ABSTRACT
In wheat, the transition of the inflorescence meristem to a terminal spikelet (IM→TS) determines the spikelet number per spike (SNS), an important yield component. In this study, we demonstrate that the plant-specific transcription factor LEAFY (LFY) physically and genetically interacts with WHEAT ORTHOLOG OF APO1 (WAPO1) to regulate SNS and floret development. Loss-of-function mutations in either or both genes result in significant and similar reductions in SNS, as a result of a reduction in the rate of spikelet meristem formation per day. SNS is also modulated by significant genetic interactions between LFY and the SQUAMOSA MADS-box genes VRN1 and FUL2, which promote the IM→TS transition. Single-molecule fluorescence in situ hybridization revealed a downregulation of LFY and upregulation of the SQUAMOSA MADS-box genes in the distal part of the developing spike during the IM→TS transition, supporting their opposite roles in the regulation of SNS in wheat. Concurrently, the overlap of LFY and WAPO1 transcription domains in the developing spikelets contributes to normal floret development. Understanding the genetic network regulating SNS is a necessary first step to engineer this important agronomic trait.
Keywords: Wheat, Inflorescence development, Spike, LFY, Spatial transcriptomics
Summary: The plant-specific transcription factor LEAFY plays an important role in the regulation of the number of spikelets per spike in wheat.
INTRODUCTION
Every year, trillions of wheat spikes mature worldwide carrying the grains that provide one-fifth of the calories and proteins consumed by the human population [according to the Food and Agriculture Organization (FAO) of the United Nations; http://www.fao.org/faostat/en/#data]. Therefore, increasing the maximum number of grains that can be produced by each spike could contribute to remedying the global needs for increased wheat productivity to feed a growing human population.
Wheat spikes, as other grass inflorescences, comprise specialized reproductive organs called spikelets, which are short indeterminate branches. Each spikelet has two proximal sterile bracts (glumes) followed by a variable number of florets. Individual florets include a lemma, which is also a bract, subtending the floral organs (one palea, two lodicules, three stamens and a pistil) (Preston et al., 2009; Debernardi et al., 2020a). The wheat inflorescence meristem (IM) produces multiple lateral spikelet meristems (SMs) in a distichous order before transitioning to a terminal spikelet (henceforth, IM→TS). The timing of this transition and the rate at which the SMs are formed determine the spikelet number per spike (SNS) and the maximum number of grains that can be formed in the spike.
The number of spikelets in a wheat spike is affected by multiple environmental conditions, including drought, salt stress, heat, and reduced nutrients, all of which result in reduced SNS (Frank and Bauer, 1982; Frank et al., 1987; Maas and Grieve, 1990). However, differences in SNS also have a strong genetic component, with broad sense heritability ranging from H2=0.84 in irrigated fields to H2=0.59 in water-stressed environments (Zhang et al., 2018). This high heritability has facilitated the identification of several wheat genes involved in the regulation of SNS. VERNALIZATION1 (VRN1), FRUITFULL2 (FUL2) and FUL3, the wheat homologs of the Arabidopsis SQUAMOSA MADS-box genes APETALA1 (AP1), CAULIFLOWER (CAL) and FUL, have been shown to be essential for spikelet development and for regulation of the IM→TS transition (Li et al., 2019). Loss-of-function mutations in vrn1 or ful2 result in normal plants with significant increases in SNS. However, in the vrn1 ful2 combined mutant the IM remains indeterminate and lateral spikelets are converted into tiller-like organs with vestigial floral organs. These vestigial floral organs disappear in the vrn1 ful2 ful3 higher-order mutant, in which spikelets revert to vegetative tillers subtended by leaves (Li et al., 2019).
Genes that regulate VRN1 expression have been shown to affect SNS. FT1, the wheat homolog of the Arabidopsis florigen FLOWERING LOCUS T (FT), binds directly to the VRN1 promoter as part of a floral activation complex, and functions as a transcriptional activator (Li and Dubcovsky, 2008; Li et al., 2015). Mutants (or knockdown transgenic plants) of FT1 (Lv et al., 2014) or its closest paralog FT2 (Shaw et al., 2019) show reduced or delayed expression of VRN1, which is associated with significant increases in SNS. In contrast, overexpression of these genes results in a precocious IM→TS transition and spikes with very few spikelets (Lv et al., 2014; Shaw et al., 2019). Mutations in PPD1 that reduce or delay FT1 expression result in SNS increases (Shaw et al., 2013), whereas mutations in ELF3 that result in the upregulation of FT1 and VRN1 expression reduce SNS (Alvarez et al., 2016). bZIPC1 encodes a protein that physically interacts with FT2, and its mutants also show a large decrease in SNS (Glenn et al., 2023).
