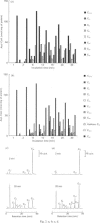
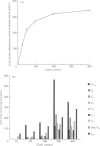
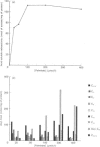
Abstract
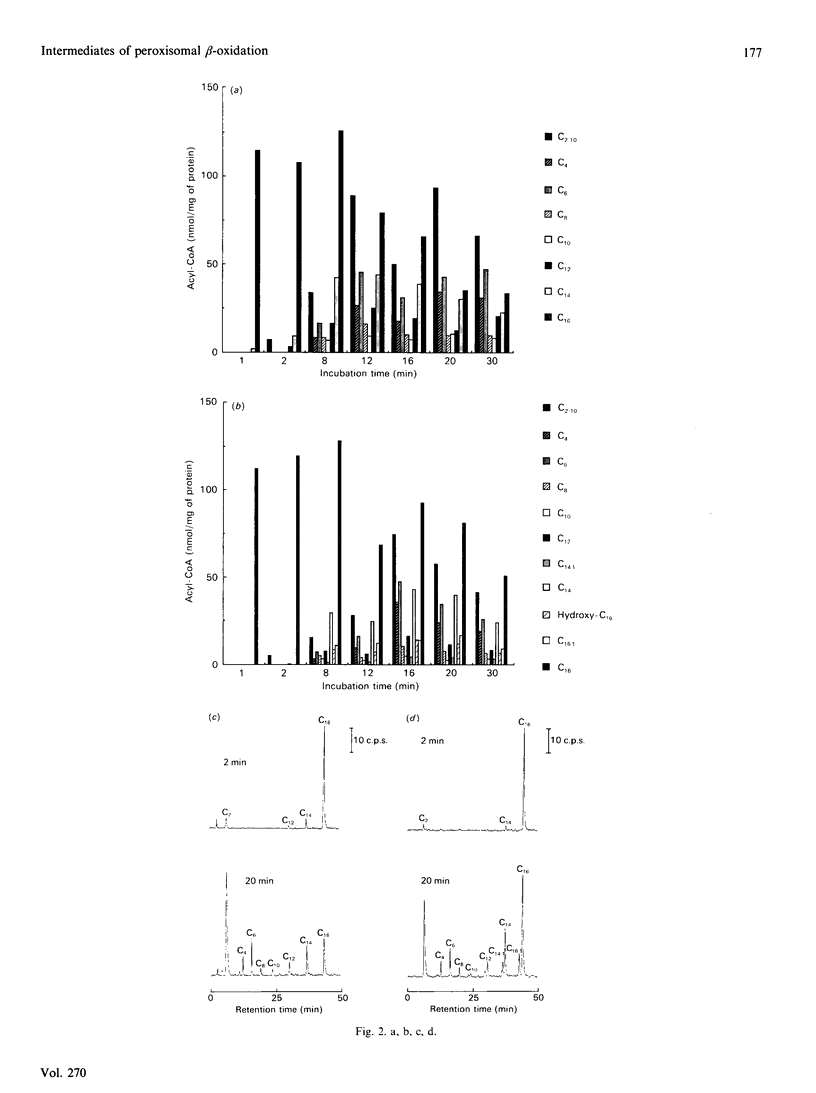
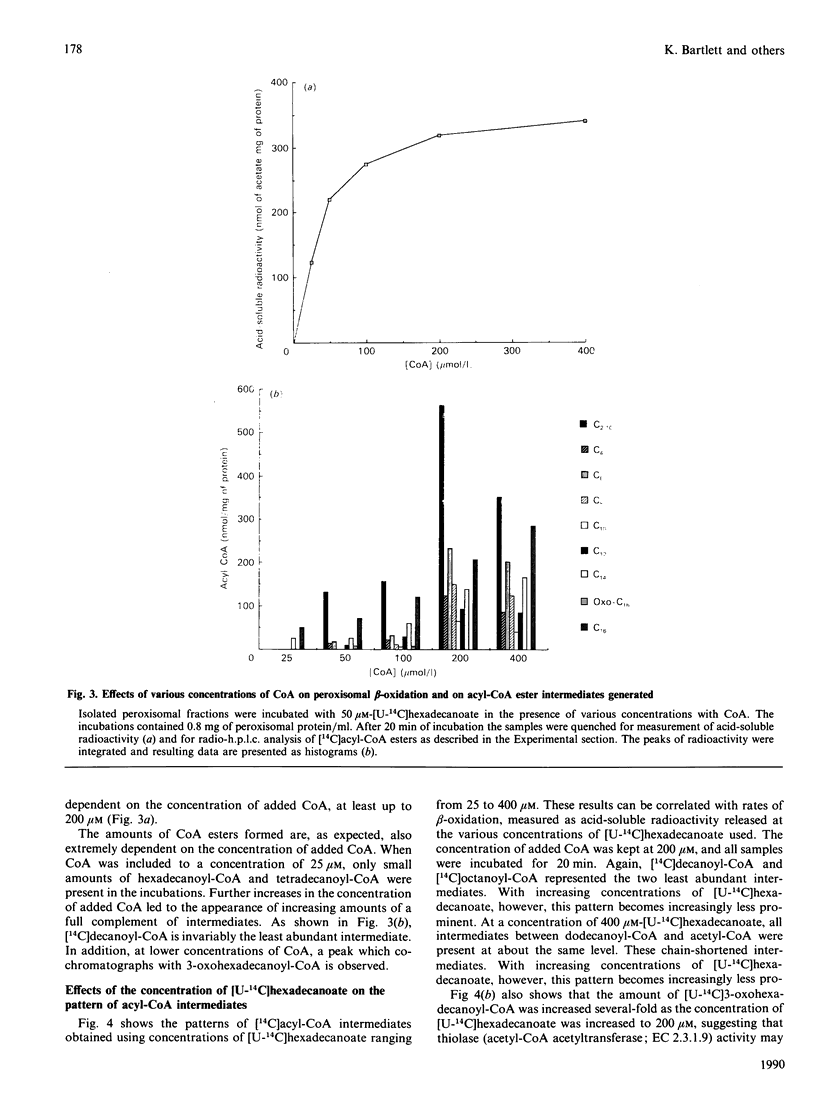
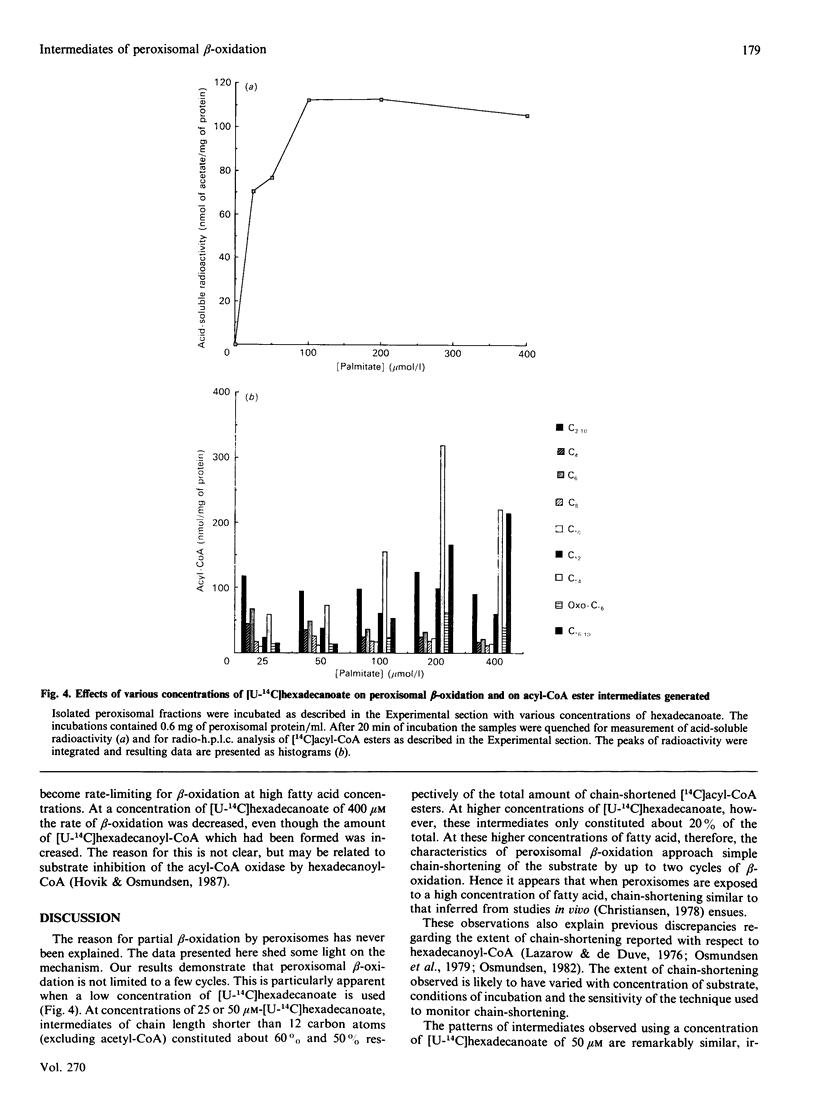
1. 14C-labelled fatty acyl-CoA esters resulting from beta-oxidation of [U-14C]hexadecanoate by peroxisomal fractions isolated from rats treated with clofibrate showed the presence of the full range of saturated intermediates down to acetyl-CoA. 2. The pattern of intermediates generated was fairly constant. At low concentrations of [U-14C]hexadecanoate (50 microM), decanoyl-CoA was present in lowest amounts. At higher concentrations of [U-14C]hexadecanoate (greater than 100 microM), all intermediates of chain length shorter than 12 carbon atoms (except acetyl-CoA) were present at similar low concentrations; the process of beta-oxidation now resembling chain-shortening of hexadecanoate by two cycles of beta-oxidation. 3. In the absence of an NAD(+)-regenerating system [pyruvate and lactate dehydrogenase (EC 1.1.1.28)] 2-enoyl- and 3-hydroxyacyl-CoA esters were generated, suggesting that re-oxidation of NADH is essential for optimal rates of peroxisomal beta-oxidation in vitro. 4. At high concentrations of [U-14C]hexadecanoate (greater than 100 microM), 3-oxohexadecanoyl-CoA was produced, suggesting that thiolase (acetyl-CoA acetyltransferase; EC 2.3.1.9) can become rate-limiting for peroxisomal beta-oxidation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexson S. E., Osmundsen