Abstract
Antifibrotic drug screening requires evaluating the inhibitory effects of drug candidates on fibrotic cells while minimizing any adverse effects on normal cells. It is challenging to create organ-specific vascularized organoids that accurately model fibrotic and normal tissues for drug screening. Our previous studies have established methods for culturing primary microvessels and epithelial cells from adult tissues. In this proof-of-concept study, we used rats as a model organism to create a two-dimensional vascularized liver organoid model that comprised primary microvessels, epithelia, and stellate cells from adult livers. To provide appropriate substrates for cell culture, we engineered ECMs with defined stiffness to mimic the different stages of fibrotic tissues and normal tissues. We examined the effects of two TGFβ signaling inhibitors, A83-01 and pirfenidone, on the vascularized liver organoids on the stiff and soft ECMs. We found that A83-01 inhibited fibrotic markers while promoting epithelial genes of hepatocytes and cholangiocytes. However, it inhibited microvascular genes on soft ECM, indicating a detrimental effect on normal tissues. Furthermore, A83-01 significantly promoted the expression of markers of stem cells and cancers, increasing the potential risk of it being a carcinogen. In contrast, pirfenidone, an FDA-approved compound for antifibrotic treatments, did not significantly affect all the genes examined on soft ECM. Although pirfenidone had minor effects on most genes, it did reduce the expression of collagens, the major components of fibrotic tissues. These results explain why pirfenidone can slow fibrosis progression with minor side effects in clinical trials. In conclusion, our study presents a method for creating vascularized liver organoids that can accurately mimic fibrotic and normal tissues for drug screening. Our findings provide valuable insights into the potential risks and benefits of using A83-01 and pirfenidone as antifibrotic drugs. This method can be applied to other organs to create organ-specific vascularized organoids for drug development.
Keywords: Liver organoid, vascularization, fibrosis, stiffness, extracellular matrix
Introduction
Fibrosis is a pathological condition that can be caused by a variety of diseases and injuries and can affect almost all the organs.1,2 During fibrosis, myofibroblasts proliferate and expand in local tissues, which leads to the deposition of excessive extracellular matrix (ECM) and causes tissue stiffening. The stiff ECM further promotes myofibroblast activation, which drives the progression of fibrosis. 3 Lack of microvessels is another important feature of fibrosis, 4 which further deteriorates organ functions. All these factors collectively contribute to organ fibrosis and eventually organ failure.
Many efforts have been done to study the mechanisms of organ fibrosis. The main cell type in liver fibrosis, myofibroblasts, can originate from multiple sources, such as epithelial cells, microvessels, stellate cells, fibroblasts, and adult stem cells.4,5 These cells can be activated by various stimuli and can differentiate into myofibroblasts through the transforming growth factor beta (TGFβ) signaling pathway, which is the master regulator of fibrosis and a potential therapeutic target.1–3 Although animal studies have made significant achievements in developing antifibrotic treatments, few of them succeeded in clinical trials.1,6,7 Pirfenidone is the only TGFβ inhibitor that has been approved by the FDA for treating fibrosis in recent years. However, it only slows the fibrosis progression. 6 In clinical trials, the major challenges are efficacy and toxicity, which arise due to the differences between animals and humans in physiology, pathology, and genetics. Therefore, it is essential to establish precise human cell culture platforms to evaluate the inhibitory effects of drug candidates on myofibroblasts, and, more importantly, potential negative effects on normal cells, such as parenchymal and microvascular cells.
Traditional in vitro fibrosis models are limited to using only one type of cells, such as myofibroblasts, fibroblasts, or stellate cells,3,8 which cannot fully replicate the in vivo multicellular microenvironment. In recent years, organoids have emerged as a promising solution. 9 There have been some reports about liver organoid models for fibrosis.10,11 Hepatic stellate cells can be cultured in three dimensions (3D) to form organoids. 12 Elbadawy et al. cocultured primary mouse hepatocytes and stellate cells in vitro to form organoids. 13 Leite et al. cocultured the hepatocyte cell line HepaRG and primary human hepatic stellate cells in 3D to make liver organoids. 14 Coll et al. generated hepatic stellate cells from pluripotent stem cells and cocultured them with HepaRG cells to form organoids for fibrosis study. 15 Ouchi et al. further developed methods for generating hepatocyte like cells, stellate like cells, and Kupffer like cells from pluripotent stem cells. 16 However, incorporating microvessels into the liver organoids remains a challenge. While there are reports about vascularized organoids generated by the induction of pluripotent stem cells, 17 it is still challenging to create vascularized organoids containing organ-specific microvessels and parenchymal cells from pluripotent stem cells.
Primary cells that are isolated from adult organs are an ideal source for creating organ-specific vascularized organoids. Our previous studies have established methods for culturing epithelial cells,18,19 microvessels, 20 and vascular stem cells 4 from adult tissues. We also engineered an extracellular matrix with defined stiffness to model fibrotic tissues. 21 In this proof-of-concept study, we aimed to establish a two-dimensional (2D) vascularized liver organoid model using rats as the model organism. This model can serve as a drug screening platform for liver fibrosis therapies. By using this model, researchers can accurately and efficiently test the effects of potential drug candidates on multiple types of cells in both fibrotic and normal tissues.
Materials and methods
Preparation of ECM substrates
The ECM substrates used in this study were mainly composed of collagen I and IV. We derived the ECM solution from decellularized porcine kidneys through pepsin digestion, as described in our earlier study. 22 The ECM solution was added to culture dishes and dried at 37°C in a sterile container to form ECM membranes. By adjusting the ECM density (µg/mm2) on the culture dish, we were able to produce ECMs with Young’s moduli of approximately 20, 6, and 1 kPa, as determined by atomic force microscopy in our previous study. 21 The culture dishes made of polystyrene had a Young’s modulus of approximately 3 GPa. We incubated the culture dishes with 0.1 mg/mL ECM solution for 1 h and washed them with PBS before seeding cells.
Isolation of primary cells from adult rat livers
All animal procedures were carried out in accordance with the Ministry of Science and Technology Guide and approved by the Animal Care and Use Committee of Qingdao University. The Sprague Dawley (SD) rats of 8–10 weeks were euthanized by an overdose of isoflurane (RWD, cat#R510-22-10). The livers were harvested and cut into 2 mm pieces and then digested in DMEM (Invitrogen, Cat#12800017) supplemented with 2 mg/mL collagenase I (Worthington, Cat#LS004196) and 5 μM Y27632 (Selleck, Cat#S1049).
Primary liver cells were collected during cyclic digestion.20,21 The cell suspension was filtered through a 150 μm strainer every 20 min, and the tissues collected on the strainer were subjected to another round of digestion until all the tissue pieces were digested. The final cell suspension was filtered through a 30 μm strainer to eliminate red blood cells and single cells. The cells that remained on the 30 μm strainer were collected for experiments.
