Introduction
Approximately 60% to 80% of patients with severe asthma have eosinophilic asthma, which is characterized by worse lung function, more severe disease, and increased exacerbation rates relative to the non-eosinophilic phenotype.1,2 Chronic obstructive pulmonary disease (COPD) is a common concomitant condition among patients with asthma. The overlap of COPD and asthma in clinical practice is not well defined as patients may present clinically in a heterogeneous manner and may present with overlapping characteristics with patients with COPD (eg, history of smoking, etc).3 The estimated prevalence of this overlap is 27–33%.3 These patients with both asthma and COPD tend to experience worse outcomes than those with either disease alone.3 For instance, patients with asthma and COPD have more respiratory symptoms, higher risk of exacerbations and comorbidities, and worse quality of life than patients with asthma or COPD alone.3 These patients are also more likely to have persistent airflow obstruction and elevated eosinophil levels.
Management of asthma and concomitant COPD involves treating both underlying conditions and aims to prevent asthma and COPD exacerbations.3 However, there are no clear treatment recommendations for patients with both conditions because of a lack of consensus on how the overlap of these heterogeneous diseases should be defined. Additionally, this patient population has historically been excluded from clinical trials of either asthma or COPD, resulting in limited high-quality clinical evidence to guide treatment decisions.3
Benralizumab is a monoclonal antibody that targets the IL-5 receptor and promotes eosinophil depletion.1 Benralizumab has proven to be effective in significantly reducing the rate of asthma exacerbations among patients with asthma in both clinical trials and real-world studies.1,4 However, there is limited evidence regarding the real-world outcomes of benralizumab among patients with asthma and concomitant COPD. Therefore, this study was conducted to describe the real-world effectiveness of benralizumab in reducing asthma exacerbations and COPD exacerbations among patients with asthma and concomitant COPD.
Methods
Data Source
Laboratory data and insurance claims data from November 2016 to June 2020 were supplied by the PatientSource® database of Source Healthcare Analytics, LLC, a Symphony Health Solutions Corporation, which covers over 80% of the US population and includes commercial, Medicare, and Medicaid claims. Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act of 1996; therefore, no reviews by an Institutional Review Board were required.
Study Design and Population
In this retrospective cohort study, the day after benralizumab initiation was defined as the index date. A pre-post design was implemented to descriptively analyze and compare asthma and COPD exacerbations between the 12-month pre-index and 12-month post-index periods.
Asthma and COPD exacerbations were defined using a claims-based algorithm. An asthma exacerbation was defined as follows based on the healthcare setting in which it occurred:
Inpatient: An asthma diagnosis code as the primary diagnosis for an inpatient stay.
Emergency department (ED): An asthma diagnosis code, ≥1 claim for a systemic corticosteroid (as a single injection or with 3 to <30 days of supply) within ±5 days of the visit, and no diagnosis codes for COPD, autoimmune disease, acute myocardial infarction, or congestive heart failure during the visit.
Outpatient: An asthma diagnosis code, ≥1 claim for a systemic corticosteroid (as a single injection or with 3 to <30 days of supply) within ±5 days of the visit, and no diagnosis codes for COPD or autoimmune disease during the visit.
A COPD exacerbation was defined as follows based on the healthcare setting in which it occurred:
Inpatient: A COPD diagnosis code as the primary diagnosis for an inpatient stay.
ED or outpatient: A COPD diagnosis code and either ≥1 claim for a systemic corticosteroid (as a single injection or with 3 to <30 days of supply) within ±5 days of the visit, and/or ≥1 claim for an antibiotic within ±5 days of the visit.
Exacerbations occurring ≥14 days apart were defined as separate episodes.
As a sensitivity analysis, an alternative definition for COPD exacerbations in the inpatient setting was created to additionally include a diagnosis code for respiratory failure or infection as the primary diagnosis with a COPD diagnosis code as a secondary diagnosis.
Patients were included in the study if they were aged ≥12 years, had ≥2 records of benralizumab, were biologic-naïve during the 12-month pre-index period, and had ≥1 asthma diagnosis and ≥1 COPD diagnosis in the pre-index period. The following two cohorts were identified based on number of COPD exacerbations in the pre-index period: (1) patients with ≥1 COPD exacerbation, and (2) patients with ≥2 COPD exacerbations. Patients with ≥1 COPD exacerbation and blood eosinophil laboratory values available pre-benralizumab initiation were additionally stratified by eosinophil level (<150 cells/µL, ≥150 cells/µL, <300 cells/µL, ≥300 cells/µL).
Study Measures and Statistical Analysis
Patient characteristics were summarized descriptively with means and standard deviations (SDs) for continuous variables and counts and proportions for categorical variables. Within each cohort, the rate of asthma and COPD exacerbations per person-year (PPY) was compared between the pre-index and post-index periods using generalized estimating equations (GEE). No comparisons between cohorts were conducted.
