Abstract
Attempts at vaccine development for feline immunodeficiency virus (FIV) have been extensive, both because this is a significant health problem for cats and because FIV may be a useful vaccine model for human immunodeficiency virus. To date, only modest success, producing only short-term protection, has been achieved for vaccine trials in controlled laboratory settings. It is unclear how relevant such experiments are to prevention of natural infection. The current study used a vaccine that employs cell-associated FIV-M2 strain fixed with paraformaldehyde. Subject cats were in a private shelter where FIV was endemic, a prevalence of 29 to 58% over an 8-year observation period. Cats roamed freely from the shelter through the surrounding countryside but returned for food and shelter. After ensuring that cats were FIV negative, they were immunized using six doses of vaccine over a 16-month period and observed for 28 months after the initiation of immunization. Twenty-six cats (12 immunized and 14 nonimmunized controls) were monitored for a minimum of 22 months. Immunized cats did not experience significant adverse effects from immunization and developed both antibodies and cellular immunity to FIV, although individual responses varied greatly. At the conclusion of the study, 0 of 12 immunized cats had evidence of FIV infection, while 5 of 14 control cats were infected. Thus, the vaccine was safe and immunogenic and did not transmit infection. Furthermore, vaccinated cats did not develop FIV infection in a limited clinical trial over an extended time period. Thus, the data suggest that a fixed, FIV-infected cell vaccine has potential for preventing natural FIV infection in free-roaming cats.
Feline immunodeficiency virus (FIV) is a serious and widespread pathogen of domestic cats and a useful model system for understanding the pathogenesis of AIDS and the development of human immunodeficiency virus (HIV) vaccines (4, 8, 24, 25, 28). A most interesting advantage of FIV is that vaccines can be tested under field conditions. Similar to HIV-1, FIV isolates have been grouped into different clades or subtypes (designated A to D) with different geographic distributions (2). For example, clade A FIV is prevalent in northern Europe and the United States, while clade B FIV is highly predominant in Italy (26).
Attempts to protect laboratory cats against FIV challenge by prior immunization with a variety of conventional, subunit, and DNA immunogens have generally given poor results (9, 27, 28). This was especially true when the postimmunization virus challenge was conducted with fully virulent FIV, freshly derived from infected animals, rather than with tissue culture-adapted strains that demonstrate diminished virulence (28). Possibly for this reason, although FIV represents an ideal animal model for testing antilentiviral vaccines in the field, there are no reports in the literature dealing with the use of experimental FIV vaccines in field cats.
Among the experimental anti-FIV vaccines that have proven most efficacious at protecting laboratory cats are those consisting of paraformaldehyde-fixed, FIV-infected cells (5, 15, 21, 31, 32). A vaccine of this type was first developed by Yamamoto and her group using the prototype clade A isolate Petaluma (FIV-Pet), which was found to be effective against homologous and slightly heterologous (different strains of clade A) FIV (31, 32). In previous reports, we demonstrated that immunization with a vaccine consisting of feline lymphoid MBM cells that were paraformaldehyde fixed during the acute phase of infection with a fresh clade B isolate of FIV (FIV-M2) was safe, well tolerated, and strongly immunogenic for specific-pathogen-free (SPF) cats (20, 21). The vaccine also conferred a robust, albeit transient, protection against intravenous challenge, administered as either cell-free or cell-associated virus, with the homologous FIV isolate derived ex vivo and never propagated in tissue culture. While challenge with cell-associated FIV was easier to protect against than cell-free virus, protective immunity was absent when challenge occurred with cell-associated virus 3 years after vaccination (20, 21).
In the present study, we report the results of a limited trial of the same anti-clade B FIV-infected, fixed-cell vaccine in field cats at high risk of contracting the infection because they were living in a setting of highly endemic clade B FIV infection. The aim of the experiment was to assess vaccine safety and ability to trigger an anti-FIV immune response when used in field cats; the results also suggest that the vaccine conferred protection against the natural acquisition of FIV infection.
MATERIALS AND METHODS
Animals.
Enrolled animals were of undetermined and various mixed breeds and resident in a private facility (shelter) that housed stray and unwanted cats near Pisa (Italy) that, for the 8 years (including 5 years prior to and 3 years following initiation of the vaccine experiment) during which it was monitored for retroviral infections, housed a population of between 76 and 164 cats in a total area of 360 m2 (Table 1). The shelter was “open” in that, following a brief adaptation period during which newly arrived cats were kept in a section of the building separated from the rest by a net, all animals were left to roam freely in the surrounding countryside. The shelter was under continuous expert veterinary control by veterinarians from the Department of Animal Pathology, University of Pisa. In the context of this control, the cats were vaccinated against feline leukemia virus (FeLV) (Leukocell 2; SmithKline Beecham, Rixensart, Belgium) and against feline calicivirus, rhinotracheitis herpesvirus, and panleukemia virus (Feligen CR/P; Virbac, Milan, Italy). A large proportion of resident cats were tested for FIV antibody using enzyme-linked immunosorbent assay (ELISA) and a Western blotting (WB) assay developed at the University of Pisa (21) and for FeLV p17 antigen using a commercial kit (Cite Combo; Agritech Systems, Portland, Maine) at least once per year.