Culture of primary liver cells
Primary liver cells were cultured on the ECMs with different stiffness in a custom-made medium. The medium was DMEM/F12 supplemented with N2 (Gibco, Cat#17502048), B27 (Gibco, Cat#17504044), 2% fetal bovine serum (FBS, Gibco, Cat#10091148), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Cat#15140122). Control medium was supplemented with 2 μM Chir99021 (Selleck, Cat#S2924), 5 μM Y27632 (Selleck, Cat#S1049), and 10 ng/mL VEGF (Peprotech, Cat#100-20), and experimental medium was supplemented with 0.2 μM A83-01 or 0.2 mg/mL pirfenidone (Selleck, Cat#S2907). The cells were cultured in an incubator at 37°C, 5% CO2, and 95% humidity.
Total RNA extraction
Total RNA was extracted from liver cells using TRIzol™ Reagent (Invitrogen, Cat#15596026). The cells were lysed in 60 mm culture dishes using 1 mL of TRIzol™ Reagent. 200 µL of chloroform was added to the lysate and left on ice for 10 min. It was then centrifuged at 12,000 rpm 4°C for 10 min. Next, 400 µL of supernatant was transferred to a new tube and 500 µL of isopropanol was added. The mixture was centrifuged at 10,000 rpm 4°C for 10 min. The RNA precipitate was washed three times with 75% ethanol at 10,000 rpm and 4°C for 5 min by centrifugation. The RNA precipitates were air-dried for 5–10 min and subsequently dissolved in 10–50 µL of DEPC water. RNA was identified and quantified using Nano Drop and Agilent 2100 Bioanalyzer.
Quantitative PCR analysis
Sample RNA was reverse transcribed to cDNA using Evo M-MLV RT Mix with gDNA Clean for qPCR Ver.2, and qPCR experiments were performed using MonAmp™ ChemoHS qPCR Mix. Fold change in mRNA expression levels was calculated by the comparative Ct method, using the formula 2−ΔΔCt and GAPDH as a calibrator. The primer list was attached to the Supplemental Materials.
Immunostaining
The cells were fixed in 4% paraformaldehyde (PFA) for 30 min, washed three times with PBS, permeabilized with 1% Triton for 10 min, blocked in 5% normal donkey serum for 1 h, and incubated with primary antibodies in a blocking solution at 4°C overnight. After primary antibody incubation, the cells were washed three times in PBS and incubated with secondary antibodies in the blocking solution for 10 h at 4°C. The primary antibodies used in this study were CD31 (Affinity, Cat#AF3628), E-cadherin (Proteintech, Cat#20874-1-AP), E-cadherin (Sangon Biotech, Cat#D194898), SMA (Santa Cruz, Cat#SC32251), Desmin (Proteintech, Cat#16520-1-AP), Collagen I (Affinity, Cat#AF7001), p-ITGB1 (Affinity, Cat#AF8384), CTGF (Affinity, Cat#AF7537). Cell nuclei were stained by DAPI (4′,6-diamidino-2-phenylindole). Confocal imaging was performed on a Leica SP8 confocal microscope.
Statistical analysis
All experimental data were presented as mean ± SD of three replicates. Immunofluorescence staining results were processed using ImageJ. One-way ANOVA was performed using Graghpad Prism 8 for single-factor data and two-way ANOVA for two-factor data followed by Bonferroni post hoc test. Mann Whitney test was performed between two groups. p < 0.05 was considered a significant difference.
Results
2D vascularized liver organoids
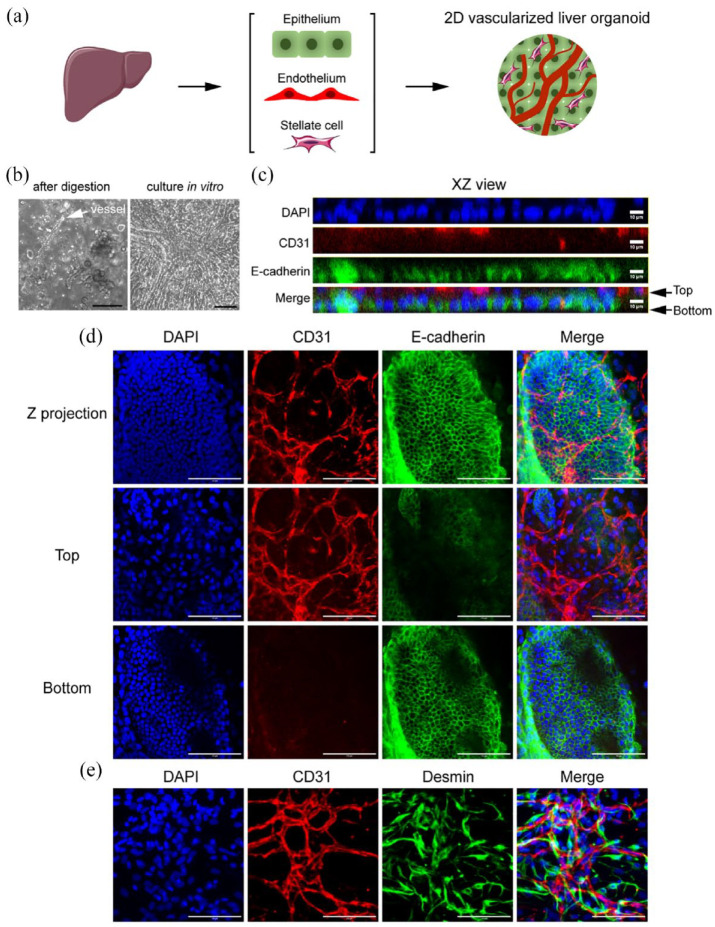
After digesting the liver tissue, we filtered the cell suspension through a 30 µm strainer to remove red blood cells and debris. The remaining microvascular segments and epithelial clusters were self-organized into multiple layers within 4 days when cultured in regular dishes with Young’s modulus of about 3 GPa (Figure 1(a) and (b)). Immunostaining showed that CD31+ microvessels grew on top of the E-cadherin+ epithelial layer (Figure 1(c) and (d)). There were plenty of Desmin+ stellate cells surrounding the microvessels (Figure 1(e)). While this 2D vascularized liver organoid does not have the typical three-dimensional spherical structure of a classical organoid, it has advantages such as the feasibility of performing immunostaining and the potential for replicating the liver’s matrix in various liver diseases.
Figure 1.
2D vascularized liver organoids. (a) Workflow illustration. (b) The phase contrast images of the primary liver cells right after digestion and those cultured in vitro in regular dishes for 4 days. The white arrow pointed to a microvessel. (c–e) Immunostaining of the 2D vascularized liver organoids cultured for 4 days in vitro. The antibodies were against CD31, E-cadherin, and Desmin. DAPI stained nuclei. Scale bars, 100 µm.