However, the underpinning mechanism by which the recently cloned gene WHEAT ORTHOLOG OF APO1 (WAPO1) (Kuzay et al., 2019, 2022) regulates SNS has not yet been elucidated. WAPO1 is orthologous to the Oryza sativa (rice) gene ABERRANT PANICLE ORGANIZATION1 (APO1), and to the Arabidopsis gene UNUSUAL FLORAL ORGANS (UFO), which are both involved in floral development (Levin and Meyerowitz, 1995; Ikeda et al., 2007; Rieu et al., 2023a). In addition to floral defects, loss-of-function mutations in WAPO1 or APO1 result in significant reductions in SNS in wheat (Kuzay et al., 2022) or in the number of branches in the rice panicle (Ikeda et al., 2005), respectively.
In Arabidopsis, UFO physically interacts with the plant-specific transcription factor LEAFY (LFY) (Lee et al., 1997; Chae et al., 2008; Rieu et al., 2023b), and the interaction is conserved between the rice homologs APO1 and APO2 (Ikeda-Kawakatsu et al., 2012). The Arabidopsis LFY protein activates the class-A MADS-box genes AP1 (Parcy et al., 1998; Wagner et al., 1999) and CAL (William et al., 2004), which are homologous to the wheat VRN1 and FUL2 genes. Because VRN1, FUL2 (Li et al., 2019) and WAPO1 (Kuzay et al., 2022) are all involved in the regulation of SNS, we investigated the role of LFY on wheat spike development.
In this study, we demonstrate that LFY physically interacts with WAPO1 and that plants carrying loss-of-function mutations in either or both genes exhibit similar floral abnormalities and similar reductions in SNS as a result of a reduced rate of SM formation. We also show significant genetic interactions for SNS between LFY and the meristem identity gene VRN1, which, together with its closest paralog FUL2, promote the IM→TS transition. Finally, we use single-molecule fluorescence in situ hybridization (smFISH) to visualize the spatiotemporal expression profiles of these genes and other floral genes during spike development. These studies reveal a tenfold increase in the ratio between the SQUAMOSA MADS-box genes (VRN1 and FUL2) and LFY in the distal part of the spike at the time of the IM→TS transition, supporting the opposing roles of these genes in the regulation of SNS.
RESULTS
Induced loss-of-function mutations in LFY reduce SNS and alter floral morphology
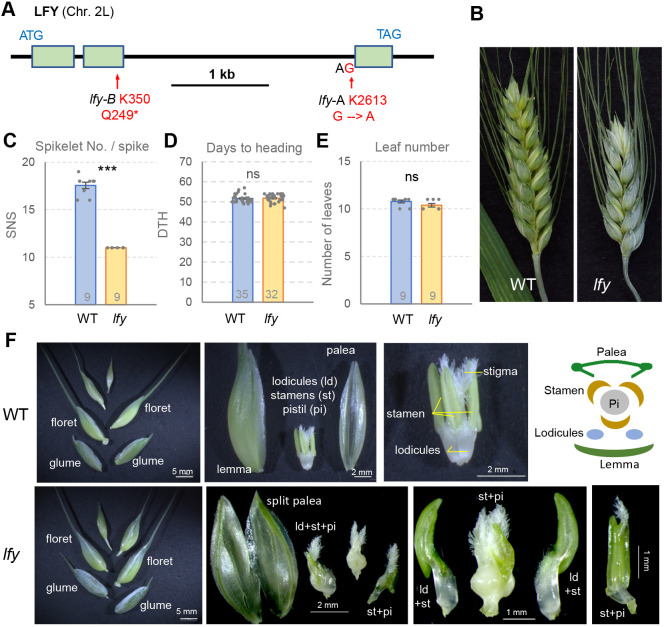
Using our sequenced Kronos mutant population (Krasileva et al., 2017), we selected truncation mutations K2613 for LFY-A (henceforth lfy-A) and K350 for LFY-B (henceforth lfy-B). The lfy-A mutant has a G>A change in the acceptor splice site of the second intron, which results in mis-splicing of the third exon, a shift in the reading frame, and a premature stop codon that eliminates 121 amino acids (31% of the total protein; Fig. 1A). The lfy-B mutant has a premature stop codon at position 249 (Q249*) that truncates 37% of the protein. The eliminated amino acids in the two wheat mutants include the highly conserved LFY DNA-binding domain, suggesting that the truncated proteins can no longer bind their target DNAs and, therefore, are most likely not functional (Maizel et al., 2005; Rieu et al., 2023b) (Fig. 1A). Primers used to track these mutations are described in Table S1. The mutants were backcrossed to Kronos to reduce background mutations, and intercrossed with each other to select sister lines homozygous for the different mutation combinations, including the wild type (WT), lfy-A, lfy-B, and the lfy-A lfy-B combined mutant, designated hereafter as lfy.
