleton C D, Arichi T, Belyakov I M. Approaches to improve engineered vaccines for human immnodeficiency virus and other viruses that cause chronic infections. Immunol Rev. 1999;177:151–172. doi: 10.1111/j.1600-065x.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 2.Boritz E, Gerlach J, Johnson J E, Rose J K. Replication-competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway. J Virol. 1999;73:6937–6945. doi: 10.1128/jvi.73.8.6937-6945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecilia D, KewalRamani V N, O'Leary J, Volsky B, Nyambi P, Burda S, Xu S, Littman D R, Zolla-Pazner S. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 7.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta- chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cel. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 8.Dulaney J T. Binding interactions of glycoproteins with lectins. Mol Cell Biochem. 1978;21:43–63. doi: 10.1007/BF00230195. [DOI] [PubMed] [Google Scholar]
- 9.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellowes O N, Dimopoullos G T, Callis J J. Isolation of vesicular stomatitis virus from an infected laboratory worker. Am J Vet Res. 1955;16:623–626. [PubMed] [Google Scholar]
- 11.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 11a.Forman J. Recombinant vesicular stomatitis viruses. M.D. thesis. New Haven, Conn: Yale University; 1998. [Google Scholar]
- 12.Fuerst T R, Earl P L, Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987;7:2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallione C J, Rose J K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983;46:162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotch F M, Koup R A, Safrit J T. New observations on cellular immune responses to HIV and T-cell epitopes. AIDS. 1997;11:S99–S107. [PubMed] [Google Scholar]
- 15.Haglund K, Foman J, Krausslich H G, Rose J K. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology. 2000;268:112–121. doi: 10.1006/viro.1999.0120. [DOI] [PubMed] [Google Scholar]
- 16.Hanson R P, Brandly C A. Epizootiology of vesicular stomatitis. Am J Public Health. 1957;47:205–209. doi: 10.2105/ajph.47.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson R P, Rasmussen A F, Jr, Brandly C A, Brown J W. Human infection with the virus of vesicular stomatitis. J Clin Lab Med. 1950;36:754–758. [PubMed] [Google Scholar]
- 18.Johnson J E, Schnell M J, Buonocore L, Rose J K. Specific targeting to CD4+cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J Virol. 1997;71:5060–5068. doi: 10.1128/jvi.71.7.5060-5068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson K M, Vogel J E, Peralta P H. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV) Am J Trop Med Hyg. 1966;15:244–246. doi: 10.4269/ajtmh.1966.15.244. [DOI] [PubMed] [Google Scholar]
- 20.Kelley J M, Emerson S U, Wagner R R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972;10:1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S K, Reed D S, Olson S, Schnell M J, Rose J K, Morton P A, Lefrancois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurstak E, Tijssen P, Kurstak C, Morisset R. Enzyme immunoassays and related procedures in diagnostic medical virology. Bull World Health Org. 1986;64:465–479. [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson N D, Stillman E A, Whitt M A, Rose J K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefrancois L, Lyles D S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982;121:168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- 25.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 26.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 27.Masters P S, Bhella R S, Butcher M, Patel B, Ghosh H P, Banerjee A K. Structure and expression of the glycoprotein gene of Chandipura virus. Virology. 1989;171:285–290. doi: 10.1016/0042-6822(89)90540-0. [DOI] [PubMed] [Google Scholar]
- 28.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas C F, 3rd, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson T M, Jr, Stryjewski B L, Broder C C, Hoxie J A, Mascola J R, Earl P L, Doms R W. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J Virol. 1996;70:753–762. doi: 10.1128/jvi.70.2.753-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts A, Buonocore L, Price R, Forman J, Rose J K. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73:3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts A, Kretzschmar E, Perkins A S, Forman J, Price R, Buonocore L, Kawaoka Y, Rose J K. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72:4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose J K, Buonocore L, Whitt M A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991;10:520–525. [PubMed] [Google Scholar]
- 35.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlereth B, Rose J K, Buonocore L, ter Meulen V, Niewiesk S. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J Virol. 2000;74:4652–4657. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnell M J, Buonocore L, Whitt M A, Rose J K. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70:2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesh R B, Peralta P H, Johnson K M. Ecological studies of vesicular stomatitis virus. I. Prevalence of infection among animals and humans living in an area of endemic VSV activity. Am J Epidem. 1969;90:255–261. doi: 10.1093/oxfordjournals.aje.a121068. [DOI] [PubMed] [Google Scholar]
- 39.Wagner R R, Rose J K. Rhabdoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1121–1135. [Google Scholar]
- 40.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 41.Zinkernagel R M, Adler B, Holland J J. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol. 1978;46:53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]