Examine the antifibrotic effect of A83-01 on 2D vascularized liver organoids on the ECM with defined stiffness mimicking fibrotic and normal microenvironments
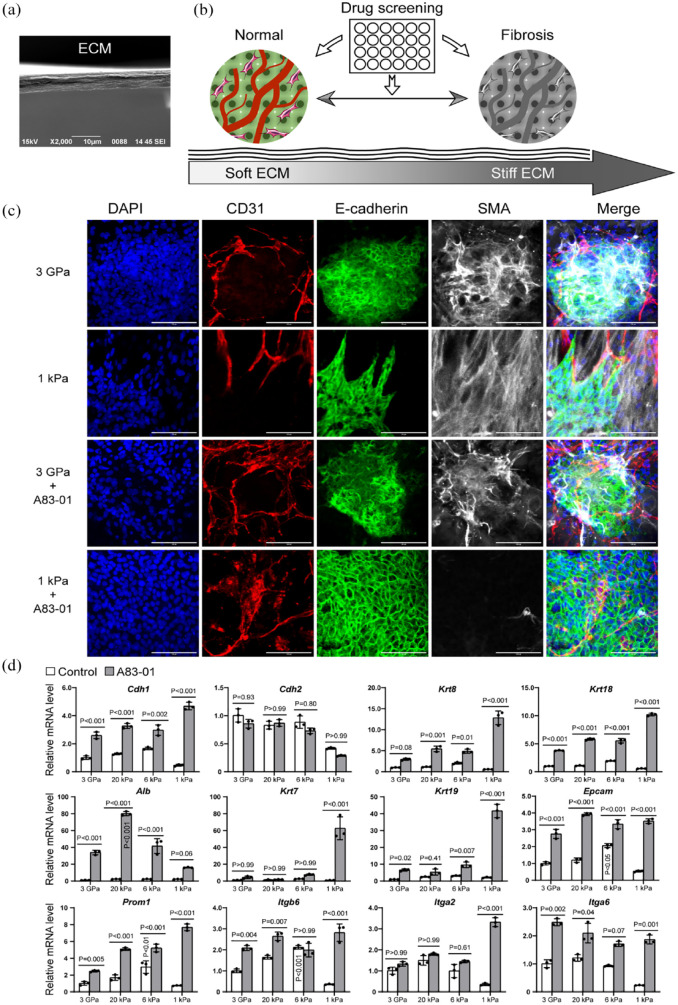
In clinical studies, it has been found that the Young’s modulus of liver tissues of advanced fibrosis is above 7 kPa, while for cirrhosis, it is higher than 13 kPa.23,24 Normal liver tissues have a Young’s modulus of about 1 kPa.25,26 To mimic these conditions, we engineered ECM substrates with Young’s moduli of approximately 20 and 6 kPa to mimic fibrotic liver tissues, as well as an ECM gel of about 1 kPa for normal liver tissues (Figure 2(a) and (b)). 21 Additionally, we also included a group of regular culture dishes with a Young’s modulus of about 3 GPa for comparison. These ECM substrates were mainly composed of collagen I and IV, without any synthetic polymers, and could better mimic the ECM in vivo. 22
Figure 2.
Drug screening using vascularized liver organoids on stiff and soft ECMs. (a) SEM image of ECM cross-section. (b) Illustration of the workflow. (c) Immunostaining of vascularized liver organoids on the ECM of 3 GPa and 1 kPa with or without A83-01. The antibodies were against CD31, E-cadherin, and SMA. DAPI stained nuclei. Scale bars, 100 µm. (d) qPCR analysis of the effect of A83-01 on vascularized liver organoids on different ECM (3 GPa, 20 kPa, 6 kPa, and 1 kPa). N = 3. Two-way ANOVA was performed on the data followed by Bonferroni post hoc tests. p < 0.05 was considered significant. The culture time was 1 week.
We first examined two extreme conditions, including the softest (1 kPa) and the stiffest (3 GPa) ECM substrates, by immunostaining. We observed that epithelial cells lost their typical cobblestone morphology on these ECMs, as shown by disorganized E-cadherin staining and strong SMA signals, suggesting epithelial-mesenchymal transition (EMT) and differentiation into myofibroblasts (Figure 2(c)). The TGFβ signaling pathway is the master regulator of organ fibrosis and EMT. The addition of a TGFβ receptor inhibitor, A83-01, restored the epithelial cell morphology (E-cadherin staining pattern) and inhibited SMA expression on the soft 1 kPa ECM (Figure 2(c)). However, A83-01 failed to reverse the epithelial morphology and SMA expression on 3 GPa ECM (Figure 2(c)), indicating that multiple signaling pathways regulate this phenotype change at high stiffness.
The quantitative analysis using qPCR showed that A83-01 treatment significantly upregulated the expression of genes of epithelial cells (Cdh1/E-cadherin), hepatocytes (Krt8, Krt18, and Alb), and cholangiocytes (Krt7 and Krt19; Figure 2(d)). All types of stiff ECMs, including 6 kPa, 20 kPa, and 3 GPa, upregulated Cdh2 expression, which is consistent with the EMT in fibrotic tissues. We found that A83-01 did not have an effect on Cdh2 expression in any of these conditions, suggesting the involvement of multiple signaling pathways in liver EMT. The greatest increase in the expression of Cdh1, Krt8, Krt18, Krt7, and Krt19 due to A83-01 treatment was observed on the 1 kPa ECM. Interestingly, in the A83-01 group, Alb expression increased with increasing ECM stiffness from 1 to 20 kPa, but then decreased on 3 GPa ECM. It is worth noting that Albumin (Alb) was reduced significantly during liver fibrosis. 27 The increase of Alb on the 20 kPa ECM may indicate a compensatory mechanism for the loss of Alb during liver fibrosis, which warrants further investigation in the future. This also suggests that engineered ECM substrates are more effective than regular culture dishes for in vitro liver fibrosis research. These results suggest that A83-01 promoted liver epithelial cell phenotype and marker expression, especially on the soft ECM of 1 kPa.
We also investigated Epcam, Prom1, Itgb6, Itga2, and Itga6 (Figure 2(d)), which have been reported to be associated with cancers. Epcam and Prom1 are known as markers of liver cancer stem cells. 28 Itgb6 has been reported to contribute to liver fibrosis and intrahepatic cholangiocarcinoma. 29 Itga6 has been found to promote hepatocellular carcinoma,30,31 while Itga2 has been linked to multiple cancers.32–34 We found that Epcam, Prom1, and Itgb6 had the highest expression on 6 kPa ECM, which corresponds to the early stages of liver fibrosis. Most cases of liver fibrosis eventually progress to cancers in the late stages. 35 The upregulation of these genes on 6 kPa ECM might be one of the mechanisms of premalignant factors, which warrants further study in the future.