Results
Patient Characteristics
The initial data set included 7276 patients with ≥1 benralizumab record and an asthma diagnosis. After applying inclusion and exclusion criteria (ie, aged ≥12 years, ≥2 benralizumab records, biologic-naïve, ≥1 asthma diagnosis, and ≥1 COPD diagnosis pre-index), a total of 1406 patients with ≥1 COPD diagnosis were included in the overall sample, of which 797 had ≥1 COPD exacerbation and 379 had ≥2 COPD exacerbations. Patients were classified based on peripheral blood eosinophil counts and included 62 patients with <150 cells/µL, 142 patients with ≥150 cells/µL, 108 patients with <300 cells/µL, and 96 patients with ≥300 cells/µL.
Across cohorts, the mean age by cohort ranged from 60.8 to 62.1 years, and the majority were female (60.2% to 62.5%). Most patients were from the South (37.5% to 53.2%), followed by the Northeast (11.5% to 31.0%) and Midwest (9.7% to 24.8%). Common comorbidities included hypertension (62.2% to 71.8%), hyperlipidemia (45.9% to 56.7%), mental disorders (44.2% to 63.1%), and allergic rhinitis (41.2% to 42.5%).
Rate of Asthma Exacerbations
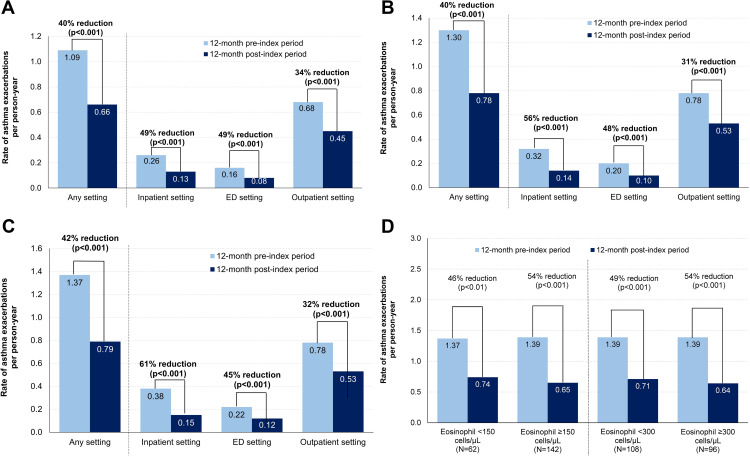
Across all cohorts and healthcare settings, the rate of asthma exacerbations decreased significantly after benralizumab initiation (all p<0.001; Figure 1). In the overall sample, there was a 40% reduction in the rate of asthma exacerbations in any setting from the pre-index period to the post-index period (1.09 to 0.66 PPY, respectively; Figure 1A). From the pre-index period to the post-index period, there was a 40% reduction in the rate of asthma exacerbations in any setting among patients with ≥1 COPD exacerbation (1.30 to 0.78 PPY, respectively; Figure 1B) and a 42% reduction among patients with ≥2 COPD exacerbations (1.37 to 0.79 PPY, respectively; Figure 1C). Consistent decreases were observed in all eosinophil subgroups of the ≥1 COPD exacerbation cohort, with rate reductions ranging from 46% to 54% (Figure 1D).
Figure 1.
Rate reductions of asthma exacerbations after benralizumab initiation across healthcare settings in the (A) overall sample, (B) ≥1 COPD exacerbation cohort, (C) ≥2 COPD exacerbations cohort, and (D) eosinophil subgroups of the ≥1 COPD exacerbation cohort.
Abbreviations: COPD, chronic obstructive pulmonary disease; ED, emergency department.
