Abstract
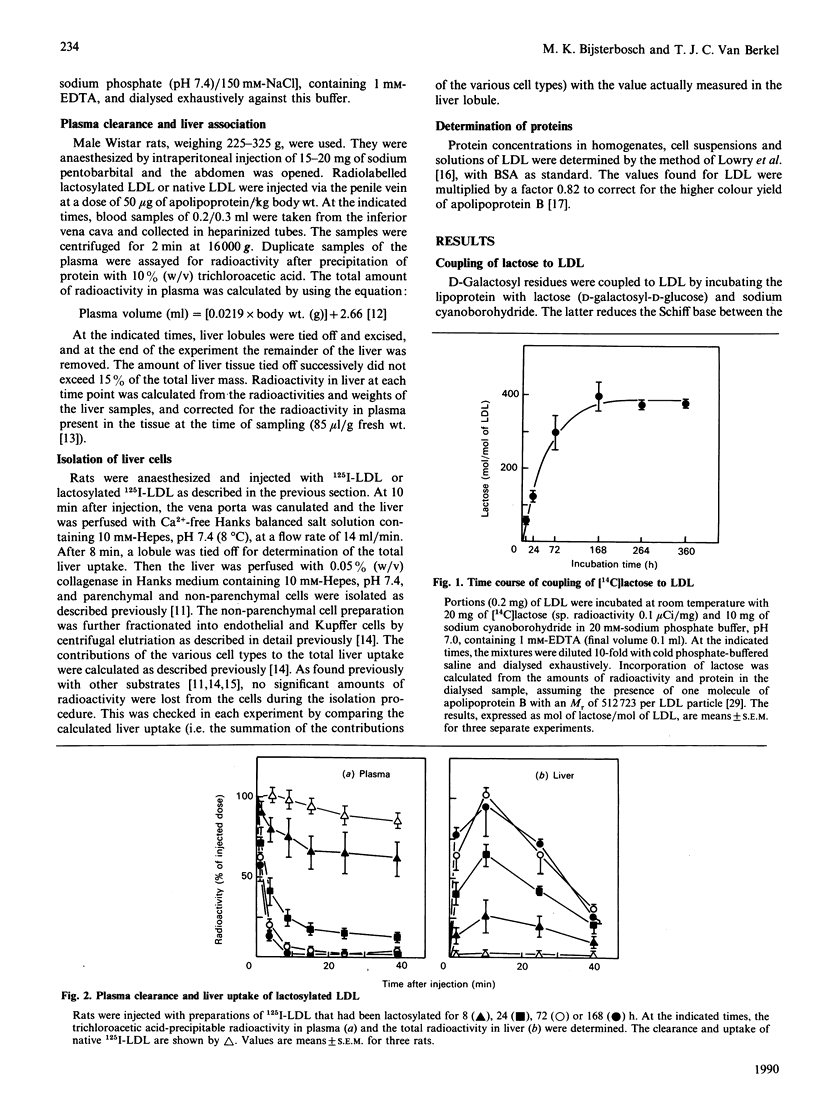
The liver contains two types of galactose receptors, specific for Kupffer and parenchymal cells respectively. These receptors are only expressed in the liver, and therefore are attractive targets for the specific delivery of drugs. We provided low-density lipoprotein (LDL), a particle with a diameter of 23 nm in which a variety of drugs can be incorporated, with terminal galactose residues by lactosylation. Radioiodinated LDL, lactosylated to various extents (60-400 mol of lactose/ mol of LDL), was injected into rats. The plasma clearance and hepatic uptake of radioactivity were correlated with the extent of lactosylation. Highly lactosylated LDL (greater than 300 lactose/LDL) is completely cleared from the blood by liver within 10 min. Pre-injection with N-acetylgalactosamine blocks liver uptake, which indicates that the hepatic recognition sites are galactose-specific. The hepatic uptake occurs mainly by parenchymal and Kupffer cells. At a low degree of lactosylation, approx. 60 lactose/LDL, the specific uptake (ng/mg of cell protein) is 28 times higher in Kupffer cells than in parenchymal cells. However, because of their much larger mass, parenchymal cells are the main site of uptake. At high degrees of lactosylation (greater than 300 lactose/LDL), the specific uptake in Kupffer cells is 70-95 times that in parenchymal cells. Under these conditions, Kupffer cells are, despite their much smaller mass, the main site of uptake. Thus not only the size but also the surface density of galactose on lactosylated LDL is important for the balance of uptake between Kupffer and parenchymal cells. This knowledge should allow us to design particulate galactose-bearing carriers for the rapid transport of various drugs to either parenchymal cells or Kupffer cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Cabana V. G., Hazzard W. R. Immunoassay of human plasma apolipoprotein B. Metabolism. 1975 Dec;24(12):1339–1351. doi: 10.1016/0026-0495(75)90050-5. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Attie A. D., Pittman R. C., Steinberg D. Metabolism of native and of lactosylated human low density lipoprotein: evidence for two pathways for catabolism of exogenous proteins in rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5923–5927. doi: 10.1073/pnas.77.10.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Ziere G. J., Van Berkel T. J. Lactosylated low density lipoprotein: a potential carrier for the site-specific delivery of drugs to Kupffer cells. Mol Pharmacol. 1989 Sep;36(3):484–489. [PubMed] [Google Scholar]
- CASTER W. O., SIMON A. B., ARMSTRONG W. D. Evans blue space in tissues of the rat. Am J Physiol. 1955 Nov;183(2):317–321. doi: 10.1152/ajplegacy.1955.183.2.317. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. R. The direct coupling of oligosaccharides to proteins and derivatized gels. Arch Biochem Biophys. 1974 Jul;163(1):426–428. doi: 10.1016/0003-9861(74)90495-0. [DOI] [PubMed] [Google Scholar]
