Abstract
Metabolic health is highly dependent on intestinal and hepatic handling of dietary and endogenous lipids and lipoproteins. Disorders of lipid and lipoprotein metabolism are commonly observed in patients with insulin resistant states such as obesity, metabolic syndrome, and type 2 diabetes. Evidence from both animal models and human studies indicates that a major underlying factor in metabolic or diabetic dyslipidemia is the overproduction of hepatic and intestinal apolipoprotein (apo)B-containing lipoprotein particles. These particles are catabolized down into highly proatherogenic remnants, which can be taken up into the arterial intima and promote plaque development. Several gut-derived peptides have been identified as key regulators of energy metabolism; one such peptide is the incretin hormone glucagon-like peptide (GLP)-1. Our laboratory has previously demonstrated that GLP-1 can signal both centrally and peripherally to reduce postprandial and fasting lipoprotein secretion. Moreover, we have demonstrated that GLP-1 receptor (GLP-1R) agonists can ameliorate diet-induced dyslipidemia. Recently, we published evidence for a novel vagal neuroendocrine signalling pathway by which native GLP-1 may exert its anti-lipemic effects. Furthermore, we demonstrated a novel role for other gut-derived peptides in regulating intestinal lipoprotein production. Overall, ample evidence supports a key role for GLP-1R on the portal vein afferent neurons and nodose ganglion in modulating intestinal fat absorption and lipoprotein production and identifies other gut-derived peptides as novel regulators of postprandial lipemia. Insights from these data may support identification of potential drug targets and the development of new therapeutics targeting treatment of diabetic dyslipidemia.
Keywords: glucagon-like peptide-1, lipid, lipoprotein, intestine, neuroendocrine
Introduction
The intestine regulates several aspects of metabolism via secretion of enteroendocrine hormones from a variety of specialized cells in the intestinal epithelium. These peptide hormones have key roles in modulating components of gastrointestinal function such as gastric acid secretion, intestinal motility, and gall bladder contraction. Importantly, several of these hormones have also been implicated in the regulation of metabolism on a global scale, influencing satiety, glucose homeostasis, thermogenesis, and nutrient mobilization [1]. Indeed, some of these peptides have already been shown to influence lipoprotein metabolism, such as glucagon-like peptide (GLP)-1 (discussed below) [2]. Importantly, these peptides can signal both locally within the GI tract and to peripheral and central nervous centers to regulate components of metabolism [3].
Glucagon-like peptides (GLPs)
GLP-1 is a 31 amino acid peptide that acts as an incretin hormone, potentiating the release of insulin. It is 50 % homologous with glucagon [4], and the majority of its presence in the circulation originates from the intestinal L-cell [5]. GLP-1 has been the focus of several pharmacological interventions for the treatment of metabolic disease due to its ability to normalize body weight, blood glucose, and blood lipids in obese and type 2 diabetic (T2D) individuals [6]. GLP-2 on the other hand is used as an intestinotrophic drug for the treatment of short bowel disease due to its ability to stimulate enterocyte growth and improve intestinal barrier function [7]. GLPs bind to their eponymous G-protein coupled receptors GLP-1 receptor (GLP-1R) and GLP-2 receptor (GLP-2R). The focus of this review will center on GLP-1 and its effects on postprandial and fasting lipoprotein production.
GLP-1 and GLP-2 are derived from the proglucagon gene, present in the pancreatic alpha cells, intestinal endocrine L-cells, and in neurons of the nucleus tractus solitarius (NTS) in the caudal brainstem [4, 8]. Post-translational processing of the 158 amino acid proglucagon polypeptide will yield a tissue-specific profile of metabolic hormones. In the intestine and brainstem, prohormone convertase (PC)-1/3 cleaves proglucagon into GLP-1, GLP-2, intervening peptide (IP)-2, oxyntomodulin, and glicentin; whereas, in the pancreatic alpha cells, PC-2 will cleave proglucagon to produce glucagon, IPs, glicentin-related polypeptide (GRPP), and major proglucagon fragment (MPFG). Importantly, GLP-1 and GLP-2 are produced at an equimolar concentration, however, have diverging metabolic effects [2]. Notably, both GLP-1 and GLP-2 are still present in the fasting state with a circulating concentration of 5–10 pmol/L of GLP-1 (in a study conducted in 2009 on children during neonatal period) [9] and 116±22 pg/mL of GLP-2 (in a study conducted in 1997 in adults (6 adults, male and female, aged 23–32) [10]. GLPs are primarily released in response to nutrient consumption and display a biphasic pattern of secretion [11]. Direct contact of nutrients with intestinal L-cells on the apical intestinal lumen produces an initial secretory peak as early as 10–15 min after consumption of a meal, followed by a secondary peak around 30–60 min post-consumption [11]. Secretion is prompted by several macronutrients, including glucose, fatty acids, and amino acids [11, 12]. Fatty acids in particular produce a more sustained elevation in GLP-1 secretion compared to glucose, mediated by intestinal L-cell expression of the long chain fatty acid receptors GPR40 and GPR120 [13] which have been shown to stimulate GLP-1 secretion [14, 15]. This is in line with evidence that intraduodenal lipid emulsion in rats increases GLP-1 secretion in a dose dependent manner [16]. However, the initial peak in GLP-1’s biphasic secretion is likely mediated not by direct nutrient contact but via a more rapid mechanism such as neural signaling, since the majority of intestinal L-cells are located in the distal ileum, and small intestinal transit time can exceed 3 h [17, 18]. This is supported by evidence that stimulation of the vagus nerve – which provides parasympathetic innervation to the gut – with a bipolar electrode potentiates GLP-1 secretion in an in-situ rat model. In turn, subdiaphragmatic truncal vagotomy has the opposite effect, abrogating early rises in GLP-1 induced by lipid load [19]. Similarly, in vivo parasympathetic blockade in humans and rats has been demonstrated to impair early GLP-1 secretion [20, 21].