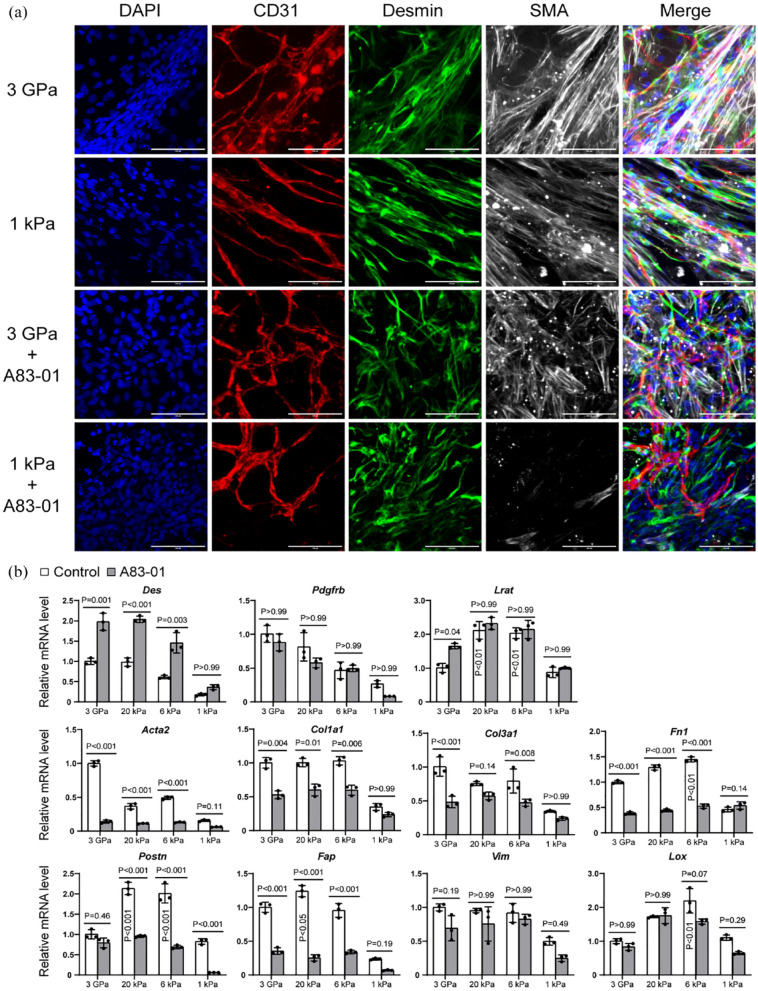
Stellate cells are an important source of myofibroblasts during liver fibrosis. 5 Our immunostaining results showed Desmin+ stellate cells around microvessels on the ECMs of 3 GPa and 1 kPa, and they exhibited strong staining for SMA (Figure 3(a)). A83-01 treatment reduced SMA expression significantly on the ECM of 1 kPa but not on 3 GPa ECM (Figure 3(a)). By qPCR analysis, we found that the stellate cell marker Desmin was upregulated on stiff ECMs and further elevated by A83-01 treatment (Figure 3(b)). Pdgfrb had higher expression on stiff ECMs but was not regulated significantly by A83-01 treatment (Figure 3(b)). Interestingly, Lrat had the highest expression on 20 and 6 kPa ECMs, which corresponds to pathological fibrotic liver tissues. Overall, most fibrotic markers were upregulated on stiff ECMs, such as Acta2 (Sma), Col1a1, Col3a1, Fn1, Postn, Fap, Vim, and Lox (Figure 3(b)). Fn1, Postn, Fap, and Lox had the highest expression on the ECMs with pathological stiffness of 20 and/or 6 kPa ECM, demonstrating the effectiveness of our engineered ECM substrates in mimicking liver fibrosis.
Figure 3.
Stellate cell and fibrotic markers of vascularized liver organoids regulated by ECM stiffness and A83-01. (a) Immunostaining of vascularized liver organoids on the ECM of 3 GPa and 1 kPa with or without A83-01 treatment. The antibodies were against CD31, Desmin, and SMA. DAPI stained nuclei. Scale bars, 100 µm. (b) qPCR analysis of vascularized liver organoids on the ECM of 3 GPa, 20 kPa, 6 kPa, and 1 kPa, with or without A83-01. N = 3. Two-way ANOVA was performed on the data, followed by Bonferroni post hoc tests. p < 0.05 was considered significant. The culture time was 1 week.
A83-01 treatment reduced the expression of most fibrotic genes on stiff ECMs, except Vim and Lox. The decrease of some genes on the soft ECM of 1 kPa was not significant possibly because of the small values (Figure 3(b)). Immunostaining results showed that COL1 was reduced by A83-01, particularly on the ECM of 1 kPa (Figure 4). These results suggest that A83-01 inhibited fibrosis effectively.
Figure 4.
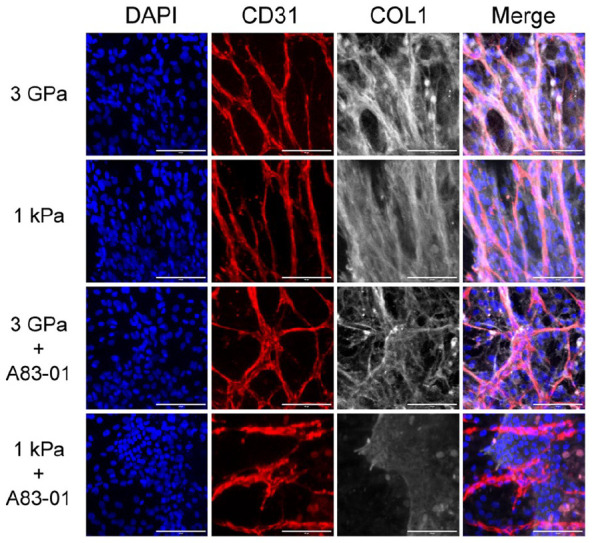
COL1 expression of vascularized liver organoids on different stiffness with or without A83-01. The primary vascularized liver organoids were cultured for 1 week before being immunostained by the antibodies against CD31 and COL1. DAPI stained nuclei. Scale bars, 100 µm.
Complex roles of A83-01 on the endothelial cells
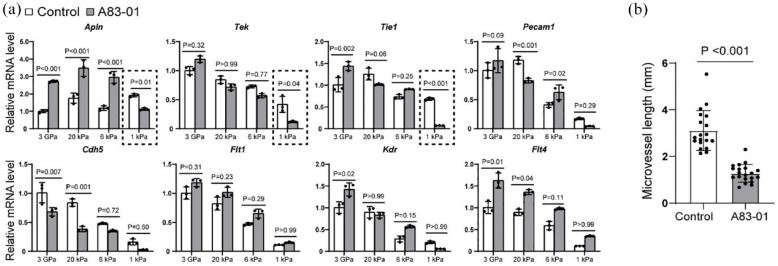
Effective antifibrotic therapies need to not only inhibit fibrosis but also restore microvessels. Therefore, we investigated the impact of A83-01 on critical microvascular genes (Figure 5). We found that the angiogenic gene Apln was significantly upregulated on stiff ECMs of 3 GPa, 20 kPa, and 6 kPa, but was downregulated on the soft ECM of 1 kPa by A83-01 (Figure 5(a)). Similarly to the results of some epithelial, stellate cell, and fibrotic genes (Figures 2(d) and 3(b)), we noticed that 3 GPa ECM had lower Apln expression than 20 kPa in both control and A83-01 groups. The other endothelial genes examined in this study had a higher expression on stiff ECMs in both control and A83-01 groups. The tyrosine kinase receptor Tek was significantly reduced on the soft 1 kPa ECM under A83-01 treatment. The trend of Tie1 was similar to Tek. Pecam1 (Cd31) showed a decrease on 20 kPa ECM but an increase on 6 kPa ECM under A83-01 treatment. Additionally, the expression of Cdh5 was found to decrease on 3 GPa and 20 kPa ECMs under A83-01 treatment. Furthermore, we observed that VEGF receptors (Flt1, Kdr, and Flt4) were upregulated on stiff ECMs to different extents by A83-01. A83-01 insignificantly affected Flt1, upregulated Kdr on 3 GPa ECM, and promoted Flt4 on 3 GPa and 20 kPa ECMs (Figure 5(a)). These results suggest that A83-01 has varying effects on endothelial cells in fibrotic and normal tissues. Considering the negative effects of A83-01 on Apln, Tek, and Tie1 on 1 kPa ECM, we measured microvascular lengths on 1 kPa ECM with and without A83-01 treatment. The results revealed that A83-01 significantly reduced microvascular growth on the soft 1 kPa ECM (Figure 5(b)), suggesting its potential to harm normal liver tissues.