Fig. 1.
Characterization of LFY loss-of-function mutants. (A) LFY gene structure and selected mutations. (B) Representative spikes of wild-type (WT) and lfy mutant plants. (C-E) Comparison between WT and lfy for spikelet number per spike (C), days to heading (D) and leaf number (E). Numbers inside bars indicate biological replicates. Dots represent individual plants; error bars represent s.e.m. ***P<0.001. ns, not significant (two-tailed t-tests). (F) Top: Images of WT spikelets and schema representing the internal structure. Bottom: Floral abnormalities in lfy. ld, lodicule; pi, pistil; st, stamen. See Table S2 for raw data.
Comparisons between the homozygous sister lines, raised in a growth chamber, revealed a highly significant decrease (37%, P<0.001) in SNS in the combined lfy mutant relative to the WT (Fig. 1B,C, Table S2). Smaller but still significant decreases in SNS were detected for the single lfy-A (12%) and lfy-B (8%) mutants (Fig. S1A, Table S3), which indicates that modification of LFY gene dosage can be used to fine-tune SNS in wheat. No significant differences in heading time or leaf number were detected between the combined lfy mutant and the WT (Fig. 1D,E, Table S2), suggesting a limited effect of LFY on the timing of the transition between the vegetative and reproductive meristems.
In addition to its effects on SNS, lfy showed severe alterations in floral organs (Fig. 1F). We quantified the frequency of the defects in 27 first and 27 second florets from spikelets located in the basal, central and distal parts of the spike (Fig. S1B,C, Table S2). The glumes and lemmas developed normally, but 18.5% of the paleas were bifurcated (Fig. 1F, Table S2). Eighty-one percent of the paleas were fused with either lodicules or stamens (Table S2). Lodicules were also fused to stamens or membranous structures. The average number of normal stamens was reduced to 1.4 (Table S2), and one-fifth of the florets showed abnormal stamens and fusions with lodicules, membranous structures or pistils. Only 9% of the florets showed single pistils (primarily those with three normal anthers) and the rest showed more than one pistil and frequent homeotic conversions between stamens and pistils (Fig. 1F, Fig. S1, Table S2).
Overexpression of LFY partially rescues the reduced SNS phenotype of lfy
To test whether LFY function was sufficient to rescue the mutant phenotypes, we generated transgenic plants expressing the LFY-A coding region fused to a C-terminal HA tag and driven by the constitutive maize UBIQUITIN promoter. Transgenic lines for the five independent UBI:LFY-HA events, all showed significantly higher LFY transcript levels in the leaves than did non-transgenic sister lines and WT Kronos, which showed no expression of endogenous LFY in this tissue (Fig. S2A, Table S4). Among 14 dissected florets, we observed missing or fused lodicules in 21%, fused stamen filaments in 43% and pistils with extra stigmas in 36% (Fig. S2B,C). Floral organ defects were less frequent and less severe than in lfy, which explains the higher fertility of the UBI:LFY-HA plants (23±10 grains/plant; mean±s.e.m.) relative to lfy (2.7±0.7 grains/plant), but its reduced fertility relative to the WT (94±25 grains/plant, P=0.019; Table S5). All errors indicated in the text and figures are s.e.m. These results indicate that the ectopic expression of LFY is associated with negative pleiotropic effects on floral organ development and fertility.
We then crossed the UBI:LFY-HA transgenic plant #4 with lfy. In the progeny, we selected sister lines homozygous for combined lfy mutations or for WT alleles, each with or without the transgenes. Among the plants without the transgene, the combined lfy mutants showed reduced SNS (6.6 spikelets, P<0.001), as in previous experiments. In the presence of the WT LFY alleles, transgenic plants showed 1.1 more spikelets per spike than non-transgenic controls (P=0.0045; Fig. 2, Table S5). The effect was larger in lfy transgenic plants, which showed four more spikelets per spike than the controls (P<0.001; Fig. 2).
Fig. 2.