GLP-1R
GLP-1 binds to the GLP-1R, a class B heterotrimeric G protein-coupled receptor (GPCR) with 463 amino acid residues that spans seven transmembrane domains [22]. The receptor exhibits diverse tissue expression but is most notably found in the pancreatic beta-cells, stomach, duodenum, vagal afferent nerves, lungs and in the CNS [23, 24]. The extracellular domain of the GLP-1R binds with the C-terminal half of GLP-1, and the third transmembrane domain interacts with the N-terminal half of GLP-1 [25]. Transmembrane topology is common to all class B GPCRs, with the N-terminal binding of G-proteins in this domain crucial for the selective recognition of peptide ligands [26]. Thus, peptides that have an N-terminal truncation such as exendin 9–39 will act as GLP-1R antagonists [27]. Upon successful binding of GLP-1, receptor-bound adenylyl cyclase will catalyze the conversion of ATP to cyclic AMP (cAMP), leading to subsequent activation of protein kinase A (PKA) and the exchange protein directly activated by cAMP (EPAC) family. In the beta-cell this increase in PKA causes ADP-dependent phosphorylation of the SUR1 K(ATP) channel subunit, ultimately triggering the insulin secretory pathway via changes in membrane depolarization [28]. Moreover, there is evidence that in addition to inhibition of ATP-regulated potassium channels PKA and EPAC can increase the activity of L-type voltage gated calcium channels (VGCCs) and opening of non-specific cation channels [28], [29], [30]. Increased phosphoinositol turnover results in additional release of intracellular Ca2+ via phospholipase-C-mediated production of inositol triphosphate (IP)3 [31]. Together leading to increased calcium influx to the cell, and calcium induced insulin secretion. Diacylglycerol generation from this pathway and elevated intracellular Ca2+ will also result in the activation of protein kinase C (PKC). Elevated PCK will phosphorylate extracellular signal-regulated kinase (ERK)1/2, which in turn phosphorylates the C-terminus of the GLP-1R [32]. Importantly, spatiotemporal control of GPCR signaling is classically mediated by receptor desensitization and cellular internalization. Wherein, the phosphorylation of the C-terminus by ERK1/2 recruits β-arrestins to sterically hinder interactions with GPCR kinases, initiating endocytosis likely via the assembly of clathrin-coated pits [24, 33]. However, there is emerging evidence that internalized GPCRs such as GLP-1R molecules may continue to signal within endosomes [34].
While there is notable overlap with the periphery, less is known about GLP-1R signaling in the CNS. It is hypothesized that GLP-1 works primarily through enhanced calcium influx through VGCCs. In the hindbrain VGCC activation is mediated by PKA, whereby, calcium influx activates mitogen-activated protein kinase (MAPK) and suppresses AMP-activated protein kinase (AMPK) [35]. Similarly, in midbrain structures such as the hypothalamus and hippocampus GLP-1R activation has been shown to increase cAMP and activate L-type VGCCs via cAMP response element binding protein (CREB) [35, 36]. PKA also has been shown to enhance glutamatergic transmission via phosphorylation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor glutamate receptor (GluR)1 subunit in the paraventricular nucleus of the hypothalamus [37].
Biological functions of GLP-1
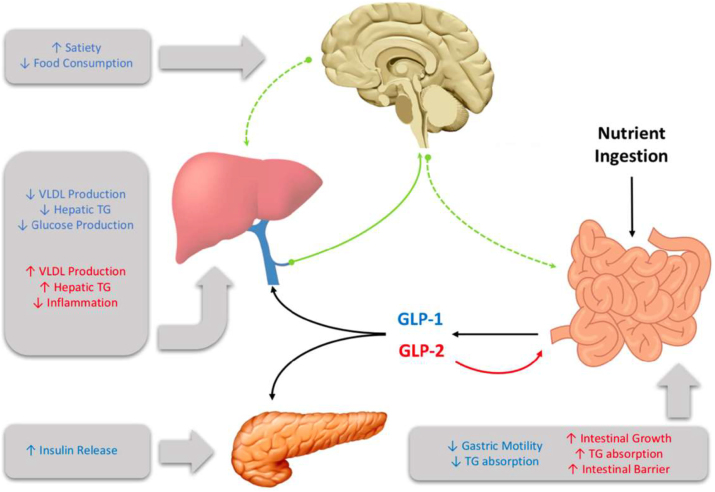
GLP-1 influences several aspects of metabolism (Figure 1). In the periphery it stimulates insulin secretion in a glucose-dependent manner, and in the brain it enhances satiety and reduces food consumption [38]. It has also been shown to have important glucoregulatory effects, with chronic treatment in T2D individuals resulting in attenuated fasting plasma glucose levels and improved hemoglobin A1c levels [39]. Moreover, patients demonstrate improved insulin sensitivity and enhanced beta cell function. Whereas, the reverse was seen when the GLP-1R antagonist exendin 9–39 was administered to healthy men, which resulted in elevations in fasting plasma glucose levels [40]. Similarly, portal vein infusion of exendin 9–39 in rats has been shown to worsen glucose tolerance [41]. Direct effects of GLP-1 include the inhibition of glucagon and somatostatin secretion, resulting in suppressed hepatic glucose production and potentiated insulin release, respectively [42], [43], [44]. In humans exenatide monotherapy has been shown to reduce plasma TG levels [45] and postprandial ApoB48 biosynthesis [46]. These observations are consistent in individuals with impaired glucose tolerance, where a single dose of the GLP-1R agonist exendin-4 reduced postprandial plasma TG, ApoB48, and remnant lipoprotein cholesterol and TG [47].