Figure 5.
Endothelial cells regulated by ECM stiffness and A83-01. (a) The primary vascularized liver organoids were cultured on the ECM of different stiffness with or without A83-01 for 1 week before harvest for qPCR analysis. N = 3. Two-way ANOVA was performed on the data, followed by Bonferroni post hoc tests. (b) The lengths of microvessels on 1 kPa ECM in control medium and A83-01 medium. Mann Whitney test was performed between the two groups. p < 0.05 was considered significant.
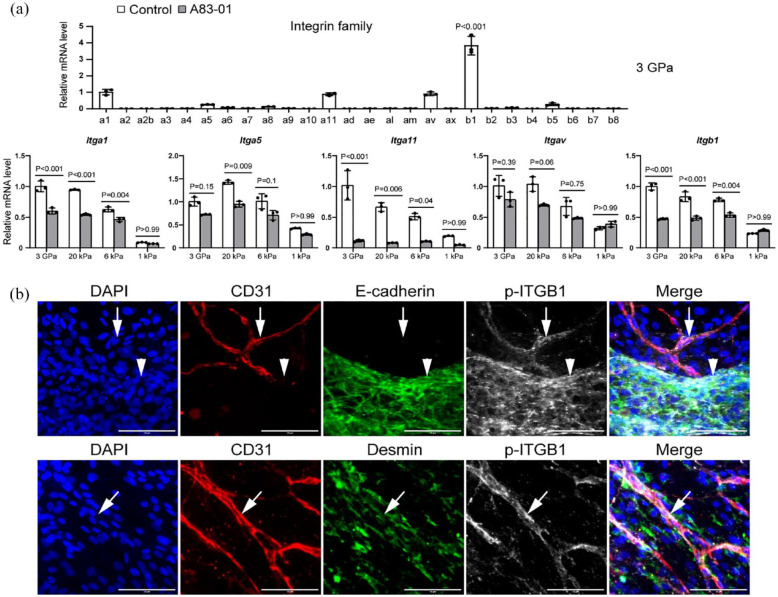
Integrins in vascularized liver organoids
Integrin family proteins are of critical importance in transducing signals from the ECM to the cells. In our study of vascularized liver organoids, we conducted a comprehensive qPCR analysis to examine the expression of Integrin proteins in the absence of A83-01 (Figure 6(a)). The results showed that among the subunits, Integrin β1 (Itgb1) exhibited the most significant upregulation in the cells of the control group that underwent fibrosis in vitro. The other subunits, namely Itga1, Itga5, Itga11, and Itgav, also showed comparatively higher expression levels than the rest (Figure 6). We further investigated these five Integrins in vascularized liver organoids on ECMs of different stiffness with or without A83-01. We found that stiff ECMs of 3 GPa, 20 kPa, and 6 kPa had higher expression of the five Integrins than 1 kPa ECM, indicating the activation of these genes during fibrosis. However, all the five Integrins were downregulated on stiff ECMs by A83-01, except that Itgav’s changes were not significant. It is worth noting that A83-01 did not influence these five Integrins on soft ECM of 1 kPa (Figure 6(a)).
Figure 6.
Integrins in vascularized liver organoids. (a) The qPCR analysis of vascularized liver organoids on the ECM of different stiffness. N = 3. One-way or two-way ANOVA was performed on the data, followed by Bonferroni post hoc tests. p < 0.05 was considered significant. (b) Immunostaining of vascularized liver organoids cultured on 3 GPa ECM in the control medium by the antibodies against CD31, E-cadherin, and p-ITGB1 (Y783). DAPI stained nuclei. Arrows pointed to CD31+ p-ITGB1+ microvascular endothelial cells. Arrowheads pointed to E-cadherin+ p-ITGB1+ epithelial cells. Scale bars, 100 µm. The cell culture time was 1 week.
Given that Itgb1 was the most abundant subunit, we performed immunostaining on vascularized liver organoids to investigate its subcellular localization. Our results showed that the phosphorylated ITGB1 (p-ITGB1; Y783) was mainly located in the endothelial and epithelial cells, but not in stellate cells, on the 3 GPa ECM in the control medium, which promoted fibrosis (Figure 6(b)). This result is in agreement with our previous study on subcutaneous microvessels, where endothelial cell p-ITGB1 (Y783) mediated mechanotransduction from ECM to endothelial cells. 21 In the liver, both endothelial cells and epithelial cells are potential mechanosensors during fibrosis.
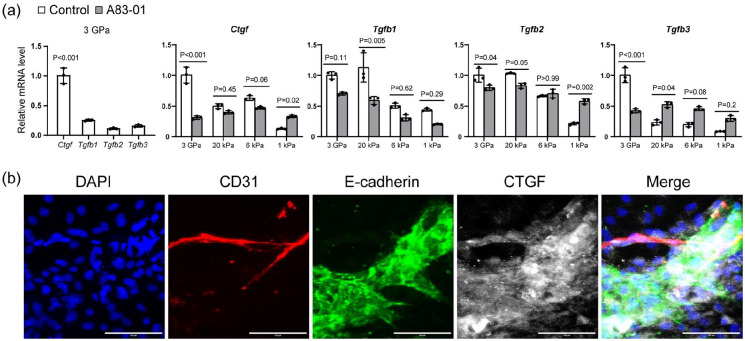
Paracrine growth factors during fibrosis
To explore the potential downstream paracrine growth factors of endothelial cells and epithelial cells, we conducted qPCR analysis of vascularized liver organoids on 3 GPa ECM in the control medium. Among the four major fibrotic growth factors, including Ctgf(Ccn2), Tgfb1, Tgfb2, and Tgfb3, Ctgf was the most expressed one (Figure 7(a)). Our immunostaining results showed that both endothelial and epithelial cells were the primary sources of CTGF (Figure 7(b)). A83-01 significantly reduced Ctgf on 3 GPa ECM while increasing it on 1 kPa ECM (Figure 7(a)). The effects of A83-01 on Tgfb1, Tgfb2, and Tgfb3 were also complex. A83-01 reduced Tgfb1 on 20 kPa ECM but not on others, reduced Tgfb2 on 3 GPa but increased it on 1 kPa, and reduced Tgfb3 on 3 GPa but increased it on 20 kPa ECM (Figure 7(a)). These results suggest that A83-01 regulates these fibrotic growth factors depending on the ECM stiffness of the local microenvironment.
Figure 7.
Paracrine growth factors during fibrosis. (a) The qPCR analysis of vascularized liver organoids on the ECM of different stiffness with or without A83-01. N = 3. One-way or two-way ANOVA was performed on the data, followed by Bonferroni post hoc tests. p < 0.05 was considered significant. (b) Immunostaining of vascularized organoids cultured on 3 GPa ECM in the control medium for 1 week. The antibodies were against CD31, E-cadherin, and CTGF. DAPI stained nuclei. Scale bars, 100 µm.