TABLE 1.
FIV diffusion in the shelter where the vaccination experiment was conducted
| Parameter | Yr
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1991 | 1992 | 1993 | 1994 | 1995 | 1996a | 1997 | 1998 | |
| No. of cats in shelter | 76 | 123 | 164 | 146 | 108 | 91 | 82 | 78 |
| No. FIV seropositive/no. examined | 22/76 | 35/83 | 45/85 | 36/62 | 29/75 | 24/74 | 27/65 | 32/61 |
| Preexisting | 21 | 34 | 33 | 23 | 16 | 19 | 26 | |
| New arrivals | 9 | 4 | 0 | 1 | 2 | 1 | 0 | |
| Seroconversions | 5 | 7 | 3 | 5 | 6 | 7 | 6 | |
| FIV prevalence (%) | 29 | 43 | 53 | 58 | 39 | 32 | 41 | 52 |
| FIV incidence (%) | 9 | 15 | 10 | 10 | 11 | 15 | 17 | |
| No. of FeLV p17 positive cats/no. examined | 1/76 | 7/83 | 7/85 | 2/62 | 9/75 | 9/74 | 7/65 | 1/60 |
Immunizations started in January 1996.
Vaccine and immunization schedule.
The fixed-cell vaccine used for immunization was prepared exactly as described (21). Briefly, it consisted of interleukin-2-dependent MBM cells infected with FIV-M2 (clade B), inactivated with 1.25% paraformaldehyde at day 8 postinfection, i.e., at the time that virus expression on the cell surface reached its peak, and dialyzed extensively. A large batch of vaccine was prepared, stored in 1-ml aliquots in liquid nitrogen until used, and checked to ensure lack of infectivity by inoculation into MBM cell cultures (19). Each vaccine dose consisted of 3 × 107 cells (approximately 60% FIV positive by membrane immunofluorescence) suspended in 1 ml of phosphate-buffered saline and mixed with an equal volume of incomplete Freund's adjuvant. The vaccine was administered subcutaneously using the schedule shown in Fig. 1. The choice of administering vaccine boosters at 4, 10, and 16 months after initiation of vaccination (three doses within 6 weeks) was based on our previous findings that protection was difficult to restimulate once it had waned (21).
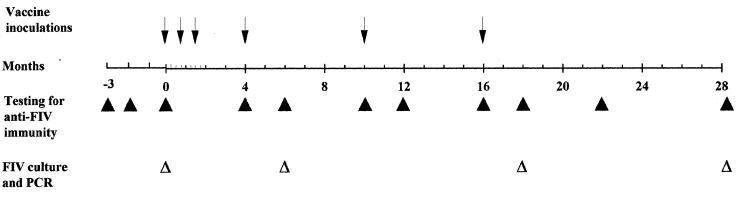
FIG. 1.
Experimental plan. Enrollment into the study began at −3 months, and time 0 marks the initiation of immunization. The experiment was terminated 28 months after the initial immunization.
FIV-specific immunological tests.
Anti-FIV immune responses were monitored using ELISA, WB, and lymphoproliferation assays that used gradient-purified, sonication-disrupted FIV, exactly as previously described (21). The virus used for antigen production was either FIV-M2 grown in MBM cells or FIV-Pet produced by chronically infected FL4 cells (30). The former was used for the ELISA and lymphoproliferation tests, and the latter was used for a second ELISA and for WB.
FACS analysis.
For fluorescence-activated cell sorting (FACS), CD4+ T lymphocytes were enumerated with monoclonal antibody FE1.7B12 (obtained from P. F. Moore, Davis, Calif.) and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). CD8+ T-lymphocyte counts were also performed but did not provide important information for this study.
FIV isolation.
Virus isolation was carried out by cocultivating 106 concanavalin A-stimulated peripheral blood mononuclear cells (PBMC) with 5 × 105 MBM cells and assaying the supernatant fluids for reverse transcriptase activity once a week for 5 weeks. Cultures were considered negative if they showed no evidence of reverse transcriptase activity during this period (11).
DNA extraction.
Genomic DNA was extracted from buffy coat cells and from isolation-positive cultures by using the QIAamp blood kit (Qiagen, Hilden, Germany).
FIV provirus detection and quantification.
The presence of FIV provirus in the study cats was investigated by amplifying 0.2 to 1 μg of extracted buffy coat DNA using a nested PCR protocol targeted to a highly conserved p25gag region and having a sensitivity of 10 FIV genomes/μg of genomic DNA. Samples that tested positive by qualitative PCR were further analyzed to quantify the proviral load by a gag-competitive PCR, which reproducibly detects ≥100 FIV genomes/μg of genomic DNA (7).
Genetic analysis of FIV isolates.