Figure 1:
Glucagon-like peptide (GLP)-1 and GLP-2 are multi-organ hormones that exert their effects through both central and peripheral signalling.
Direct impact of GLP-1 on hepatic functions
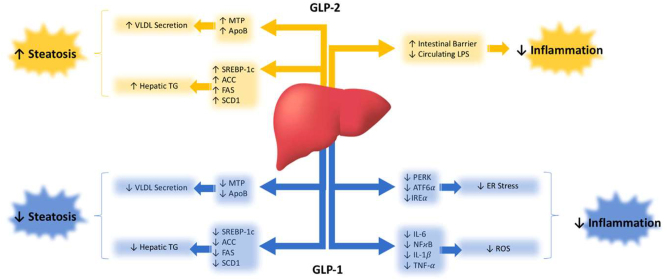
The complete understanding of the metabolic repercussions of GLP-1 and GLP-2 on hepatic lipid and lipoprotein metabolism is still pending (Figure 2). Nonetheless, both play a significant role in influencing hepatic health. When primary hepatocytes and immortalized cell lines are exposed to GLP-1R agonists, the outcomes appear to be diverse. Despite reports indicating the expression and functional activity of GLP-1Rs in immortalized hepatocyte cell lines like HuH7 and HepG2, as well as in primary human hepatocytes [48], the effects vary. Upon treatment with the GLP-1R agonist exendin-4, steatotic HuH7 and HepG2 cells exhibited reduced lipid accumulation compared to vehicle controls, as evidenced by oil red O staining [48]. Furthermore, HepG2 cells treated with liraglutide demonstrated a dose-dependent decrease in protein and mRNA levels of proprotein convertase subtilisin/kexin type 9 (PCSK9), a pro-hypercholesterolemic factor [49]. In contrast, McArdle cells treated with palmitic acid displayed increased expression of genes involved in de novo lipogenesis (SREBP-1c, FAS, SCD1, and ACC). Co-incubation with exendin-4, however, mitigated this steatotic phenotype [50]. Surprisingly, ex vivo treatment of primary hamster hepatocytes with exendin-4 did not alter cAMP production, an indicator of GLP-1R signaling [51]. This is unexpected since GLP-1R activation is anticipated to elevate cAMP within hepatocytes, leading to increased phosphorylation of AMPK, a key enzymatic suppressor of lipogenesis [52]. Consequently, conflicting evidence exists regarding the presence of GLP-1Rs on hepatocytes [53], [54], [55], [56]. Interestingly, however, a direct effect of GLP-1 infusion on endogenous glucose production has been demonstrated in humans under conditions where plasma insulin and glucagon are not allowed to change and glucose concentrations are matched, suggesting a potential direct effect on hepatocytes [57].
Figure 2:
Glucagon-like peptide (GLP)-1 and GLP-2 modulate liver health through various metabolic signalling cascades.
Similar uncertainties surround GLP-2R, which is acknowledged to be expressed in the nervous system and on enteric neurons, myofibroblasts, and enteroendocrine cells of the gut. However, its presence in the liver is contentious, with some studies suggesting its presence [58] and others indicating its absence [59] in hepatocytes. A study investigating the localization of intravenously injected radiolabeled 125 I-GLP-2 (1–33) proposed that the primary site of GLP-2R-specific binding is within the small intestine. The liver and kidney were proposed to metabolize GLP-2 through a non-specific metabolic pathway [60]. Consequently, further research is imperative to definitively establish whether GLP-1 and GLP-2 directly exert their metabolic effects on the liver.
Direct intestinal effects of GLP-1
In humans GLP-1 has been shown to slow digestion by suppressing gastric acid secretion [61] and slowing gastric emptying [62, 63]. In rats GLP-1 has been shown to lower plasma lipid accumulation after intraduodenal fat load by suppressing intestinal lymph flow, triglyceride absorption, and ApoB production [64]. Similarly, GLP-1R agonist treatment in Syrian golden hamsters suppressed hepatic very-low density lipoprotein (VLDL) and TRL TG accumulation, with tandem reductions in TRL ApoB48 [65]. Peripheral exendin-4 treatment has also been shown to depress intestinal microsomal triglyceride transfer protein (MTP) activity, decreasing the lipidation and secretion of ApoB48 particles after lipid load [66]. Recently, the GLP-1R has been found in human colon cell culture models, and in the colonic epithelium [67]. Similarly, in CD1 mice GLP-1R expression was found by immunohistochemical staining to be localized to the mucosal villi layer of the ileum and colon [68]. Interestingly, while GLP-1Rs are not found on Villin+enterocytes and are predominantly localized to intraepithelial lymphocytes (IELs) [69]. Regardless, GLP-1R agonist treatment has been shown to reduce ApoB48 secretion from primary hamster enterocyte cultures [65]. Similarly, exendin-4 has been shown to directly increase cAMP activity in these IELs, however, their role in modulating enterocyte function has yet to be fully explored [69]. GLP-1R mRNA has also been found in acid secreting parietal cells [70] where they may block gastric acid secretion and in specialized mucus secreting cells in the proximal duodenum called Brunners glands [71].
GLP-1R expression is also prominent in the enteric nervous system (ENS). In reporter mice expressing yellow fluorescent protein (YFP) under a GLP-1R-Cre, expression was found in a subpopulation of enteric neurons, the action potential frequency of which could be modulated ex vivo by GLP-1 administration [23]. Interestingly, a significant proportion of these ENS neurons were positive for neuronal nitric oxide synthase (nNOS) expression, which has been linked to the pro-lipemic properties of GLP-2 [72]. RNA seq data suggests that these neurons may be secretomotor/vasodilatory in nature [73]. However, their relative importance to GLP-1s anti-lipemic effects may be minimal as GLP-1R KO in the enteric neurons of mice did not affect plasma TG accumulation after an oral fat load [74].