Our study has shown that A83-01 can reduce fibrotic genes, elevate epithelial genes, and increase endothelial genes on stiff ECM but reduce them on soft ECM. We have also observed that it reduced paracrine fibrotic growth factor genes on stiff ECM but promoted them on soft ECM. Furthermore, it can promote the expression of genes related to tumor formation. While the TGFβ signaling pathway participates in many biological processes, it is difficult to target them solely as an antifibrotic target without affecting other processes. Our findings are significant as they provide crucial insights into the use of A83-01 as a potential antifibrotic drug. There have been clinical trials for targeting the downstream factors, such as CTGF and TGFβ members. Among these drug candidates, a TGFβ inhibitor, pirfenidone, has been approved by the FDA for treating idiopathic pulmonary fibrosis. We next examined the effects of pirfenidone on the vascularized liver organoids.
The effects of pirfenidone on vascularized liver organoids
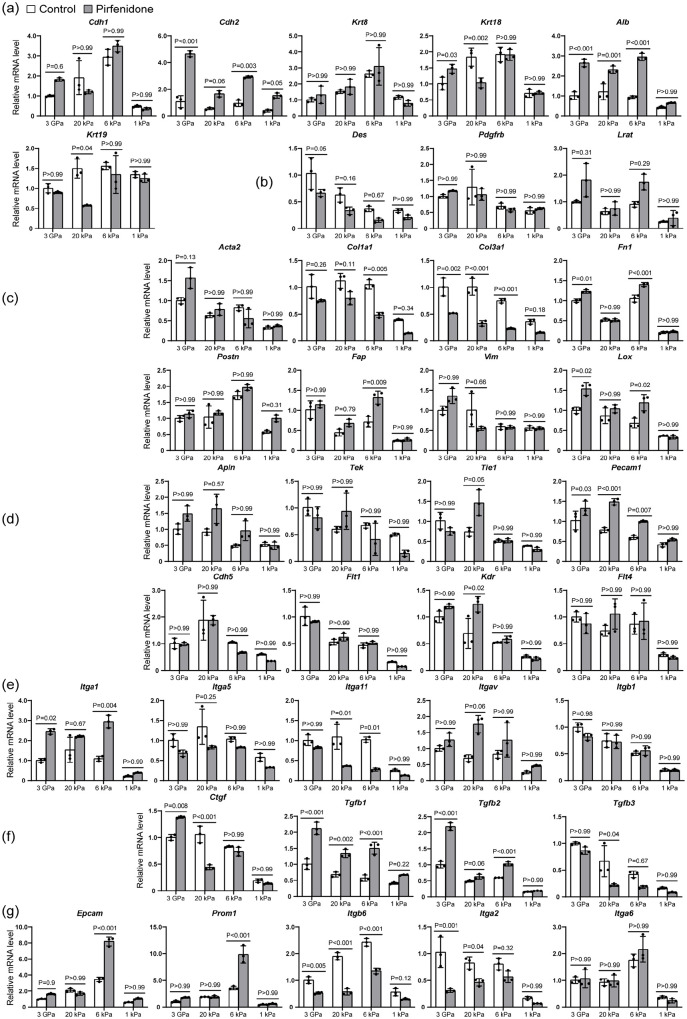
We found that pirfenidone did not significantly affect the expression of all genes examined on 1 kPa ECM, indicating that it may not impact normal tissues while inhibiting fibrosis in fibrotic regions of the liver (Figure 8). However, it had complex effects on these genes on the stiff ECMs of 3 GPa, 20 kPa, and 6 kPa, representing different stages of liver fibrosis (Figure 8).
Figure 8.
The effects of pirfenidone on vascularized liver organoids on stiff and soft ECM. The qPCR analysis of vascularized liver organoids cultured on the ECM of different stiffness with or without pirfenidone for 1 week. N = 3. Two-way ANOVA was performed on the data, followed by Bonferroni post hoc tests. p < 0.05 was considered significant: (a) epithelia, (b) stellate cells, (c) myofibroblasts, (d) endothelia, (e) fibrotic integrins, (f) paracrine factors, and (g) stem cells and cancers.
Epithelia: Pirfenidone did not significantly regulate Cdh1 or Krt8, but it did upregulate Alb expression in all three stiff conditions (Figure 8(a)). It increased Cdh2 on 3 GPa and 6 kPa ECMs, increased Krt18 on 3 GPa ECM but reduced it on 20 kPa ECM. Pirfenidone reduced Krt19 on 20 kPa ECM, but not in other groups (Figure 8(a)). These findings suggest that pirfenidone’s effect on liver epithelial cells depends on ECM stiffness, which is correlated with the stage of fibrotic diseases.
Stellate cells: Pirfenidone did not significantly affect the expression of stellate cell markers in all conditions of this study (Figure 8(b)).
Myofibroblasts: Pirfenidone did not significantly affect Acta2, Postn, or Vim in all conditions, and even increased Fn1 on 3 GPa and 6 kPa, Fap on 6 kPa, and Lox on 3 GPa and 6 kPa ECMs. However, pirfenidone reduced Col1a1 on 6 kPa ECM, and reduced Col3a1 on all stiff ECMs, indicating that it can inhibit liver fibrosis, especially in the early stages of liver fibrosis (Figure 8(c)).
Endothelia: Pirfenidone did not affect most endothelial genes significantly, but it did increase Pecam1 on all the stiff ECMs and increased Kdr on 20 kPa ECM (Figure 8(d)).
Fibrotic integrins: Pirfenidone increased Itga1 on 3 GPa and 6 kPa ECMs, but reduced Itga11 on 20 kPa and 6 kPa ECMs. It had no significant effects on Itga5, Itgav, or Itgb1 (Figure 8(e)).
Paracrine factors: Pirfenidone increased Ctgf (Ccn2) on 3 GPa ECM but reduced it on 20 kPa ECM, increased Tgfb1 on all stiff ECMs, increased Tgfb2 on 3 GPa and 6 kPa ECMs, and reduced Tgfb3 on 20 kPa ECM (Figure 8(f)).
Stem cells and cancers: Pirfenidone increased Epcam and Prom1 expression on 6 kPa ECM, reduced Itgb6 on all the stiff ECMs, reduced Itga2 on 3 GPa and 20 kPa, and had no significant effect on Itga6 in all conditions (Figure 8(g)). These findings suggest that pirfenidone can upregulate Epcam and Prom1 in the early stage of liver fibrosis, indicating the potential risk of cancer formation.
Discussion
Creating vascularized organoids is a complex task. While pluripotent stem cells have been used to generate microvessels, parenchymal cells, and mesenchymal cells for developing vascularized organoids,36,37 obtaining organ-specific microvessels and parenchymal cells from these cells is quite challenging. This is why primary cells from adult tissues are considered the ideal sources for making vascularized organoids. Our research group has been working on in vitro culture models for adult epithelial stem cells,18,19 microvessels,20,22 and vascular stem cells.4,38 In this proof-of-concept study, we have presented a method for culturing vascularized liver organoids from adult livers. The vascularized liver organoids contained primary liver epithelial cells, including hepatocytes and cholangiocytes, microvessels, and stellate cells, presenting a multicellular drug-screening platform for liver diseases.