Nested PCR p25gag region products obtained from isolation-positive cultures were sequenced using an automated DNA sequencer (ALF DNA Sequencer; Amersham-Pharmacia Biotech, Uppsala, Sweden) using a cycle sequencing method. Sequences of 308 bp were aligned with those of selected strains representative of the A, B, and D subtypes (at the time of writing, no clade C gag sequences are available in the genomic data banks) and of the Italian isolates described in a previous report (26). Pairwise nucleotide and amino acid genetic distances were estimated using Kimura's two-parameter and p distance methods, respectively. Phylogenetic relationships were computed from nucleotide distances by using the Fitch-Margoliash algorithm included in the DAMBE software package (version 3.7.48) (29). Trees were built using the TREEVIEW program (version 1.5.2) (23).
RESULTS
Prevalence of FIV infection in the cat shelter.
FIV infection was highly widespread among the cats of the study shelter. As determined by testing a large proportion of animals for 8 consecutive years, FIV seroprevalence rates ranged from 29 to 58% and annual seroconversion rates from 9 to 17% in different years (Table 1). In a previous study, we had reported that all 32 FIV isolates obtained in Italy belonged to clade B. Nine such isolates were from cats present in this shelter in 1995 and, in the gag region considered, were found to have amino acid distances of up to 7% (26). Thus, clade B FIV circulated widely within the shelter. In contrast, FeLV prevalence was consistently relatively low (Table 1), most likely because the majority of cats were vaccinated against this pathogen.
Enrollment of prospective vaccinees and control cats.
When the experiment was initiated, we enrolled at random 15 prospective vaccinees and 23 unvaccinated controls among 51 animals in good clinical health that had repeatedly tested FeLV and FIV seronegative, were in approximately the same age range, and had been vaccinated against FeLV. Prior to initiation of the experiment, all enrolled animals were confirmed to be FIV free by virus isolation and a sensitive PCR test as well as by retesting with WB. As a result, immunization of the vaccinees started 3 months after enrollment and monitoring for signs of FIV infection (Fig. 1).
The experiment was terminated 28 months after initiation of immunizations. Due to the open nature of the shelter, some enrolled cats were lost earlier. Only the cats that could be monitored for at least 22 months or that had become FIV infected before they were lost were considered sufficiently informative and are discussed below. These were 12 vaccinees (7 females and 5 males; mean age, 5.9 years; range, 4 to 13 years) and 14 unvaccinated controls (9 females and 5 males; mean age, 5.3 years; range, 4 to 13 years).
Reactions to the vaccine.
As judged from general behavior, frequent physical examination, and routine hematochemical analysis, none of the immunized cats showed significant adverse reactions to the vaccine. A slight swelling at the site of inoculation was observed in some animals, but as a rule this appeared to be well tolerated by the animals and waned within a few days. This was true both for the primary immunization series and for the boosters given at months 4, 10, and 16. Most importantly, none of the vaccinees developed markers of FIV infection (see below), confirming the total inactivation of viral infectivity in the vaccine batch used for immunization.
Anti-FIV immune responses and markers of FIV infection in the vaccinees.
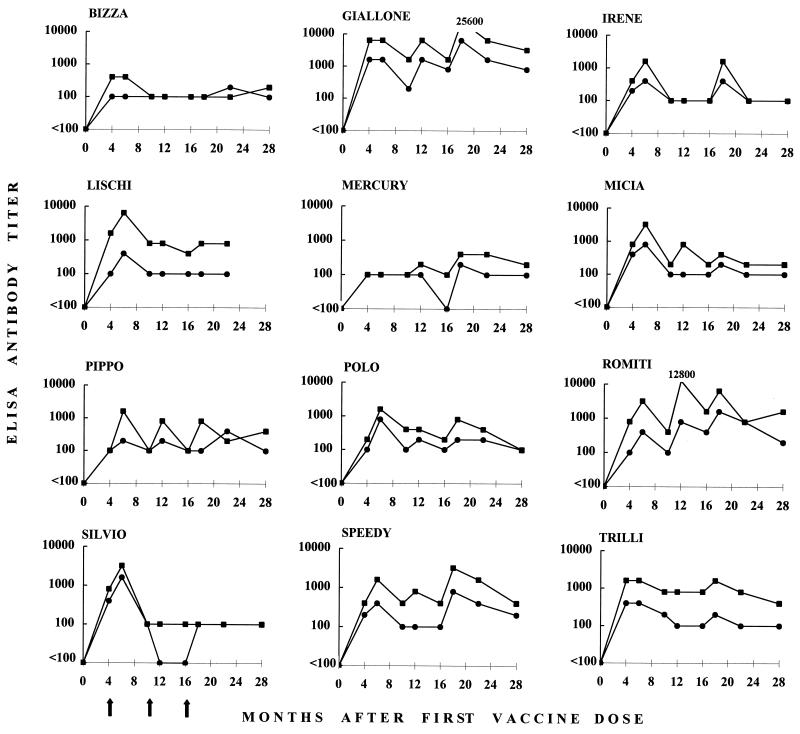
The vaccinees were monitored for anti-FIV immune responses and for markers of FIV infection at selected time points throughout the postimmunization observation period. As shown in Fig. 2, all vaccinees produced some ELISA anti-FIV antibodies, although the kinetics and titers observed varied widely between individual animals. All cats were found to be antibody positive at all times tested with at least one of the FIV antigens used, and antibody titers usually peaked at the samplings performed 2 months after vaccine boosters. Three cats (Bizza, Irene, and Mercury) mounted weak humoral responses, although mostly they exhibited a similar trend. Finally, one cat (Silvio) responded well to the 4-month booster but failed to respond to the following boosters. ELISA titers varied considerably also, depending on the viral source of test antigen, in that FIV-Pet antigen generally yielded higher antibody titers than FIV-M2. This was attributed to an especially high representation of viral capsid antigens relative to envelope antigens in the specific FIV-Pet preparation used in the ELISA (data not shown). Following the response to the last booster given at 16 months, ELISA antibodies had a general tendency to decline; however, low titers were still detected in all cats at one or more time points thereafter up to 28 months.
