Abstract
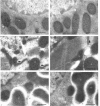
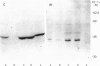
Observation and quantification of the catalytic subunit C of cyclic AMP-dependent protein kinases by immuno-gold electron microscopy suggested a high concentration of cyclic AMP-dependent protein kinases in mitochondria from liver, kidney, heart and skeletal muscle, pancreas, parotid gland and brain cells. The position of gold particles pointed to a localization in the inner membrane/matrix space. A similar distribution was obtained by immunolocalization of the cyclic AMP-dependent protein kinase regulatory subunits RI and RII in liver, pancreas and heart cells. The results indicated the presence of both the type I and the type II cyclic AMP-dependent protein kinases in mitochondria of hepatocytes, and the preferential occurrence of the type I protein kinase in mitochondria from exocrine pancreas and heart muscle. The immunocytochemical results were confirmed by immunochemical determination of cyclic AMP-dependent protein kinase subunits in fractionated tissues. Determinations by e.l.i.s.a. of the C-subunit in parotid gland cell fractions indicated about a 4-fold higher concentration of C-subunit in the mitochondria than in a crude 1200 g supernatant. Immunoblot analysis of subfractions from liver mitochondria supported the localization in situ of cyclic AMP-dependent protein kinases in the inner membrane/matrix space and suggested that the type I enzyme is anchored by its regulatory subunit to the inner membrane. In accordance with the immunoblot data, the specific activity of cyclic AMP-dependent protein kinase measured in the matrix fraction was about twice that measured in whole mitochondria. These findings indicate the importance of cyclic AMP-dependent protein kinases in the regulation of mitochondrial functions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. G., Schneider B. G., Papermaster D. S. Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem. 1984 Nov;32(11):1217–1223. doi: 10.1177/32.11.6436366. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Cyclic AMP stimulation of calcium efflux from isolated mitochondria: a negative report. J Membr Biol. 1976 Oct 20;29(1-2):209–210. doi: 10.1007/BF01868961. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Cyclic AMP stimulation of calcium efflux from kidney, liver and heart mitochondria. J Membr Biol. 1974;16(3):221–236. doi: 10.1007/BF01872416. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Burgess J. W., Yamada E. W. cAMP-dependent protein kinase isozymes with preference for histone H2B as substrate in mitochondria of bovine heart. Biochem Cell Biol. 1987 Feb;65(2):137–143. doi: 10.1139/o87-019. [DOI] [PubMed] [Google Scholar]
- Cameron R. S., Castle J. D. Isolation and compositional analysis of secretion granules and their membrane subfraction from the rat parotid gland. J Membr Biol. 1984;79(2):127–144. doi: 10.1007/BF01872117. [DOI] [PubMed] [Google Scholar]
- Caron M. G., Goldstein S., Savard K., Marsh J. M. Protein kinase stimulation of a reconstituted cholesterol side chain cleavage enzyme system in the bovine corpus luteum. J Biol Chem. 1975 Jul 10;250(13):5137–5143. [PubMed] [Google Scholar]
- Cercek B., Houslay M. D. Submitochondrial localization and asymmetric disposition of two peripheral cyclic nucleotide phosphodiesterases. Biochem J. 1982 Oct 1;207(1):123–132. doi: 10.1042/bj2070123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. J., Walsh D. A. Multiple forms of hepatic adenosine 3':5'-monophosphate dependent protein kinase. Biochemistry. 1971 Sep 14;10(19):3614–3621. doi: 10.1021/bi00795a020. [DOI] [PubMed] [Google Scholar]
- Churchill P. F., Kimura T. Topological studies of cytochromes P-450scc and P-45011 beta in bovine adrenocortical inner mitochondrial membranes. Effects of controlled tryptic digestion. J Biol Chem. 1979 Oct 25;254(20):10443–10448. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Defaye G., Monnier N., Guidicelli C., Chambaz E. M. Phosphorylation of purified mitochondrial cytochromes P-450 (cholesterol desmolase and 11 beta-hydroxylase) from bovine adrenal cortex. Mol Cell Endocrinol. 1982 Jul;27(2):157–168. doi: 10.1016/0303-7207(82)90105-8. [DOI] [PubMed] [Google Scholar]
- Deviller P., Vallier P., Bata J., Saez J. M. Distribution and characterization of cAMP-dependent protein kinase isoenzymes in bovine adrenal cells. Mol Cell Endocrinol. 1984 Nov;38(1):21–30. doi: 10.1016/0303-7207(84)90141-2. [DOI] [PubMed] [Google Scholar]
- Dimino M. J., Bieszczad R. R., Rowe M. J. Cyclic AMP-dependent protein kinase in mitochondria and cytosol from different-sized follicles and corpora lutea of porcine ovaries. J Biol Chem. 1981 Nov 10;256(21):10876–10882. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K. [Cyclic AMP-dependent protein kinase in rat adrenal mitochondrial fraction--studies on the mechanism of ACTH action (II) (author's transl)]. Nihon Naibunpi Gakkai Zasshi. 1977 Sep 20;53(9):1094–1105. doi: 10.1507/endocrine1927.53.9_1094. [DOI] [PubMed] [Google Scholar]