H., Berge R. K. The presence of acyl-CoA hydrolase in rat brown-adipose-tissue peroxisomes. Biochem J. 1989 Aug 15;262(1):41–46. doi: 10.1042/bj2620041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen E. N., Thomassen M. S., Christiansen R. Z., Osmundsen H., Norum K. R. Metabolism of erucic acid in perfused rat liver: increased chain shortening after feeding partially hydrogenated marine oil and rapeseed oil. Lipids. 1979 Oct;14(10):829–835. doi: 10.1007/BF02534124. [DOI] [PubMed] [Google Scholar]
- Christiansen R. Z. The effect of clofibrate-feeding on hepatic fatty acid metabolism. Biochim Biophys Acta. 1978 Sep 28;530(3):314–324. doi: 10.1016/0005-2760(78)90151-0. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K., Kärki T., Hassinen I. E., Osmundsen H. beta-Oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2,4-dienoyl-coenzyme A reductase in peroxisomal beta-oxidation. J Biol Chem. 1986 Dec 15;261(35):16484–16493. [PubMed] [Google Scholar]
- Hovik R., Osmundsen H. Peroxisomal beta-oxidation of long-chain fatty acids possessing different extents of unsaturation. Biochem J. 1987 Nov 1;247(3):531–535. doi: 10.1042/bj2470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Leighton F., Bergseth S., Rørtveit T., Christiansen E. N., Bremer J. Free acetate production by rat hepatocytes during peroxisomal fatty acid and dicarboxylic acid oxidation. J Biol Chem. 1989 Jun 25;264(18):10347–10350. [PubMed] [Google Scholar]