- Harkes L., Van Berkel J. C. Quantitative role of parenchymal and non-parenchymal liver cells in the uptake of [14C]sucrose-labelled low-density lipoprotein in vivo. Biochem J. 1984 Nov 15;224(1):21–27. doi: 10.1042/bj2240021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I. Distribution of 125I-ligands among the liver cell types. J Cell Biol. 1979 Oct;83(1):47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb-Bachofen V., Schlepper-Schäfer J., Vogell W., Kolb H. Electron microscopic evidence for an asialoglycoprotein receptor on Kupffer cells: localization of lectin-mediated endocytosis. Cell. 1982 Jul;29(3):859–866. doi: 10.1016/0092-8674(82)90447-0. [DOI] [PubMed] [Google Scholar]
- Kuiper J., Otter M., Rijken D. C., van Berkel T. J. Characterization of the interaction in vivo of tissue-type plasminogen activator with liver cells. J Biol Chem. 1988 Dec 5;263(34):18220–18224. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law S. W., Grant S. M., Higuchi K., Hospattankar A., Lackner K., Lee N., Brewer H. B., Jr Human liver apolipoprotein B-100 cDNA: complete nucleic acid and derived amino acid sequence. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8142–8146. doi: 10.1073/pnas.83.21.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of catabolism of rat and human low density lipoproteins in rats. Biochim Biophys Acta. 1982 Jan 15;710(1):7–14. doi: 10.1016/0005-2760(82)90183-7. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Roos P. H., Hartman H. J., Schlepper-Schäfer J., Kolb H., Kolb-Bachofen V. Galactose-specific receptors on liver cells. II. Characterization of the purified receptor from macrophages reveals no structural relationship to the hepatocyte receptor. Biochim Biophys Acta. 1985 Oct 30;847(1):115–121. doi: 10.1016/0167-4889(85)90161-2. [DOI] [PubMed] [Google Scholar]
- Roos P. H., Kolb-Bachofen V., Schlepper-Schäfer J., Monsigny M., Stockert R. J., Kolb H. Two galactose-specific receptors in the liver with different function. FEBS Lett. 1983 Jul 4;157(2):253–256. doi: 10.1016/0014-5793(83)80556-0. [DOI] [PubMed] [Google Scholar]
- Schlepper-Schäfer J., Hülsmann D., Djovkar A., Meyer H. E., Herbertz L., Kolb H., Kolb-Bachofen V. Endocytosis via galactose receptors in vivo. Ligand size directs uptake by hepatocytes and/or liver macrophages. Exp Cell Res. 1986 Aug;165(2):494–506. doi: 10.1016/0014-4827(86)90602-6. [DOI] [PubMed] [Google Scholar]
- Shaw J. M., Shaw K. V., Yanovich S., Iwanik M., Futch W. S., Rosowsky A., Schook L. B. Delivery of lipophilic drugs using lipoproteins. Ann N Y Acad Sci. 1987;507:252–271. doi: 10.1111/j.1749-6632.1987.tb45806.x. [DOI] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Wilson G. Effect of reductive lactosamination on the hepatic uptake of bovine pancreatic ribonuclease A dimer. J Biol Chem. 1978 Apr 10;253(7):2070–2072. [PubMed] [Google Scholar]
- van Berkel T. J., Dekker C. J., Kruijt J. K., van Eijk H. G. The interaction in vivo of transferrin and asialotransferrin with liver cells. Biochem J. 1987 May 1;243(3):715–722. doi: 10.1042/bj2430715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Spanjer H. H., Nagelkerke J. F., Harkes L., Kempen H. J. The effect of a water-soluble tris-galactoside-terminated cholesterol derivative on the fate of low density lipoproteins and liposomes. J Biol Chem. 1985 Mar 10;260(5):2694–2699. [PubMed] [Google Scholar]
- van der Sluijs P., Bootsma H. P., Postema B., Moolenaar F., Meijer D. K. Drug targeting to the liver with lactosylated albumins: does the glycoprotein target the drug or is the drug targeting the glycoprotein? Hepatology. 1986 Jul-Aug;6(4):723–728. doi: 10.1002/hep.1840060431. [DOI] [PubMed] [Google Scholar]