Gut-brain axis
Recent data has demonstrated a strong role for bidirectional neuronal governance over intestinal and hepatic lipid metabolism. The intestine itself boasts its own independent neural network called the enteric plexus, which is comprised of over 100 million neurons that can operate autonomously, or in tandem with afferent and efferent sensory feedback from the CNS [75]. To complicate this, the intestine is the largest endocrine organ in the body, secreting over 100 bioactive peptides, which can act in an autocrine, paracrine, and neuroendocrine manner [76]. These hormonal signals when released upon nutrient consumption can signal through binding to vagal or somatosensory afferent nerve fibers which project to the dorsal vagal complex in the brainstem, in turn relaying sensory information to key metabolic regulatory nuclei in the hypothalamus. Alternatively, some hormones may be able to circulate and directly bind to receptors in hypothalamic nuclei, either by active transport through the blood brain barrier, or via access to circumventricular organs [77], [78], [79], [80]. This gut-brain axis has already been implicated in the signaling of several key hormones released by the gut such as GLP-1, CCK, and PYY. These hormones act as intermediate messengers to signal information via peripheral nerves about meal size and composition to the brain [1, 81]. Hypothalamic centers in the brain then act on these signals to alter key components of metabolism such as energy expenditure, food intake, and gastrointestinal function [82, 83]. Many have demonstrated the integral role of the vagus nerve in mediating this system, as CCK-related satiation is dependent on intact gastric and duodenal vagal afferent signaling [84, 85]. Similarly, the inhibitory effects of PYY and GLP-1 and the stimulatory effects of ghrelin on feeding, were lost after vagotomy [86], [87], [88]. In the same vein, the anorectic effects of GLP-1 by intraperitoneal exendin-4 administration were also lost after capsaicin-mediated vagal denervation [89]. Loss of certain subpopulations of vagal nerves shows similar results, where selective denervation of CCK receptor-containing vagal afferents abolished the satiating effects of GLP-1 and CCK, as well as feeding-induced c-fos expression in the NTS [90]. The relative contributions of the peripheral and central nervous systems are discussed further below.
Peripheral neural control of metabolism
The peripheral nervous system consists of nerves and associates ganglia which lie outside the brain and spinal cord. Autonomic signals from these nerves deliver sympathetic and parasympathetic drive to the body wall and viscera. The sympathetic system provides the “fight or flight” response, and its effects are mediated through stimulatory neurotransmitters such as norepinephrine and epinephrine. Pre-ganglionic sympathetic motor neurons originate from the ventral horn of the spinal cord, they then travel through ventral rootlets to the white rami communicantes of spinal nerves, where they synapse on post-ganglionic sympathetic neurons in sympathetic chain ganglia. Contributions of several spinal levels spanning thoracic spinal nerve 5 (T5) to T12 form sympathetic splanchnic nerves which innervate the viscera [91]. In contrast, the parasympathetic system provides the “rest and digest” response, mediated by release of the neurotransmitter acetylcholine. Parasympathetic neurons which innervate the viscera do not originate from the spinal cord, but from cranial nerves which project directly from the brainstem. Together, the sympathetic greater and lesser splanchnic nerves, and parasympathetic anterior and posterior vagal trunks coalesce in the celiac plexus (solar plexus), which is a network of interconnecting fibers that innervate the abdominal contents, both uniquely influencing metabolism and lipoprotein production [87].
Neural control of hepatic lipoprotein metabolism by GLP-1
It has long been known that GLP-1 and GLP-2 can act as neurotransmitters in the brain [92]. Early studies have shown that hindbrain pre-proglucagon (PPG) producing neurons in the NTS can be activated even without gut-associated hormone release, just by simple mechanical distention of the stomach [93]. This, paired with the observation that GLP-1 can act as a neuroendocrine signalling peptide in the circulation tightly links central and peripheral GLP-1 signalling to the regulation of energy homeostasis and satiety. Although the effect of GLP-1 in regulating satiety has been known for some time, the relative contribution of GLP-1 to hepatic and intestinal lipid homeostasis is only recently emerging. Intracerebroventricular (ICV) injection of active GLP-1(7–37) peptide into the brains of HFD-fed mice resulted in enhanced Akt-mediated hepatic insulin signalling during hyperinsulinemic-euglycemic clamp experiments. This was associated with elevations in insulin secretion, improved glucose tolerance, and decreased hepatic TG accumulation in the livers of HFD-fed mice. In contrast, ICV injection of the GLP-1R antagonist exendin 9–39 impaired the suppressive effects of insulin on hepatic glucose production, suggesting that central GLP-1R inhibition deteriorates hepatic insulin signalling. Moreover, central GLP-1R agonism selectively attenuated hepatic TG accumulation in HFD-fed mice, with no observed change in muscle, white adipose tissue (WAT), or plasma TG during hyperinsulinemia [93]. ICV injections of exendin-4 have also been shown to lower fasting hypertriglyceridemia and hepatic VLDL production in a dietary fructose-induced dyslipidemic hamster model [50]. Similarly, both acute and chronic treatment with ICV exendin-4 has been shown to reduce circulating plasma TG, cholesterol, and low-density lipoprotein cholesterol (LDLc) in addition to hepatic lipids. This was further associated with reductions in hepatic expression of sterol regulatory element binding protein (SREBP)-1c and elevated LDL receptor expression, which occurred independent of food consumption [94].