Rate of COPD Exacerbations
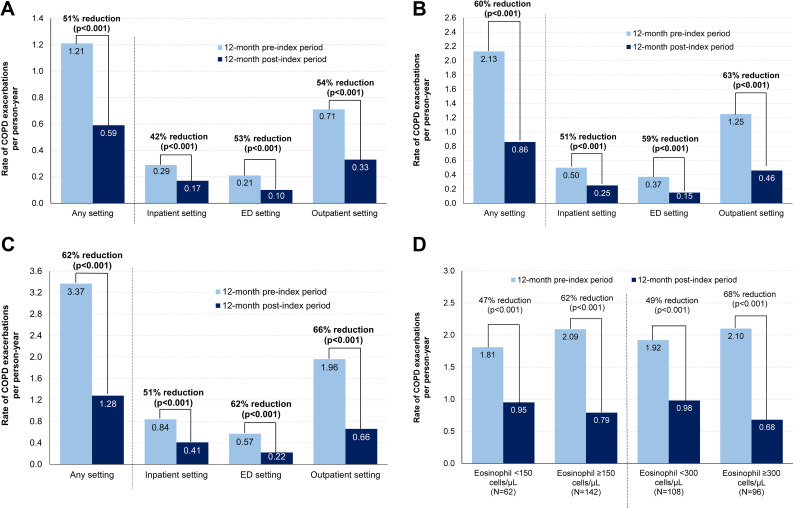
Across all cohorts and healthcare settings, the rate of COPD exacerbations decreased significantly after benralizumab initiation (all p<0.001; Figure 2). In the overall sample, there was a 51% reduction in the rate of COPD exacerbations in any setting from the pre-index period to the post-index period (1.21 to 0.59 PPY, respectively; Figure 2A). Based on the sensitivity definition, there was also a 51% reduction in the rate of COPD exacerbations in any setting from the pre-index period to the post-index period (any setting: 1.26 to 0.62 PPY; inpatient: 0.34 to 0.20 PPY; ED: 0.21 to 0.10 PPY; outpatient: 0.70 to 0.32 PPY; all p<0.001). From the pre-index period to the post-index period, there was a 60% reduction in the rate of COPD exacerbations in any setting among patients with ≥1 COPD exacerbation (2.13 to 0.86 PPY, respectively; Figure 2B) and a 62% reduction among patients with ≥2 COPD exacerbations (3.37 to 1.28 PPY, respectively; Figure 2C). Consistent decreases were observed in all eosinophil subgroups of the ≥1 COPD exacerbation cohort, with rate reductions ranging from 47% to 68% (Figure 2D).
Figure 2.
Rate reductions of COPD exacerbations after benralizumab initiation across healthcare settings in the (A) overall sample, (B) ≥1 COPD exacerbation cohort, (C) ≥2 COPD exacerbations cohort, and (D) eosinophil subgroups of the ≥1 COPD exacerbation cohort.
Abbreviations: COPD, chronic obstructive pulmonary disease; ED, emergency department.
Discussion
In this real-world, pre-post study of patients with asthma and concomitant COPD, benralizumab initiation was associated with significantly reduced rates of both asthma and COPD exacerbations across all healthcare settings. Consistent decreases were observed among patients with prior COPD exacerbations and across all baseline eosinophil levels.
To our knowledge, this is the first study to evaluate the effect of benralizumab on asthma and COPD exacerbations among patients who have coexistent coding for both conditions, thus contributing important insight to the limited breadth of literature involving this patient population. This population continues to pose a challenge in clinical practice as it is not as well defined, partially due to differing definitions of patients who have both asthma and COPD.3 The population with severe asthma may present clinically in a heterogenous manner and may have overlapping characteristics with patients with COPD, such as those with a smoking history or persistent airflow obstruction on spirometry (FEV1/FVC <70%).3 However, patients with asthma and concomitant COPD, when compared to patients with either disease, are known to have worse outcomes, and understanding the impact of treatment for these patients is of particular importance.3 Benralizumab has previously been evaluated in clinical trials of patients with either asthma or COPD.1,5 The benefits of benralizumab in severe eosinophilic asthma have been well characterized and treatment resulted in significantly lower annual asthma exacerbation rates, decreased need for systemic corticosteroids, and improvements in asthma symptom scores compared to placebo.1 With regards to COPD, a post hoc analysis of the randomized, double-blind, placebo-controlled, Phase 3 clinical trials GALATHEA and TERRANOVA demonstrated that benralizumab significantly reduced the risk of moderate or severe COPD exacerbations by 60% or 42% compared with placebo during the 30-day or 90-day periods, respectively, following an initial exacerbation among a responder population of patients with high eosinophil counts and frequent COPD exacerbations (p<0.01).5 However, the aforementioned clinical trials, and other clinical trials, exclude patients with both asthma and COPD.
The few studies evaluating biologics in patients with asthma and concomitant COPD are largely in the real-world setting.3 Shim et al recently used data from the prospective, observational PRISM study to identify a cohort of 94 patients with severe asthma (81 with asthma only, 13 with asthma and concomitant COPD) who were treated with biologics (omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab) in clinical practice.6 Both the asthma and asthma plus concomitant COPD groups experienced improvements in asthma symptom scores and exacerbation frequency after 6 months of biologic treatment, with no significant differences between the two patient groups, suggesting that patients with both diseases have similar treatment response patterns to those with asthma only.6 In a small claims-based study of 221 patients with asthma and COPD, significant reductions in the mean rate of asthma exacerbations and oral corticosteroid use were observed after 12 months of treatment with mepolizumab (p<0.001).7 While the use of different biologics in these prior studies limits comparability, they are nonetheless aligned with the current study findings in supporting the use of biologics in patients with asthma and concomitant COPD. Further research is warranted to provide evidence-based treatment guidance for this especially vulnerable population of patients with both conditions.