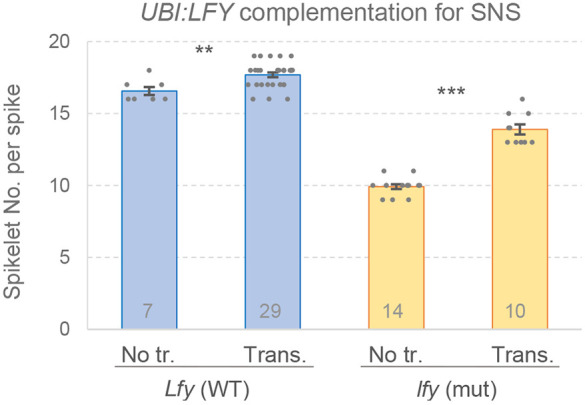
Effect of UBI:LFY-HA on spikelet number per spike (SNS). Effect of the UBI:LFY-HA transgene on SNS in WT and lfy. Numbers within bars indicate biological replicates. Dots represent individual plants; error bars represent s.e.m. **P<0.01, ***P<0.001 (two-tailed t-tests). ns, not significant. See Table S5 for raw data.
Wheat LFY and WAPO1 show physical and genetic interactions
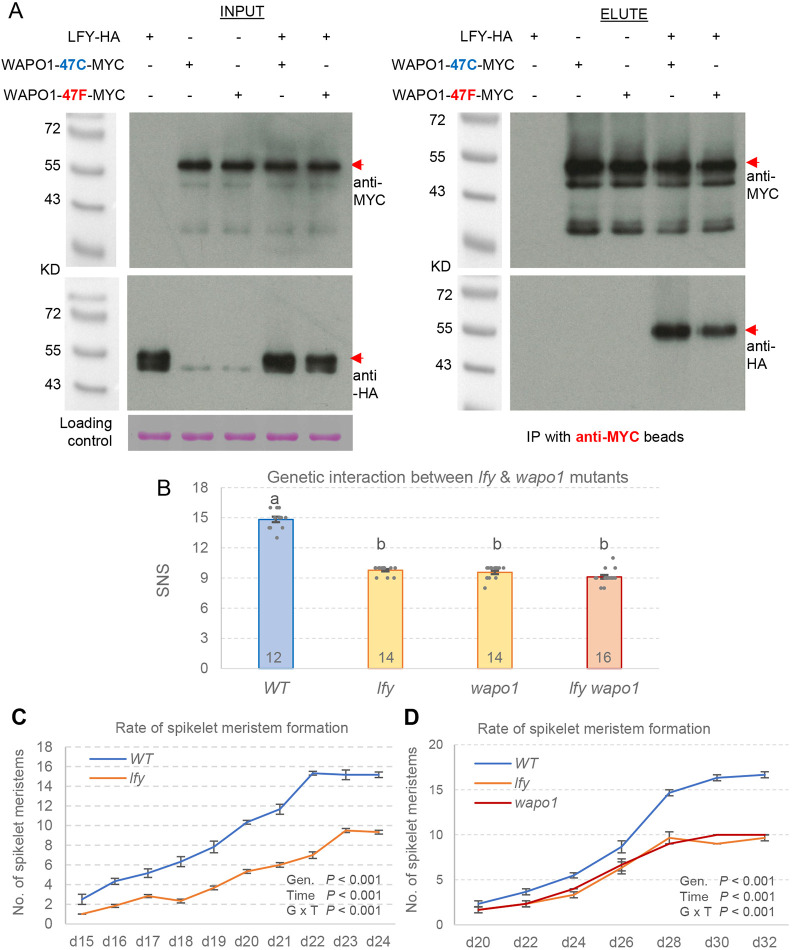
Because LFY and WAPO1 mutants are both associated with similar reductions in SNS (Kuzay et al., 2022), and their homologous proteins interact with each other in Arabidopsis (Chae et al., 2008) and rice (Ikeda-Kawakatsu et al., 2012), we tested the ability of LFY and WAPO1 proteins to interact physically with each other in wheat. We used co-immunoprecipitation (Co-IP) to test the interaction between LFY-A and two WAPO-A1 natural alleles that differ in the presence of a cysteine or a phenylalanine at position 47 to determine whether this polymorphism affects the interaction (Kronos carries the 47C allele). The WAPO-A1-47F allele was previously associated with higher SNS than the WAPO-A1-47C allele (Kuzay et al., 2022). We co-transformed wheat leaf protoplasts with UBI:LFY-HA combined with either UBI:WAPO1-47C-MYC or UBI:WAPO1-47F-MYC. After immunoprecipitation with anti-MYC beads, we detected LFY-HA using an anti-HA antibody in both the WAPO1-47C-MYC and WAPO1-47F-MYC precipitates (Fig. 3A). These results indicate that LFY can interact with both WAPO-A1 variants in wheat.