Separate experiments have shown that bilateral injection of active GLP-1(7–37) peptide into the dorsomedial hypothalamus (DMH) of mice results in increased TG mobilization from the liver. Alternatively, GLP-1R knockdown in the DMH induced hepatic steatosis, coupled with elevated de novo lipogenesis, and the development of insulin resistance [95]. This is in line with previous observations that ICV administration of exendin-4 increases sympathetic outflow to brown adipose tissue (BAT) and WAT depots, resulting in increased thermogenesis and uptake of fatty acid (FA) to these tissues. Interestingly, this effect on WAT was seen to be blunted in a diet-induced obese mouse model [96]. Recently, chronic ICV infusion of a GLP-1R and glucagon receptor co-agonist has been shown to significantly decrease plasma and liver lipids in a cholesterol-fed hamster model of dyslipidemia. Co-agonist treated hamsters also showed increased hepatic TG excursion, and depressed expression of hepatic lipogenic factors such as SREBP-1c, 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, stearoyl-CoA desaturase (SCD)-1, FA synthase, and acetyl-CoA carboxylase. Interestingly, these effects could be partially blocked by co-administration of the GLP-1R antagonist exendin 9–39, and completely abrogated by surgical vagotomy [97] - further reinforcing the importance of central GLP-1R activity, and its influence on hepatic lipid metabolism.
Genetic ablation of specific neuronal GLP-1R-containing populations has recently been achieved in mice. Ablation in Wnt1 expressing neurons, representing neurons in the hypothalamus, brainstem, and ENS was compared to ablation in Phox2b-expressing neurons, representing peripheral autonomic nerves. Strikingly, plasma TG following an oral fat load was unaffected in either model, nor were the anti-lipemic effects of several GLP-1R agonists [74]. While lipid tolerance was unaffected, the distinct kinetics of excursion and clearance were not assessed; nor were intestinal and hepatic lipoproteins delineated. Thus, the exact signaling cascade resulting in central modulation of lipoprotein secretion by GLP-1 has yet to be fully elucidated.
The most compelling data for central control over intestinal lipoprotein metabolism comes from animal studies examining the effects of central GLP-1R activation. In the Syrian golden hamster ICV injections of exendin 9–39 into the third ventricle acutely depressed postprandial TRL-TG and ApoB48 secretion by approximately 55 %. Antagonism of central GLP-1R with exendin 9–39 did not completely abrogate the effects of peripheral exendin-4 administration, suggesting at least partial independence of these systems under conditions of prolonged GLP-1 activation. Importantly, depressions in postprandial lipoprotein metabolism via central GLP-1R activation were mediated via increased sympathetic outflow, as pharmacological blockade abolished the effects of ICV GLP-1. While no changes were seen in the activity of lipogenic genes, jejunal MTP activity was significantly reduced, explaining how sympathetic outflow may exert rapid temporal control over chylomicron lipidation and secretion [66].
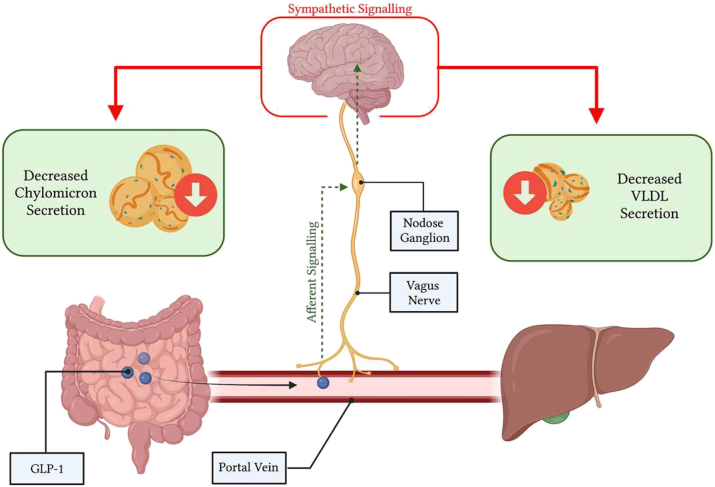
However, endogenous GLP-1 is rapidly cleaved in the circulation, leading to recent doubts regarding its endocrine potential to signal central metabolic regulatory nuclei and peripheral organs [98]. That said, native GLP-1 is secreted into the portal vein, which is richly innervated with vagal afferent nerve terminals containing GLP-1Rs [41]; constituting a rapid mechanism by which GLP-1 may exert its anti-lipemic effects within its short life-span. The GLP-1R containing vagal afferents overlying the portal bed house their cell bodies in the nodose ganglia where GLP-1R expression has also been observed [99]. Moreover, primary isolated nodose neurons show action potential generation, coupled with increases in intracellular Ca2+ when exposed to GLP-1 [100]. The role of these GLP-1R-containing NG neurons in regulating peripheral metabolism has been evidenced previously by GLP-1R knockdown in the vagal afferent nerves of rats, which display increased food consumption, accelerated gastric emptying, and post-meal glycemia coupled with depressed insulin secretion [101, 102]. Moreover, vagal afferent neurons project to GLP-1 producing neurons in the caudal brainstem which secrete GLP-1 into hypothalamic nuclei which regulate energy metabolism [103]. Importantly, activation of GLP-1Rs in the CNS shows similar anti-lipemic actions as peripheral administration, where ICV injection of exendin-4 markedly suppressed chylomicron excursion [66]. Together, suggesting that a portal-vagal axis may explain how endogenous GLP-1 modulates lipoprotein production. Indeed, recently we have demonstrated the importance of this portal-vagal axis in lipid homeostasis in Syrian golden hamsters (Figure 3). Wherein, portal but not jugular or caval administration of active GLP-1(7–37) caused significant reductions in postprandial lipids [104]. This reduction was lost upon surgical or pharmacological vagal deafferentation, or under conditions of adrenergic blockade – corroborating previous reports that GLP-1 both signals via the vagus and reduces plasma lipids via sympathetic signalling [66, 101]. Strikingly, this axis was sensitive to diet-induced insulin resistance, and portal GLP-1 resistance rapidly developed under high-fructose diet feeding [104]. Suggesting that loss of endogenous GLP-1 signalling may be a contributing or initiating factor in the development of hypertriglyceridemia.