FIG. 2.
Titers of anti-FIV ELISA antibody in vaccinated cats at different time points postimmunization. Sera were tested against FIV-M2 antigen (●) and FIV-Pet antigen (■). The results are expressed as the reciprocal of the highest serum dilution that produced optical density readings higher than the average value obtained with 20 control FIV-negative sera plus three times the standard deviation. Sera that proved unreactive at a 1:100 dilution, the lowest dilution tested, are indicated as having titers of <100. Arrows indicate the times that vaccine boosters were administered to all cats.
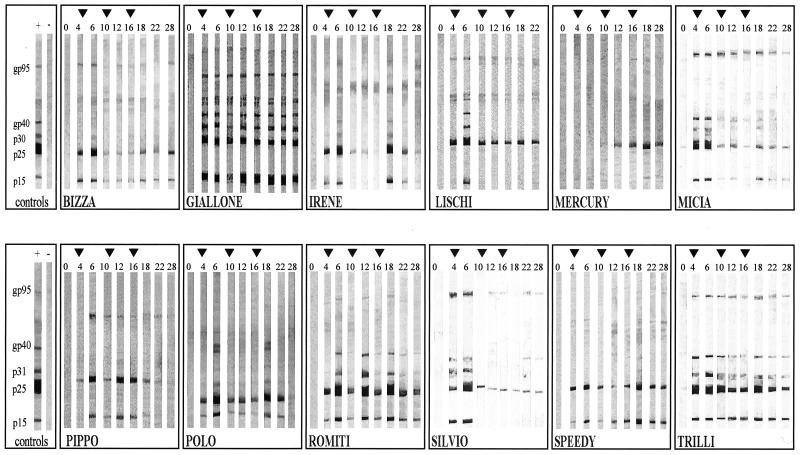
WB analysis was carried out using FIV-Pet antigen due to ready availability and, in general, gave results that correlated well with the ELISA results. Sequential sera showed that the bands produced by the capsid proteins p25 and p15 were highly dominant over the ones produced by the envelope glycoproteins gp95 and gp40 (Fig. 3). WB analysis also provided evidence that the antibody response mounted by individual cats varied not only in strength but also in the pattern and kinetics of viral antigens recognized: some vaccinees showed multiple bands that changed slightly throughout the observation period, others initially exhibited multiple bands but subsequently reacted to a few antigens only, and one proved reactive only after the booster at 10 months. For the 11 cats that could be monitored for 28 months, 8 showed at least one or two faint WB bands, confirming that vaccine-induced antibody responses remained but usually declined 1 year after the last booster.
FIG. 3.
Anti-FIV WB profiles of vaccinated cats at different time points. Arrowheads indicate the times that vaccine boosters were administered. Results for positive and negative controls, as defined in the text, are shown at the left.
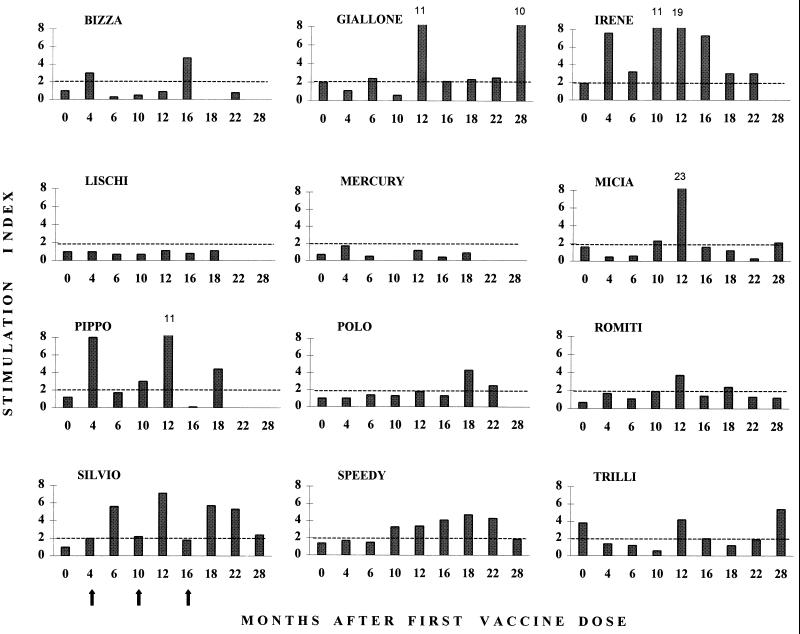
Figure 4 reports the results of testing the PBMC from vaccinated cats for their ability to proliferate in vitro in response to FIV-M2 antigen stimulation. This parameter also varied extensively among individual animals: in some cats, the response was clearly evident and durable, while in others there was no indication that the vaccine had elicited a detectable cell-mediated anti-FIV response, as measurable by this assay. In responding cats, the highest stimulation indices were often observed 2 months after the boosters, similar to antibody responses.