There have been several methods for inducing liver fibrosis in vitro, such as the addition of Allyl alcohol, 14 Methotrexate, 14 free fatty acid, 16 TGFβ, 15 or thioacetamide. 15 Our study manufactured an ECM with defined stiffness that mimics the different stages of liver fibrosis. 21 To induce liver fibrosis, we cultured vascularized liver organoids on stiff ECM, leading to epithelial cells undergoing EMT and stellate cells differentiating into myofibroblasts, expressing high levels of SMA and collagens. There were also microvessels growing in the coculture system. Our engineered ECM provides a well-defined and reliable fibrotic microenvironment, making it a proper in vitro liver model for investigating multiple types of cells, in both normal tissues and fibrotic tissues at different stages.
The TGFβ signaling pathway is the master regulator of fibrosis and a promising target for therapeutic intervention.1–3 Despite numerous clinical trials targeting this pathway, however, very few have been successful.1–3 Pirfenidone is the only antifibrotic drug approved by the FDA in recent years that can inhibit TGFβ signaling, although its mechanism of action remains unclear. 39 In our study, we investigated the efficacy of two TGFβ signaling inhibitors: A83-01 and pirfenidone. Our results showed that A83-01, an ALK4/5/7 inhibitor, significantly inhibited myofibroblast genes while promoting liver epithelial genes of hepatocytes and cholangiocytes. However, A83-01 inhibited microvascular genes on soft ECM of 1 kPa, suggesting that it may have negative effects on the microvessels in healthy tissues. Moreover, A83-01 significantly increased the expression of genes associated with cancers, implying its potential as a carcinogen.
In contrast, we found that pirfenidone did not have a significant effect on all the genes of vascularized liver organoids examined on the soft ECM of 1 kPa. These results suggest that it may not be toxic to the healthy liver tissues. Although pirfenidone did not have a strong inhibitory role in most fibrotic genes, it did inhibit collagen genes, including Col1a1 and Col3a1, which encode the major components of fibrotic ECM. Therefore, it can be concluded that pirfenidone can slow fibrotic progression with minor side effects and was finally approved by the FDA. It is worth noting that our study found that pirfenidone significantly promoted Epcam and Prom1 expression on the ECM of 6 kPa. This indicates a potential risk of tumor formation in the local areas at the early stage of liver fibrosis. Overall, our study provides new insights into the efficacy and safety of TGFβ signaling inhibitors, which could pave the way for improved therapeutic strategies for fibrosis.
The Integrin family proteins play a crucial role in transducing physical signals from the ECM to cells. 40 Our previous study has identified ITGB1 as the major subunit involved in microvascular mechanotransduction. 21 Stiff ECM can induce the phosphorylation of ITGB1 (p-ITGB1) of endothelial cells, which were activated to secret paracrine factors including CTGF and TGFβ1-3 to induce pericyte differentiation into myofibroblasts. 21 In this study, we have also found that p-ITGB1 and CTGF were located in both endothelial and epithelial cells, indicating their critical role in regulating liver fibrosis. Although some clinical trials have tested antifibrotic treatments targeting integrins 41 and CTGF, 42 there have been no positive reports yet. Further investigation is necessary to understand the regulatory mechanisms of endothelial and epithelial cells in liver fibrosis.
Fibrosis is a complex process that involves multiple types of cells and signaling pathways.1–3 Our study has demonstrated that antifibrotic treatments must consider multiple cell types in the organs. It is crucial to thoroughly assess the antifibrotic efficacy in fibrotic tissues while also taking into account the potential negative effects on healthy cells. Our study has shown that the effects of TGFβ signaling inhibitors on vascularized liver organoids are dependent on the stiffness of the ECM. This highlights the importance of developing drug strategies that target different stages of tissue fibrosis. Further study is warranted to investigate the mechanisms underlying ECM stiffness regulation of antifibrotic effects.
Our study has some limitations that need to be addressed in future research. Firstly, the cells of vascularized liver organoids were grown in a 2D culture, which may not accurately reflect the conditions in a 3D microenvironment. Our future work will focus on developing 3D vascularized liver organoids with a defined stiffness to better mimic the in vivo microenvironment. Secondly, the cells were in a static culture, which means that blood flow shear stress was not considered. Blood flow plays a crucial role in regulating microvessels. Therefore, future research should incorporate blood flow into the organoid system to better simulate the in vivo conditions. Moreover, there were no immune cells in the vascularized liver organoids, which should be addressed in future studies. Finally, this proof-of-concept study utilized animal cells, which had limited implications in translational medicine. It is necessary to culture primary human vascularized liver organoids in future studies.
Conclusion
We have developed an adult vascularized liver organoid model to evaluate the effects of drug candidates on liver epithelial cells, including hepatocytes and cholangiocytes, microvessels, and stellate cells, in both normal tissues and fibrotic tissues at different stages. We tested two TGFβ signaling inhibitors, A83-01 and the FDA-approved antifibrotic drug pirfenidone. Our findings showed that A83-01 inhibited fibrotic genes but also inhibited microvessels and promoted cancer-related genes in normal healthy tissues, indicating severe side effects. On the other hand, pirfenidone inhibited the expression of collagens and did not affect the cells in normal tissues, explaining its clinical performance. Our 2D vascularized liver organoid model provides a more comprehensive assessment of drug candidates and has the potential to be extended to other organs for drug development.
Supplemental Material
Supplemental material, sj-docx-1-tej-10.1177_20417314241268344 for Two-dimensional vascularized liver organoid on extracellular matrix with defined stiffness for modeling fibrotic and normal tissues by Lei Ma, Lin Yin, Hai Zhu, Jing Li and Dong Wang in Journal of Tissue Engineering
Supplemental material, sj-docx-2-tej-10.1177_20417314241268344 for Two-dimensional vascularized liver organoid on extracellular matrix with defined stiffness for modeling fibrotic and normal tissues by Lei Ma, Lin Yin, Hai Zhu, Jing Li and Dong Wang in Journal of Tissue Engineering
Acknowledgments
The authors thank Zhishang Chang, Qian Wen, and Xuxia Song of the Laboratory of Biomedical Center, Qingdao University, for their technical help in confocal microscopy and qPCR. Thanks to Lulu Song and Guangdong Mei for their help in the animal facility of Qingdao University.
Footnotes
Author’s Note: Hai Zhu is also affiliated to Department of Urology, Qingdao Municipal Hospital, University of Health and Rehabilitation Sciences, Qingdao, China.
Author contributions: DW designed the experiments. LM performed primary cell isolation, immunostaining, and confocal imaging. LM and LY performed qPCR analysis. LM fabricated the ECMs for cell culture. LM, JL, HZ, and DW analyzed the data. DW and LM wrote the manuscript.
Availability of data and materials: The data required to reproduce these findings can be made available upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Natural Science Foundation of Shandong Province (ZR2023MH327 to Z.H. and ZR2019LZL001 to D.W.), the National Nature Science Foundation of China (32101020 to J.L.), the Natural Science Foundation of Qingdao (23-2-1-193-zyyd-jch to H.Z.), and the “Medicine+” Program of Medical College of Qingdao University to D.W.