- Neat C. E., Thomassen M. S., Osmundsen H. Effects of high-fat diets on hepatic fatty acid oxidation in the rat. Isolation of rat liver peroxisomes by vertical-rotor centrifugation by using a self-generated, iso-osmotic, Percoll gradient. Biochem J. 1981 Apr 15;196(1):149–159. doi: 10.1042/bj1960149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmundsen H. Factors which can influence beta-oxidation by peroxisomes isolated from livers of clofibrate treated rats. Some properties of peroxisomal fractions isolated in a self-generated Percoll gradient by vertical rotor centrifugation. Int J Biochem. 1982;14(10):905–914. doi: 10.1016/0020-711x(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Neat C. E., Norum K. R. Peroxisomal oxidation of long chain fatty acids. FEBS Lett. 1979 Mar 15;99(2):292–296. doi: 10.1016/0014-5793(79)80975-8. [DOI] [PubMed] [Google Scholar]
- Watmough N. J., Bhuiyan A. K., Bartlett K., Sherratt H. S., Turnbull D. M. Skeletal muscle mitochondrial beta-oxidation. A study of the products of oxidation of [U-14C]hexadecanoate by h.p.l.c. using continuous on-line radiochemical detection. Biochem J. 1988 Jul 15;253(2):541–547. doi: 10.1042/bj2530541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watmough N. J., Turnbull D. M., Sherratt H. S., Bartlett K. Measurement of the acyl-CoA intermediates of beta-oxidation by h.p.l.c. with on-line radiochemical and photodiode-array detection. Application to the study of [U-14C]hexadecanoate oxidation by intact rat liver mitochondria. Biochem J. 1989 Aug 15;262(1):261–269. doi: 10.1042/bj2620261. [DOI] [PMC free article] [PubMed] [Google Scholar]