FIG. 4.
Anti-FIV lymphoproliferative responses of vaccinated cats at different time points. The stimulation index was calculated as the ratio of [3H]thymidine incorporated by PBMC in the presence of FIV antigen to that in the presence of mock antigen. Arrows indicate the times that vaccine boosters were administered to all cats. Only a value of ≥2 was considered indicative of FIV-specific lymphoproliferation (broken lines). The mean stimulation index ± standard error for 23 assays carried out in the unvaccinated control cats that did not become infected was 1.6 ± 0.3.
Vaccinees were monitored for direct markers of FIV infection at 6, 12, 18, and 28 months after initiation of immunization (Table 2). None of the 12 cats was found to carry infectious virus or provirus in their PBMC at any of the times tested, as determined using sensitive tissue culture (11) and PCR (7) methods. Because reports have shown that CD8+ T lymphocytes can inhibit FIV isolation in vitro (10, 16), the PBMC harvested at month 28 were also cultured following depletion of such cells using a previously published method (16); all cultures remained FIV negative (data not shown). Also, although serology was of limited value as a marker of infection in these cats due to the presence of vaccine-induced antibodies, serological findings remained consistent with vaccine booster administration and did not indicate ongoing infection at any time during the study.
TABLE 2.
Markers of FIV infection in vaccinated and unvaccinated cats
| Cat group | No. of cats positive/no. examineda
|
Total no. of infected cats at end of expt | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 mo
|
12 mo
|
18 mo
|
28 mo
|
||||||||||
| VI | PCR | Serology | VI | PCR | Serology | VI | PCR | Serology | VI | PCR | Serology | ||
| Vaccinated | 0/12 | 0/12 | NA | 0/12 | 0/12 | NA | 0/11 | 0/12 | NA | 0/11 | 0/11 | NA | 0/12 |
| Unvaccinated | 0/12 | 1/12 | 1/12 | 0/13 | 2/13 | 3/13 | 1/13 | 4/13 | 4/13 | 2/11 | 3/11 | 4/11 | 5/14 |
At time zero, all cats were negative for all tests after 3 months of assessment. VI, virus isolation. Serology, confirmed positives using ELISA followed by WB. At some time points, not all cats were examined due to inability to bleed some animals. NA, not applicable.
Markers of FIV infection in control unvaccinated cats.
Unvaccinated control cats were subjected to the same follow-up protocol as the vaccinees to assess the presence of infection. As stated above, at the start of the experiment none of these animals showed serological or virological markers of FIV infection. However, over time, several control cats became isolation, PCR, and/or antibody positive for FIV (Tables 2 and 3). Based on the fact that, when dealing with free-living cats, some FIV serological data can be difficult to interpret (14) and virus isolation is successful in only a proportion of cases (18), we scored as FIV infected only those animals that both developed a WB-confirmed FIV-positive serology and were positive for infectious virus and/or provirus. On the basis of these criteria, five control unvaccinated cats had become FIV infected when the experiment was terminated. Infected cats had provirus loads that ranged from 230 to 850 viral genomes/μg of PBMC DNA (Table 3). These values were lower than observed in experimentally infected cats examined with the same quantitative PCR protocol used here, possibly reflecting the fact that, in nature, cats are most likely exposed to small FIV inocula or that conservation of the region targeted by the PCR assay is not absolute among FIV isolates (26).
TABLE 3.
Detailed analysis of unvaccinated control cats that showed positive markers of FIV infection during follow-up
| Cat | Test resulta
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 mo
|
12 mo
|
18 mo
|
28 mo
|
|||||||||
| VI | PCR | Serology | VI | PCR | Serology | VI | PCR | Serology | VI | PCR | Serology | |
| Ciopi | − | − | − | − | + (450) | + | − | + (360) | + | NLA | ||
| Gassata | − | − | − | − | − | − | + | + (850) | + | + | + (560) | + |
| Giuditta | − | − | − | − | − | − | − | + (730) | + | − | + (230) | + |
| Pentolina | − | + (650) | + | − | + (480) | + | NLA | |||||
| Umberto | − | − | − | − | − | − | − | + (230) | + | + | + (280) | + |
VI, virus isolation. PCR, qualitative PCR. The proviral load in 1 μg of PBMC DNA as assessed by competitive PCR is shown in parentheses. Serology, WB-confirmed positives. NLA, no longer available: these cats stopped frequenting the shelter for undetermined reasons.
T-cell subset counts in vaccinated and control cats.
FACS analysis showed that CD4+ lymphocyte counts were within the normal range at all times, and no significant differences were noted between the vaccinated and unvaccinated cats, with one exception (data not shown). One of the unvaccinated control cats that became infected (Umberto) showed a significant fall in CD4+ T-cells from 39 to 19%, which occurred at month 18 and was followed by a return to preinfection values by month 28. This was consistent with our unpublished studies showing that CD4+ T-cell counts decline very slowly in field cats naturally infected with FIV.