Figure 3:
Glucagon-like peptide (GLP)-1 works through a portal-vagal signalling axis to modulate postprandial and fasting lipids. Native GLP-1 works in a site-specific manner within the portal vein, binding to GLP-1R on vagal afferent nerves. Vagal afferents project to the nodose ganglion to integrate viscerosensory data, then impulses are propagated to the brainstem, and central metabolic regulator nuclei. Changes in efferent sympathetic tone alter postprandial and fasting lipoprotein secretion and lipemia. Image created with BioRender.com.
Concluding remarks
Dyslipidemia is a common co-morbidity in insulin resistant states, resulting in the overproduction of ApoB-containing chylomicron particles, VLDL particles, and elevated plasma TG levels [100]. This leads to the generation of atherogenic remnant particles, precursors to the development of atherosclerosis and CVD, the leading cause of death in T2D [105, 106]. Several hormones have been shown to regulate intestinal and hepatic lipid metabolism such as insulin and gut-derived incretin hormones released upon nutrient consumption [50, 107]. Key viscerosensory peptides released from the gut are the incretin hormones GLP-1 and gastric inhibitory peptide (GIP), CCK, and PYY [74, 84, 108], [109], [110]. Notably, GLP-1 has been shown by our laboratory and others to modulate intestinal and hepatic lipoprotein production through a complex gut-brain-liver axis [66, 111], [112], [113].
Footnotes
Research ethics: This is a review article and does not require ethical approval.
Informed consent: Not applicable.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Research funding: Canadian Institutes of Health Research (CIHR) (FDN-148396).
Data availability: Not applicable.
References
- 1.Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15:226–37. doi: 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- 2.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62:373–81. doi: 10.2337/db12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–9. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 4.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880–9. doi: 10.1016/s0021-9258(18)67324-7. [DOI] [PubMed] [Google Scholar]
- 5.Glucagon like peptide – an overview. Science Direct Topics; 2023. [30 Jan 2024]. https://www.sciencedirect.com/topics/medicine-and-dentistry/glucagon-like-peptide [Internet] Available from. Accessed. [Google Scholar]
- 6.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metabol. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kounatidis D, Vallianou NG, Tsilingiris D, Christodoulatos GS, Geladari E, Stratigou T, et al. Therapeutic potential of GLP-2 analogs in gastrointestinal disorders: current knowledge, nutritional aspects, and future perspectives. Curr Nutr Rep. 2022;11:618–42. doi: 10.1007/s13668-022-00433-0. [DOI] [PubMed] [Google Scholar]
- 8.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–70. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 9.Padidela R, Patterson M, Sharief N, Ghatei M, Hussain K. Elevated basal and post-feed glucagon-like peptide 1 (GLP-1) concentrations in the neonatal period. Eur J Endocrinol. 2009;160:53–8. doi: 10.1530/eje-08-0807. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker PL, Crivici A, Izzo A, Ehrlich P, Tsai CH, Drucker DJ. Circulating and tissue forms of the intestinal growth factor, glucagon-like peptide-2. Endocrinology. 1997;138:4837–43. doi: 10.1210/endo.138.11.5482. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–26. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 12.Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2010;35:9–16. doi: 10.1139/h09-119. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, et al. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 14.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–4. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 16.Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol. 2009;297:G299–305. doi: 10.1152/ajpgi.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- 18.Kim SK. Small intestine transit time in the normal small bowel study. Am J Roentgenol Radium Ther Nucl Med. 1968;104:522–4. doi: 10.2214/ajr.104.3.522. [DOI] [PubMed] [Google Scholar]
- 19.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–94. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 20.Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002;143:2420–6. doi: 10.1210/en.143.6.2420. [DOI] [PubMed] [Google Scholar]
- 21.Balks HJ, Holst JJ, von zur Mühlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab. 1997;82:786–90. doi: 10.1210/jc.82.3.786. [DOI] [PubMed] [Google Scholar]
- 22.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 23.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–33. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manchanda Y, Bitsi S, Kang Y, Jones B, Tomas A. Spatiotemporal control of GLP-1 receptor activity. Curr Opin Endocr Metab Res. 2021;16:19–27. doi: 10.1016/j.coemr.2020.07.003. [DOI] [Google Scholar]
- 25.Hällbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochim Biophys Acta. 2001;1546:79–86. doi: 10.1016/s0167-4838(00)00270-3. [DOI] [PubMed] [Google Scholar]
- 26.Couvineau A, Laburthe M. The family B1 GPCR: structural aspects and interaction with accessory proteins. Curr Drug Targets. 2012;13:103–15. doi: 10.2174/138945012798868434. [DOI] [PubMed] [Google Scholar]
- 27.Wootten D, Reynolds CA, Smith KJ, Mobarec JC, Koole C, Savage EE, et al. The extracellular surface of the GLP-1 receptor is a molecular trigger for biased agonism. Cell. 2016;165:1632–43. doi: 10.1016/j.cell.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol Baltim Md. 2002;16:2135–44. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- 29.Britsch S, Krippeit-Drews P, Lang F, Gregor M, Drews G. Glucagon-like peptide-1 modulates Ca2+ current but not K+ATP current in intact mouse pancreatic B-cells. Biochem Biophys Res Commun. 1995;207:33–9. doi: 10.1006/bbrc.1995.1149. [DOI] [PubMed] [Google Scholar]
- 30.Leech CA, Habener JF. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J Biol Chem. 1997;272:17987–93. doi: 10.1074/jbc.272.29.17987. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE. Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology. 1993;133:57–62. doi: 10.1210/endo.133.1.8391428. [DOI] [PubMed] [Google Scholar]