- Henriksson T., Jergil B. Protein kinase activity and endogenous phosphorylation in subfractions of rat liver mitochondria. Biochim Biophys Acta. 1979 Dec 11;588(3):380–391. doi: 10.1016/0304-4165(79)90346-5. [DOI] [PubMed] [Google Scholar]
- Jahn R., Unger C., Söling H. D. Specific protein phosphorylation during stimulation of amylase secretion by beta-agonists or dibutyryl adenosine 3',5'-monophosphate in the rat parotid gland. Eur J Biochem. 1980 Nov;112(2):345–352. doi: 10.1111/j.1432-1033.1980.tb07211.x. [DOI] [PubMed] [Google Scholar]
- Joachim S., Schwoch G. Immunoelectron microscopic localization of catalytic and regulatory subunits of cAMP-dependent protein kinases in the parotid gland. Eur J Cell Biol. 1988 Aug;46(3):491–498. [PubMed] [Google Scholar]
- Joachim S., Schwoch G. Localization of cAMP-dependent protein kinase subunits along the secretory pathway in pancreatic and parotid acinar cells and accumulation of the catalytic subunit in parotid secretory granules following beta-adrenergic stimulation. Eur J Cell Biol. 1990 Feb;51(1):76–84. [PubMed] [Google Scholar]
- Kleitke B., Sydow H., Wollenberger A. Evidence for cyclic AMP-dependent protein kinase activity in isolated guinea pig and rat liver mitochondria. Acta Biol Med Ger. 1976;35(3-4):K9–K17. [PubMed] [Google Scholar]
- Kulinskii V. I., Zobova N. V. Submitokhondrial'noe raspredelenie cAMP pri ego inkubatsii s mitokhondriiami pecheni krys. Biokhimiia. 1985 Sep;50(9):1546–1552. [PubMed] [Google Scholar]
- Kulinsky V. I., Trufanova L. V., Medvedev A. E. Catecholamine control of enzymes involved in isocitrate oxidation of rat liver mitochondria. FEBS Lett. 1984 Nov 5;177(1):143–145. doi: 10.1016/0014-5793(84)80999-0. [DOI] [PubMed] [Google Scholar]
- Lieberman S. J., Wasco W., MacLeod J., Satir P., Orr G. A. Immunogold localization of the regulatory subunit of a type II cAMP-dependent protein kinase tightly associated with mammalian sperm flagella. J Cell Biol. 1988 Nov;107(5):1809–1816. doi: 10.1083/jcb.107.5.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- Müller G., Bandlow W. cAMP-dependent protein kinase activity in yeast mitochondria. Z Naturforsch C. 1987 Nov-Dec;42(11-12):1291–1302. doi: 10.1515/znc-1987-11-1224. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Beasley A., Corbin J. D. Regulatory subunits of bovine heart and rabbit skeletal muscle cAMP-dependent protein kinase isozymes. Methods Enzymol. 1983;99:55–62. doi: 10.1016/0076-6879(83)99040-7. [DOI] [PubMed] [Google Scholar]
- Richards J. S., Rolfes A. I. Hormonal regulation of cyclic AMP binding to specific receptor proteins in rat ovarian follicles. Characterization by photoaffinity labeling. J Biol Chem. 1980 Jun 10;255(11):5481–5489. [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Hamann A. Determination and comparative analysis of the catalytic subunit of adenosine 3',5'-cyclic phosphate-dependent protein kinase by an enzyme-linked immunosorbent assay. Biochem J. 1982 Oct 15;208(1):109–117. doi: 10.1042/bj2080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Hamann A., Hilz H. Antiserum against the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Reactivity towards various protein kinases. Biochem J. 1980 Oct 15;192(1):223–230. doi: 10.1042/bj1920223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Lohmann S. M., Walter U., Jung U. Determination of cyclic AMP-dependent protein kinase subunits by an immunoassay reveals a different subcellular distribution of the enzyme in rat parotid than does determination of the enzyme activity. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(3):247–258. [PubMed] [Google Scholar]
- Siess E. A., Fahimi F. M., Wieland O. H. Evidence that glucagon stabilizes rather than activates mitochondrial functions in rat liver. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1643–1651. doi: 10.1515/bchm2.1981.362.2.1643. [DOI] [PubMed] [Google Scholar]
- Siess E. A. Influence of isolation media on the preservation of mitochondrial functions. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):279–289. doi: 10.1515/bchm2.1983.364.1.279. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Regulation of NAD-dependent glutamate dehydrogenase by protein kinases in Saccharomyces cerevisiae. J Biol Chem. 1984 Jan 25;259(2):1288–1293. [PubMed] [Google Scholar]
- Verdanis A. Protein kinase activity at the inner membrane of mammalian mitochondria. J Biol Chem. 1977 Feb 10;252(3):807–813. [PubMed] [Google Scholar]
- Weber W., Hilz H. Stoichiometry of cAMP binding and limited proteolysis of protein kinase regulatory subunits R I and R II. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1074–1081. doi: 10.1016/0006-291x(79)91935-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K. Glucagon stimulation of mitochondrial respiration. J Biol Chem. 1975 Oct 10;250(19):7924–7930. [PubMed] [Google Scholar]