Genotype and phylogenetic analysis of FIV strains isolated from unvaccinated cats.
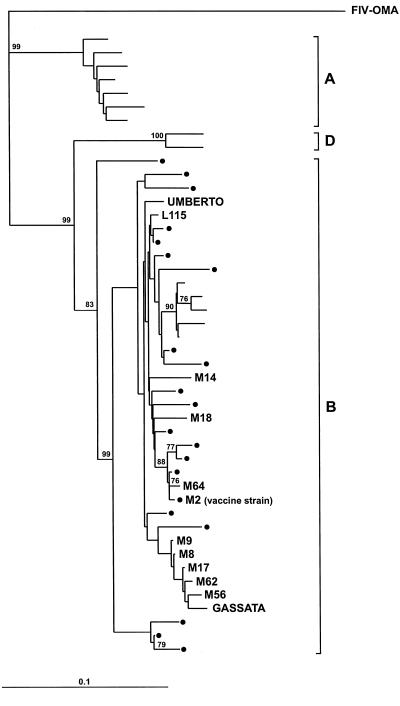
The two FIV strains that were cultured in vitro from the unvaccinated controls that became infected during the course of the experiment were sequenced in an informative segment of the p25gag region. The nucleotide sequences thus obtained were then compared to those of Italian FIV isolates that had been characterized in a previous study (26), including nine isolates obtained from the shelter where the vaccination experiment was conducted, as well as some reference strains deposited in gene banks. Similar to all other Italian isolates included in the study, the two isolates were found to belong to clade B. Also, they segregated separately from each other and from the FIV-M2 isolate used for vaccine preparation (Fig. 5). In particular, amino acid divergences were 6.9% between the isolates from Gassata and Umberto and 8 and 1% between these isolates and FIV-M2, respectively. Because FIV-M2 is the only clade B isolate that is routinely propagated in our laboratory, this ruled out laboratory contamination and suggested that the cats from which the two isolates were obtained had acquired the infection naturally. The genetic disparity of the two isolates also suggests that they were acquired from different sources.
FIG. 5.
Phylogenetic analysis of FIV isolates cultured from two of the unvaccinated control cats that became infected during the course of the experiment (Gassata and Umberto). All other isolates have been previously described (26) except FIV-OMA, a nondomestic Pallas' cat FIV isolate (3) that was used as an outgroup. M and L followed by numbers indicate isolates obtained from the shelter prior to initiation of this experiment except for M2, which was isolated from a cat from the Pisa area but at a site distant from the shelter and which had no known contacts with cats at the shelter. Dots represent Italian isolates from other locations. Unnamed isolates are reference sequences. Fitch-Margoliash tree based on a 308-bp sequence (nucleotides 1130 to 1438) of the gag gene. Bootstrap values above 75 out of 100 are shown at branch points. Bar indicates the number of nucleotide substitutions per site.
DISCUSSION
This report provides the results of a limited trial of an experimental FIV vaccine that was conducted in field cats primarily to determine safety and immunogenicity in a natural setting. All previously reported experiments had been carried out in laboratory cats living under controlled conditions that are very different from those of free-living cats, especially those whose environment and habits make them more likely to become exposed to and infected with FIV (24). The vaccine used consisted of lymphoid cells infected with an FIV strain of clade B (the predominant FIV subtype in Italy) and then paraformaldehyde fixed. The vaccine had previously been proven to be completely safe and devoid of adverse effects and to exert a powerful but limited protective action in laboratory SPF cats. The vaccine could protect against cell-free virus challenge for less than 1 year, and protection against cell-associated virus was lost by 3 years postvaccination (20, 21).
The trial was carried out in cats living in a shelter that provided a suitable environment for several reasons: (i) it was a crowded cat community where most animals were not neutered and were left free to roam around and that accepted new arrivals regardless of their health status, ideal conditions for the frequent transmission of FIV; (ii) for many years, it had been under continuous surveillance by well-trained veterinarians who understood the risks associated with retroviral infections; (iii) FIV serological monitoring for the 5 years that preceded the experiment had shown that FIV was endemic to the shelter, with high prevalence and annual incidence rates in resident cats, ranging from 29 to 58% and 9 to 15%, respectively; (iv) characterization of FIV isolates obtained from the shelter had shown that they belonged to clade B and exhibited considerable genetic diversity within this clade; (v) most cats had been vaccinated against several important cat pathogens, reducing the possibility that outbreaks of these infections might interfere with the success of the experiment; and, last but not least, (vi) the owner of the shelter had a clear appreciation of the hazards of FIV for the health of her cats and was very eager to collaborate.
The vaccination schedule consisted of an initial series of three immunizations within 6 weeks, followed by boosters given at 4, 10, and 16 months after the first immunization. A major deviation from the schedule used previously in SPF cats is that it included two additional boosters (10 and 16 months) aimed at prolonging the duration of vaccine-induced protection. Experiments in laboratory cats had in fact shown that protection against cell-free virus, although not against cell-associated virus, had waned 1 year after the only booster administered at 4 months after initiation of immunization and that protection was not recalled by a booster given 22 months later (22). On the other hand, frequent boosting is a common practice in routine veterinary vaccinations, e.g., FeLV and Feligen CR/P boosters are given annually.