- 32.Thompson A, Kanamarlapudi V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gαq pathway. Biochem Pharmacol. 2015;93:72–84. doi: 10.1016/j.bcp.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen ARB, Plouffe B, Cahill TJ, Shukla AK, Tarrasch JT, Dosey AM, et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166:907–19. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metabol. 2011;13:320–30. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilman CP, Perry T, Furukawa K, Grieg NH, Egan JM, Mattson MP. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J Neurochem. 2003;87:1137–44. doi: 10.1046/j.1471-4159.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96:897–909.e5. doi: 10.1016/j.neuron.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–9. doi: 10.1172/jci112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet Lond Engl. 2002;359:824–30. doi: 10.1016/s0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 40.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101:1421–30. doi: 10.1172/jci1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–73. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 42.Fehmann HC, Habener JF. Functional receptors for the insulinotropic hormone glucagon-like peptide-I(7-37) on a somatostatin secreting cell line. FEBS Lett. 1991;279:335–40. doi: 10.1016/0014-5793(91)80182-3. [DOI] [PubMed] [Google Scholar]
- 43.Heller RS, Kieffer TJ, Habener JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes. 1997;46:785–91. doi: 10.2337/diabetes.46.5.785. [DOI] [PubMed] [Google Scholar]
- 44.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–6. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- 45.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–70. doi: 10.1161/circulationaha.117.028136. [DOI] [PubMed] [Google Scholar]
- 46.Xiao C, Bandsma RHJ, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol. 2012;32:1513–9. doi: 10.1161/atvbaha.112.246207. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. 2010;212:217–22. doi: 10.1016/j.atherosclerosis.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 48.Gupta NA, Kolachala VL, Jiang R, Abramowsky C, Romero R, Fifadara N, et al. The glucagon-like peptide-1 receptor agonist exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis. Am J Pathol. 2012;181:1693–701. doi: 10.1016/j.ajpath.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang SH, Xu RX, Cui CJ, Wang Y, Du Y, Chen ZG, et al. Liraglutide downregulates hepatic LDL receptor and PCSK9 expression in HepG2 cells and db/db mice through a HNF-1a dependent mechanism. Cardiovasc Diabetol. 2018;17:48. doi: 10.1186/s12933-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khound R, Taher J, Baker C, Adeli K, Su Q. GLP-1 elicits an intrinsic gut–liver metabolic signal to ameliorate diet-induced VLDL overproduction and insulin resistance. Arterioscler Thromb Vasc Biol. 2017;37:2252–9. doi: 10.1161/atvbaha.117.310251. [DOI] [PubMed] [Google Scholar]
- 51.Taher J, Baker CL, Cuizon C, Masoudpour H, Zhang R, Farr S, et al. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol Metabol. 2014;3:823–33. doi: 10.1016/j.molmet.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–23. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 53.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology. 2013;154:127–39. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 54.Baggio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, et al. GLP-1 receptor expression within the human heart. Endocrinology. 2018;159:1570–84. doi: 10.1210/en.2018-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–78. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 56.Liu D, Pang J, Shao W, Gu J, Zeng Y, He HH, et al. Hepatic fibroblast growth factor 21 is involved in mediating functions of liraglutide in mice with dietary challenge. Hepatology. 2021;74:2154–69. doi: 10.1002/hep.31856. [DOI] [PubMed] [Google Scholar]
- 57.Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, et al. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia. 2013;56:156–61. doi: 10.1007/s00125-012-2738-3. [DOI] [PubMed] [Google Scholar]
- 58.El-Jamal N, Erdual E, Neunlist M, Koriche D, Dubuquoy C, Maggiotto F, et al. Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G274–85. doi: 10.1152/ajpgi.00389.2012. [DOI] [PubMed] [Google Scholar]
- 59.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744–55. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 60.Thulesen J, Hartmann B, Ørskov C, Jeppesen PB, Holst JJ, Poulsen SS. Potential targets for glucagon-like peptide 2 (GLP-2) in the rat: distribution and binding of i.v. injected 125I-GLP-2. Peptides. 2000;21:1511–7. doi: 10.1016/s0196-9781(00)00305-3. [DOI] [PubMed] [Google Scholar]
- 61.O’Halloran DJ, Nikou GC, Kreymann B, Ghatei MA, Bloom SR. Glucagon-like peptide-1 (7-36)-NH2: a physiological inhibitor of gastric acid secretion in man. J Endocrinol. 1990;126:169–73. doi: 10.1677/joe.0.1260169. [DOI] [PubMed] [Google Scholar]
- 62.Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/jci118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schirra J, Kuwert P, Wank U, Leicht P, Arnold R, Göke B, et al. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc Assoc Am Phys. 1997;109:84–97. [PubMed] [Google Scholar]
- 64.Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, et al. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G943–949. doi: 10.1152/ajpgi.00303.2004. [DOI] [PubMed] [Google Scholar]
- 65.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53:552–61. doi: 10.1007/s00125-009-1611-5. [DOI] [PubMed] [Google Scholar]
- 66.Farr S, Baker C, Naples M, Taher J, Iqbal J, Hussain M, et al. Central nervous system regulation of intestinal lipoprotein metabolism by glucagon-like peptide-1 via a brain–gut Axis. Arterioscler Thromb Vasc Biol. 2015;35:1092–100. doi: 10.1161/atvbaha.114.304873. [DOI] [PubMed] [Google Scholar]
- 67.Grau-Bové C, González-Quilen C, Cantini G, Nardini P, Espina B, Bani D, et al. GLP1 exerts paracrine activity in the intestinal lumen of human colon. Int J Mol Sci. 2022;23:3523. doi: 10.3390/ijms23073523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kedees MH, Guz Y, Grigoryan M, Teitelman G. Functional activity of murine intestinal mucosal cells is regulated by the glucagon-like peptide-1 receptor. Peptides. 2013;48:36–44. doi: 10.1016/j.peptides.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64:2537–49. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 70.Broide E, Bloch O, Ben-Yehudah G, Cantrell D, Shirin H, Rapoport MJ. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem Off J Histochem Soc. 2013;61:649–58. doi: 10.1369/0022155413497586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–90. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 72.Grande EM, Raka F, Hoffman S, Adeli K. GLP-2 regulation of dietary fat absorption and intestinal chylomicron production via neuronal nitric oxide synthase (nNOS) signaling. Diabetes. 2022;71:1388–99. doi: 10.2337/db21-1053. [DOI] [PubMed] [Google Scholar]