Ethical approval: The animal procedure was conducted according to the Ministry of Science and Technology guide for laboratory animal care and use and approved by the animal care and use committee of Qingdao University.
ORCID iD: Dong Wang  https://orcid.org/0000-0003-3489-915X
https://orcid.org/0000-0003-3489-915X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature 2020; 587: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rockey DC, Bell PD, Hill JA. Fibrosis—a common pathway to organ injury and failure. N Engl J Med 2015; 372: 1138–1149. [DOI] [PubMed] [Google Scholar]
- 3. Long Y, Niu Y, Liang K, et al. Mechanical communication in fibrosis progression. Trends Cell Biol 2022; 32: 70–90. [DOI] [PubMed] [Google Scholar]
- 4. Wang D, Wang A, Wu F, et al. Sox10+ adult stem cells contribute to biomaterial encapsulation and microvascularization. Sci Rep 2017; 7: 40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HY, Sakane S, Eguileor A, et al. The origin and fate of liver myofibroblasts. Cell Mol Gastroenterol Hepatol 2024; 17: 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castelli G, Cocconcelli E, Bernardinello N, et al. Idiopathic pulmonary fibrosis: 8 years on after nintedanib and pirfenidone approval—what is on the horizon? Curr Pulmonol Rep 2023; 12: 113–124. [Google Scholar]
- 7. Odagiri N, Matsubara T, Sato-Matsubara M, et al. Anti-fibrotic treatments for chronic liver diseases: the present and the future. Clin Mol Hepatol 2021; 27: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee YS, Seki E. In vivo and in vitro models to study liver fibrosis: mechanisms and limitations. Cell Mol Gastroenterol Hepatol 2023; 16: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takebe T, Wells JM. Organoids by design. Science 2019; 364: 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bao YL, Wang L, Pan HT, et al. Animal and organoid models of liver fibrosis. Front Physiol 2021; 12: 666138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut 2019; 68: 2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brovold M, Keller D, Soker S. Differential fibrotic phenotypes of hepatic stellate cells within 3D liver organoids. Biotechnol Bioeng 2020; 117: 2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elbadawy M, Yamanaka M, Goto Y, et al. Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model. Biomaterials 2020; 237: 119823. [DOI] [PubMed] [Google Scholar]
- 14. Leite SB, Roosens T, El Taghdouini A, et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 2016; 78: 1–10. [DOI] [PubMed] [Google Scholar]
- 15. Coll M, Perea L, Boon R, et al. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell 2018; 23: 101–113.e7. [DOI] [PubMed] [Google Scholar]
- 16. Ouchi R, Togo S, Kimura M, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab 2019; 30: 374–384.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salewskij K, Penninger JM. Blood vessel organoids for development and disease. Circ Res 2023; 132: 498–510. [DOI] [PubMed] [Google Scholar]
- 18. Wang D, Wang E, Liu K, et al. Roles of TGFβ and FGF signals during growth and differentiation of mouse lens epithelial cell in vitro. Sci Rep 2017; 7: 7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Mu J, Zhou Z, et al. Expansion of mouse castration-resistant intermediate prostate stem cells in vitro. Stem Cell Res Ther 2022; 13: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song X, Yu Y, Leng Y, et al. Expanding tubular microvessels on stiff substrates with endothelial cells and pericytes from the same adult tissue. J Tissue Eng 2022; 13: 20417314221125310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Y, Leng Y, Song X, et al. Extracellular matrix stiffness regulates microvascular stability by controlling endothelial paracrine signaling to determine pericyte fate. Arterioscler Thromb Vasc Biol 2023; 43: 1887–1899. [DOI] [PubMed] [Google Scholar]
- 22. Shen L, Song X, Xu Y, et al. Patterned vascularization in a directional ice-templated scaffold of decellularized matrix. Eng Life Sci 2021; 21: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hezode C, Castera L, Roudot-Thoraval F, et al. Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment Pharmacol Ther 2011; 34: 656–663. [DOI] [PubMed] [Google Scholar]
- 24. Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134: 960–974. [DOI] [PubMed] [Google Scholar]
- 25. Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 2011; 301: G110–G118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9: 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carvalho JR, Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol 2018; 17: 547–560. [DOI] [PubMed] [Google Scholar]
- 28. Liang N, Yang T, Huang Q, et al. Mechanism of cancer stemness maintenance in human liver cancer. Cell Death Dis 2022; 13: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Z, Wang Z, Liu T, et al. Exploring the role of ITGB6: fibrosis, cancer, and other diseases. Apoptosis 2024; 29: 570–585. [DOI] [PubMed] [Google Scholar]
- 30. Zheng G, Bouamar H, Cserhati M, et al. Integrin alpha 6 is upregulated and drives hepatocellular carcinoma progression through integrin α6β4 complex. Int J Cancer 2022; 151: 930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan X, Li H, Wang M, et al. PSMC2/ITGA6 axis plays critical role in the development and progression of hepatocellular carcinoma. Cell Death Discov 2021; 7: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ren D, Zhao J, Sun Y, et al. Overexpressed ITGA2 promotes malignant tumor aggression by up-regulating PD-L1 expression through the activation of the STAT3 signaling pathway. J Exp Clin Cancer Res 2019; 38: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adorno-Cruz V, Hoffmann AD, Liu X, et al. ITGA2 promotes expression of ACLY and CCND1 in enhancing breast cancer stemness and metastasis. Genes Dis 2021; 8: 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo P, Moses-Gardner A, Huang J, et al. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci Rep 2019; 9: 6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Ann Rev Pathol 2017; 12: 153–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang S, Wan Z, Kamm RD. Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip 2021; 21: 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu H, Zhang X, Liu J, et al. Vascularization of engineered organoids. BMEMat 2023; 1: e12031. [Google Scholar]
- 38. Wang D, Li LK, Dai T, et al. Adult stem cells in vascular remodeling. Theranostics 2018; 8: 815–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruwanpura SM, Thomas BJ, Bardin PG. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Respir Cell Mol Biol 2020; 62: 413–422. [DOI] [PubMed] [Google Scholar]
- 40. Chastney MR, Conway JRW, Ivaska J. Integrin adhesion complexes. Curr Biol 2021; 31: R536–R542. [DOI] [PubMed] [Google Scholar]
- 41. Slack RJ, Macdonald SJF, Roper JA, et al. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov 2022; 21: 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raghu G, Scholand MB, de Andrade J, et al. FG-3019 anti-connective tissue growth factor monoclonal antibody: results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J 2016; 47: 1481–1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tej-10.1177_20417314241268344 for Two-dimensional vascularized liver organoid on extracellular matrix with defined stiffness for modeling fibrotic and normal tissues by Lei Ma, Lin Yin, Hai Zhu, Jing Li and Dong Wang in Journal of Tissue Engineering
Supplemental material, sj-docx-2-tej-10.1177_20417314241268344 for Two-dimensional vascularized liver organoid on extracellular matrix with defined stiffness for modeling fibrotic and normal tissues by Lei Ma, Lin Yin, Hai Zhu, Jing Li and Dong Wang in Journal of Tissue Engineering