Enrolled animals were in good health and FIV infection free by all available parameters over 3 months of testing prior to the start of immunization. Since vaccinees and controls were left free to roam similar to the other cats cared for at the shelter, some were lost during the 28 months of follow-up. Since only the ones that could be monitored for at least 22 months were deemed sufficiently informative, by the end of the experiment, the study groups comprised 12 vaccinees and 14 matched controls. Similar to what has been observed in SPF cats (20, 21), in field cats the vaccine produced no major local or general reactions in spite of repeated inoculation and was well tolerated, and absolutely free of residual infectivity. As determined by ELISA and WB analysis of sequential sera, all vaccinated field cats exhibited an FIV-specific antibody response that was mainly directed to viral capsid antigens but was clearly evident also against the viral envelope glycoproteins gp40 and gp95. Overall, antibody response was more variable and generally weaker than previously observed for SPF cats (20); however, relatively good antibody levels were maintained in several cats even prior to boosting. Lymphoproliferative responses of PBMC to FIV antigen were essentially in the same range as previously detected for SPF cats given the same vaccine and also appeared to be somewhat recalled by the boosters. Generally, cats that had strong antibody responses, as measured by both ELISA and WB, also had strong lymphoproliferative responses to FIV antigen. By contrast, a few cats, most notably Lischi and Romiti, had a divergence of the humoral and cellular immune responses. The significance of the similarity or differences in immune parameters is unclear, since it is still not known what the correlates of protective immunity are in lentivirus infections (12, 13). For example, extensive attempts to identify the correlates of protection induced by the vaccine used here have failed to show a relationship to neutralizing antibody or any other measured immunological parameter (22). In essence, collectively these findings showed that field cats responded to the vaccine in a manner not very dissimilar from SPF cats even though, probably due to their greater genetic and environmental diversity, the immune response mounted was less stereotyped.
The fact that the cats enrolled in this study lived in an environment of hyperendemic clade B FIV infection allowed us to also collect preliminary information with regard to the protective efficacy of the fixed-cell vaccine under natural conditions of contagion. During the 28 months of follow-up, 5 of the 14 unvaccinated control cats developed unequivocal markers of FIV infection. This corresponded to an annual incidence rate of approximately 15%, which was consistent with historical data obtained for the shelter during the 5 years that preceded this experiment. In contrast, none of the 12 vaccinated cats became FIV infected during the same period, despite epidemiological data that would predict two to four infections. Given the small sample size, it is unclear how far these data can be interpreted. At a minimum, they show that vaccination did no harm and that no evidence of virus infection was noted as a consequence of vaccination.
These results raise several interesting questions. How valid are laboratory trials of vaccines using empirically selected amounts of challenge virus that may be relatively high compared to natural exposure? What route(s) of infection is appropriate to simulate natural infection? How important were the two additional boosters used in this trial compared to previous laboratory studies? How much (or little) immunity is sufficient to protect against a natural exposure to virus based on the disparate results in immune parameters in the protected cats?
Thus, although the present results involve too small a study to be regarded as conclusive about the protective efficacy of the vaccine in naturally exposed cats, they nevertheless encourage further testing of fixed-cell anti-FIV vaccines under field conditions. The formidable challenges represented by the development of safe and effective FIV and HIV vaccines (1, 6, 17) stress the need for additional experimentation of this kind.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministero della Sanità—Istituto Superiore di Sanità, “Programma per l'AIDS.”
We are indebted to Letizia Montanari for lovingly operating the cat shelter in which the experiment was performed and for her attentive collaboration.