- 73.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–21. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varin EM, Mulvihill EE, Baggio LL, Koehler JA, Cao X, Seeley RJ, et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 2019;27:3371–84.e3. doi: 10.1016/j.celrep.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 75.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 76.Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol Off J Eur Soc Med Oncol. 2001;12(2 Suppl):S63–68. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- 77.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Therapeut. 2003;306:948–53. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- 78.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/jmn:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 79.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Therapeut. 2002;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 80.Imbernon M, Saponaro C, Helms HCC, Duquenne M, Fernandois D, Deligia E, et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metabol. 2022;34:1054–63.e7. doi: 10.1016/j.cmet.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Lartigue G, Diepenbroek C. Novel developments in vagal afferent nutrient sensing and its role in energy homeostasis. Curr Opin Pharmacol. 2016;31:38–43. doi: 10.1016/j.coph.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 83.Lam CKL, Chari M, Lam TKT. CNS regulation of glucose homeostasis. Physiol Bethesda Md. 2009;24:159–70. doi: 10.1152/physiol.00003.2009. [DOI] [PubMed] [Google Scholar]
- 84.Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- 85.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–7. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 86.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 87.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 88.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JRC, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–56. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 90.Diepenbroek C, Quinn D, Stephens R, Zollinger B, Anderson S, Pan A, et al. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am J Physiol Gastrointest Liver Physiol. 2017;313:G342–52. doi: 10.1152/ajpgi.00095.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Netter M, Frank H. Atlas of human anatomy. 4th ed. Saunders; 2006. [Google Scholar]
- 92.Hoosein NM, Gurd RS. Human glucagon-like peptides 1 and 2 activate rat brain adenylate cyclase. FEBS Lett. 1984;178:83–6. doi: 10.1016/0014-5793(84)81245-4. [DOI] [PubMed] [Google Scholar]
- 93.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 94.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab. 2012;302:E334–43. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- 95.Patel V, Joharapurkar AA, Kshirsagar SG, Patel KN, Bahekar R, Shah G, et al. Central GLP-1 receptor activation improves cholesterol metabolism partially independent of its effect on food intake. Can J Physiol Pharmacol. 2016;94:161–7. doi: 10.1139/cjpp-2014-0457. [DOI] [PubMed] [Google Scholar]
- 96.Lee SJ, Sanchez-Watts G, Krieger JP, Pignalosa A, Norell PN, Cortella A, et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metabol. 2018;11:33–46. doi: 10.1016/j.molmet.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patel V, Joharapurkar A, Kshirsagar S, Sutariya B, Patel M, Patel H, et al. Central administration of coagonist of GLP-1 and glucagon receptors improves dyslipidemia. Biomed Pharmacother. 2018;98:364–71. doi: 10.1016/j.biopha.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 98.Aulinger BA, Perabo M, Seeley RJ, Parhofer KG, D’Alessio DA. Rapid hepatic metabolism blunts the endocrine action of portally infused GLP-1 in male rats. Am J Physiol Endocrinol Metab. 2020;318:E189–97. doi: 10.1152/ajpendo.00298.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Egerod KL, Petersen N, Timshel PN, Rekling JC, Wang Y, Liu Q, et al. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metabol. 2018;12:62–75. doi: 10.1016/j.molmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ridgway N. Biochemistry of lipids, lipoproteins and membranes. San Diego, CA, USA: Elsevier Science; 2015. [Google Scholar]
- 101.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65:34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 102.Krieger JP, Langhans W, Lee SJ. Vagal mediation of GLP-1’s effects on food intake and glycemia. Physiol Behav. 2015;152:372–80. doi: 10.1016/j.physbeh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Singh I, Wang L, Xia B, Liu J, Tahiri A, El Ouaamari A, et al. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 2022;12:178. doi: 10.1186/s13578-022-00914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoffman S, Alvares D, Adeli K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol Metabol. 2022;65:101590. doi: 10.1016/j.molmet.2022.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21:21. doi: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 106.Langsted A, Nordestgaard BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51:131–41. doi: 10.1016/j.pathol.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 107.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, et al. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–25. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 108.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJPM, Wishart J, et al. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287:R524–33. doi: 10.1152/ajpregu.00039.2004. [DOI] [PubMed] [Google Scholar]
- 109.Vrang N, Madsen AN, Tang-Christensen M, Hansen G, Larsen PJ. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R367–375. doi: 10.1152/ajpregu.00726.2005. [DOI] [PubMed] [Google Scholar]
- 110.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13:700–9. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Farr S, Taher J, Adeli K. Central nervous system regulation of intestinal lipid and lipoprotein metabolism. Curr Opin Lipidol. 2016;27:1–7. doi: 10.1097/mol.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 112.Farr S, Taher J, Adeli K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc Hematol Disord: Drug Targets. 2014;14:126–36. doi: 10.2174/1871529x14666140505125300. [DOI] [PubMed] [Google Scholar]
- 113.Taher J, Farr S, Adeli K. Central nervous system regulation of hepatic lipid and lipoprotein metabolism. Curr Opin Lipidol. 2017;28:32–8. doi: 10.1097/mol.0000000000000373. [DOI] [PubMed] [Google Scholar]