A major strength of this study is the inclusion of a large sample size from a data source that covers 80% of the US population; therefore, this study sample is likely representative of patients with asthma and concomitant COPD in the US. Despite this strength, the study findings should be interpreted within the context of some limitations. A claims-based algorithm was used to identify asthma and COPD exacerbations, which may be subject to misclassification. Further, there may also be misclassification due to the incorrect recording of ICD codes by physicians. This study evaluated the treatment effect of benralizumab using a pre-post study design and did not include a control arm to adjust for potential temporal changes. While any free doses of benralizumab obtained by patients would not have been visible in the claims data, this would have been rare and unlikely to affect the results that were observed. Future studies including more variables of interest (eg, immunoglobulin E levels, exhaled nitric oxide, smoking history, and lung function) for additional patients would be valuable.
Conclusion
In this real-world study, significant and consistent reductions in both asthma and COPD exacerbations were observed after benralizumab initiation among patients with asthma and concomitant COPD, including those with prior COPD exacerbations and across all baseline eosinophil levels. These findings provide new insights into the benefits of benralizumab in reducing both asthma and COPD exacerbations in patients with asthma and concomitant COPD.
Acknowledgments
The authors thank Christine Tam, MWC, of Analysis Group, Inc., for providing professional medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP 2022) (https://www.ismpp.org/gpp-2022) guidelines.
Part of the material in this manuscript was presented at the American College of Allergy, Asthma and Immunology (ACAAI) Annual Scientific Meeting 2023, held November 9–13 in Anaheim, CA, USA and the Western Society of Allergy, Asthma & Immunology (WSAAI) 61st Annual Scientific Session 2024, held February 4–8 in Koloa, HI, USA as poster presentations.
Funding Statement
This study was funded by AstraZeneca. AstraZeneca was involved in several aspects of the research, including the study design and interpretation of data. The authors were involved in the writing of the manuscript and decision to submit the manuscript for publication. AstraZeneca reviewed the publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy, and the protection of intellectual property.
Data Sharing Statement
The data that support the findings of this study are available from Source Healthcare Analytics, LLC, a Symphony Health Solutions Corporation. Restrictions apply to the availability of these data, which were used under license for this study. Data may be requested directly through Source Healthcare Analytics, LLC.
Ethics Statement
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act of 1996; therefore, no review by an Institutional Review Board was required.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
DC, JKD, and YC are employees and shareholders of AstraZeneca, which funded the development and conduct of this study and manuscript. DY, EEC, FM, and JC are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to AstraZeneca, which funded the development and conduct of this study and manuscript. DJM received consultant/speaker fees from AstraZeneca, GSK, Amgen, Sanofi/Regeneron. The authors report no other conflicts of interest in this work.
References
- 1.Cushen B, Menzies-Gow A. Benralizumab: an updated treatment of eosinophilic asthma. Expert Rev Respir Med. 2020;14(5):435–444. doi: 10.1080/17476348.2020.1739526 [DOI] [PubMed] [Google Scholar]
- 2.Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160(3):814–830. doi: 10.1016/j.chest.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 3.Maselli DJ, Hanania NA. Management of asthma COPD overlap. Ann Allergy Asthma Immunol. 2019;123(4):335–344. doi: 10.1016/j.anai.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 4.Carstens D, Maselli DJ, Mu F, et al. Real-world effectiveness study of benralizumab for severe eosinophilic asthma: ZEPHYR 2. J Allergy Clin Immunol Pract. 2023;11(7):2150–2161e2154. doi: 10.1016/j.jaip.2023.04.029 [DOI] [PubMed] [Google Scholar]
- 5.Singh D, Criner GJ, Agusti A, et al. Benralizumab prevents recurrent exacerbations in patients with chronic obstructive pulmonary disease: a post hoc analysis. Int J Chron Obstruct Pulmon Dis. 2023;18:1595–1599. doi: 10.2147/COPD.S418944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim JS, Kim H, Kwon JW, et al. A comparison of treatment response to biologics in asthma-COPD overlap and pure asthma: findings from the PRISM study. World Allergy Organ J. 2023;16(12):100848. doi: 10.1016/j.waojou.2023.100848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casale T, Molfino NA, Silver J, et al. Real-world effectiveness of mepolizumab in patients with severe asthma and associated comorbidities. Ann Allergy Asthma Immunol. 2021;127(3):354–362e352. doi: 10.1016/j.anai.2021.05.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Source Healthcare Analytics, LLC, a Symphony Health Solutions Corporation. Restrictions apply to the availability of these data, which were used under license for this study. Data may be requested directly through Source Healthcare Analytics, LLC.