REFERENCES
- 1.Almond N M, Heeney J C. AIDS vaccine development in primate models. AIDS. 1998;12:S133–S144. [PubMed] [Google Scholar]
- 2.Bachman M H, Sodora D L, Mathhiason-Dubard C, Learn G H, Rodrigo A G, Mazzetti P, Hoover E A, Mullins J I. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates between envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr M C, Zou L, Hozschu D L, Phillips L, Scott F W, Casey J W, Avery R J. Isolation of a highly cytopathic lentivirus from a nondomestic cat. J Virol. 1995;69:7371–7374. doi: 10.1128/jvi.69.11.7371-7374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop S A, Stokes C R, Gruffydd-Jones T J, Whiting C V, Humphries J E, Osborne R, Papanastasopoulou M, Harbour D A. Vaccination with fixed feline immunodeficiency virus (FIV) infected cells: protection, breakthrough and specificity of response. Vaccine. 1996;14:1243–1250. doi: 10.1016/s0264-410x(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 6.Burton D R, Moore J P. Why do we not have an HIV vaccine and how can we make one? Nat Med. 1998;4:495–498. doi: 10.1038/nm0598supp-495. [DOI] [PubMed] [Google Scholar]
- 7.Cammarota G, Da Prato L, Nicoletti E, Matteucci D, Bendinelli M, Pistello M. Quantitation of feline immunodeficiency proviruses in doubly infected cats using competitive PCR and a fluorescence-based RFLP. J Virol Methods. 1996;62:21–31. doi: 10.1016/0166-0934(96)02085-x. [DOI] [PubMed] [Google Scholar]
- 8.Elder J H, Dean G A, Hoover E A, Hoxie J A, Malim M H, Mathes L, Neil J C, North T W, Sparger E, Tompkins M B, Tompkins W A F, Yamamoto J, Yuhki N, Pedersen N C, Miller R H. Lesson from the cat: the immunodeficiency virus as a tool to develop intervention strategies against human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1998;8:495–498. doi: 10.1089/aid.1998.14.797. [DOI] [PubMed] [Google Scholar]
- 9.Elyar J S, Tellier M C, Soos J M, Yamamoto J K. Perspectives on FIV vaccine development. Vaccine. 1997;15:1437–1444. doi: 10.1016/s0264-410x(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 10.Flynn J N, Cannon A, Sloan D, Neil J C, Jarrett O. Suppression of feline immunodeficiency virus replication in vitro by a soluble factor secreted by CD8+ T lymphocytes. Immunology. 1996;96:220–229. doi: 10.1046/j.1365-2567.1999.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannecchini S, Matteucci D, Mazzetti P, Bendinelli M. Incubation time for feline immunodeficiency virus cultures. J Clin Microbiol. 1996;34:2036–2038. doi: 10.1128/jcm.34.8.2036-2038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeney J L, Bruck C, Goudsmit J, Montagnier L, Schultz A, Tyrrell D, Zolla-Pazner S. Immune correlates of protection from HIV infection and AIDS. Immunol Today. 1997;18:4–8. doi: 10.1016/s0167-5699(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 13.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosie M J, Jarrett O. Serological responses of cats to feline immunodeficiency virus. AIDS. 1990;4:215–220. doi: 10.1097/00002030-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Hosie M J, Jarrett O. Analysis of the protective immunity induced by feline immunodeficiency virus vaccination. Adv Vet Med. 1999;41:325–332. doi: 10.1016/s0065-3519(99)80024-x. [DOI] [PubMed] [Google Scholar]
- 16.Jeng C R, English R V, Childers T, Tompkins M B, Tompkins W A F. Evidence for CD8+ antiviral activity in cats infected with feline immunodeficiency virus. J Virol. 1996;70:2474–2480. doi: 10.1128/jvi.70.4.2474-2480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy J A, editor. HIV and the pathogenesis of AIDS. 2nd ed. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 18.Matteucci D, Baldinotti F, Mazzetti P, Pistello M, Bandecchi P, Ghilarducci R, Poli A, Tozzini F, Bendinelli M. Detection of feline immunodeficiency virus in saliva and plasma by cultivation and polymerase chain reaction. J Clin Microbiol. 1993;31:494–501. doi: 10.1128/jcm.31.3.494-501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matteucci D, Mazzetti P, Baldinotti F, Zaccaro L, Bendinelli M. The feline lymphoid cell line MBM and its use for feline immunodeficiency virus isolation and quantitation. Vet Immunol Immunopathol. 1995;46:71–82. doi: 10.1016/0165-2427(94)07007-t. [DOI] [PubMed] [Google Scholar]
- 20.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Lonetti I, Zaccaro L, Pollera C, Specter S, Bendinelli M. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J Virol. 1997;71:8368–8376. doi: 10.1128/jvi.71.11.8368-8376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro L, Bandecchi P, Tozzini F, Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzetti P, Giannecchini S, Del Mauro D, Matteucci D, Portincasa P, Merico A, Chezzi C, Bendinelli M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: detailed analysis of the humoral immune response to a protective vaccine. J Virol. 1999;73:1–10. doi: 10.1128/jvi.73.1.1-10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computer. Comp Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen N C. Feline immunodeficiency virus infection. In: Levy J A, editor. The retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 181–228. [Google Scholar]
- 25.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 26.Pistello M, Cammarota G, Nicoletti E, Matteucci D, Curcio M, Del Mauro D, Bendinelli M. Analysis of genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates high prevalence and heterogeneity of subtype B. J Gen Virol. 1997;78:2247–2257. doi: 10.1099/0022-1317-78-9-2247. [DOI] [PubMed] [Google Scholar]
- 27.Richardson J, Moraillon A, Baud S, Cuisinier A-M, Sonigo P, Pancino G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J Virol. 1995;71:9640–9646. doi: 10.1128/jvi.71.12.9640-9649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 29.Xia X. Data analysis in molecular biology and evolution. Boston, Mass: Kluwer Academic Publishers; 2000. [Google Scholar]
- 30.Yamamoto J K, Ackley C D, Zochlinski H, Louie H, Pembroke E, Torten M, Hansen H, Munn R, Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto J K, Hohdatsu T, Holmsted R A, Pu R, Louie H, Zochlinski H A, Acevedo V, Johnson H M, Soulds G A, Gardner M B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto J K, Okuda T, Ackley C D, Louie H, Pembroke E, Zochlinski H, Munn R J, Gardner M B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991;7:911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]