Fig. 3.
Physical and genetic interactions between WAPO1 and LFY. (A) Interaction between LFY and WAPO1 alleles 47F-47C in Kronos leaf protoplasts by co-immunoprecipitation. (B) Genetic interaction between lfy and wapo1. Different letters indicate significant differences in Tukey tests (P<0.05), and numbers inside bars biological replications. Dots represent individual plants; error bars represent s.e.m. (C,D) Changes in the number of SMs with time. d, days from germination. (C) WT and lfy mutant grown under long days (n=6). (D) WT, and lfy and wapo1 mutants grown for 8 days under short days and then long days (n=3). P-values correspond to a repeated measures analysis. Error bars are s.e.m. See Table S6 for raw data.
To investigate whether the physical interaction between LFY and WAPO1 is reflected in a genetic interaction for SNS, we intercrossed lfy with a loss-of-function wapo1 mutant containing early truncation mutations in both WAPO-A1 and WAPO-B1 (Kuzay et al., 2022). Owing to the reduced fertility of the homozygous lfy mutants (Table S5), we first selected lines homozygous for the lfy-A mutant allele and heterozygous for lfy-B among F2 and F3 progenies. We then screened a large F4 segregating population and selected the four homozygous classes (WT, lfy, wapo1, and combined lfy wapo1) using molecular markers (primers in Table S1). Plants homozygous for mutations in either LFY (lfy) or WAPO1 (wapo1) showed large and similar reductions in SNS relative to the WT (34% and 35% reduction, respectively; Fig. 3B). Interestingly, the combined lfy wapo1 mutant showed a reduction of 38%, relative to the WT, which was not significantly different from the reductions observed in the single mutants (Fig. 3B, Table S6). The genetic epistatic interaction for SNS was highly significant in a factorial ANOVA, and the contrasts for the simple effects showed no significant differences in SNS for LFY or WAPO1 in the presence of the mutant allele of the other gene (Table S6). These results indicate a reciprocal recessive epistatic interaction between these two genes, and that LFY and WAPO1 need each other to regulate SNS.
To determine whether the lfy reduction in SNS was due to a premature IM→TS transition or a reduced rate of SM formation, we dissected developing spikes and recorded the variation in SM number per day (sm/d). The first experiment (long days), showed a similar IM→TS transition time but a significantly faster rate of spikelet formation in the WT (1.83 sm/d) than in lfy (0.86 sm/d) (Fig. 3C, Table S6). In the second experiment, we grew the seedlings for 8 days under short days and then transferred them to long days to synchronize the reproductive transition. In this experiment, the rate of spikelet meristem formation in the WT (1.40 sm/d) was also faster than in the lfy (0.73 sm/d) and wapo1 (0.83 sm/d) (Fig. 3D). In both experiments, different rates of SM formation were observed from the earliest stages of spike development (Fig. 3C,D). Repeated measures analyses revealed highly significant differences between mutant and WT genotypes, time points, and genotype×time interactions, which indicates a differential response in time (Table S6). There was no significant difference in the rate of SM formation when comparing the lfy and wapo1 mutants alone (Table S6).
LFY and WAPO1 show dynamic expression profiles during wheat spike development
A previous RNA-sequencing (RNAseq) study including different tissues at different developmental stages in Chinese Spring (CS) wheat detected LFY transcripts in developing spikes and elongating stems (Choulet et al., 2014) (Fig. S3A). A separate RNAseq study including five spike developmental stages in tetraploid wheat Kronos (VanGessel et al., 2022) demonstrated the presence of transcripts for both LFY and WAPO1 at all five stages. LFY transcript levels were more abundant than those of WAPO1, and both genes showed lower transcript levels in the apical region at the vegetative stage than at the double-ridge to floret primordia stages (Fig. S3, Table S7). These studies indicate that LFY and WAPO1 are present at the same stages of spike development. To refine the localization of LFY and WAPO1 transcripts within the developing spike, we examined their dynamic spatial patterns using smFISH (Fig. 4). For all the smFISH studies, we only compared hybridization signals across developmental stages for individual genes because comparisons of total expression levels among genes are affected by probe sensitivity and can be misleading.
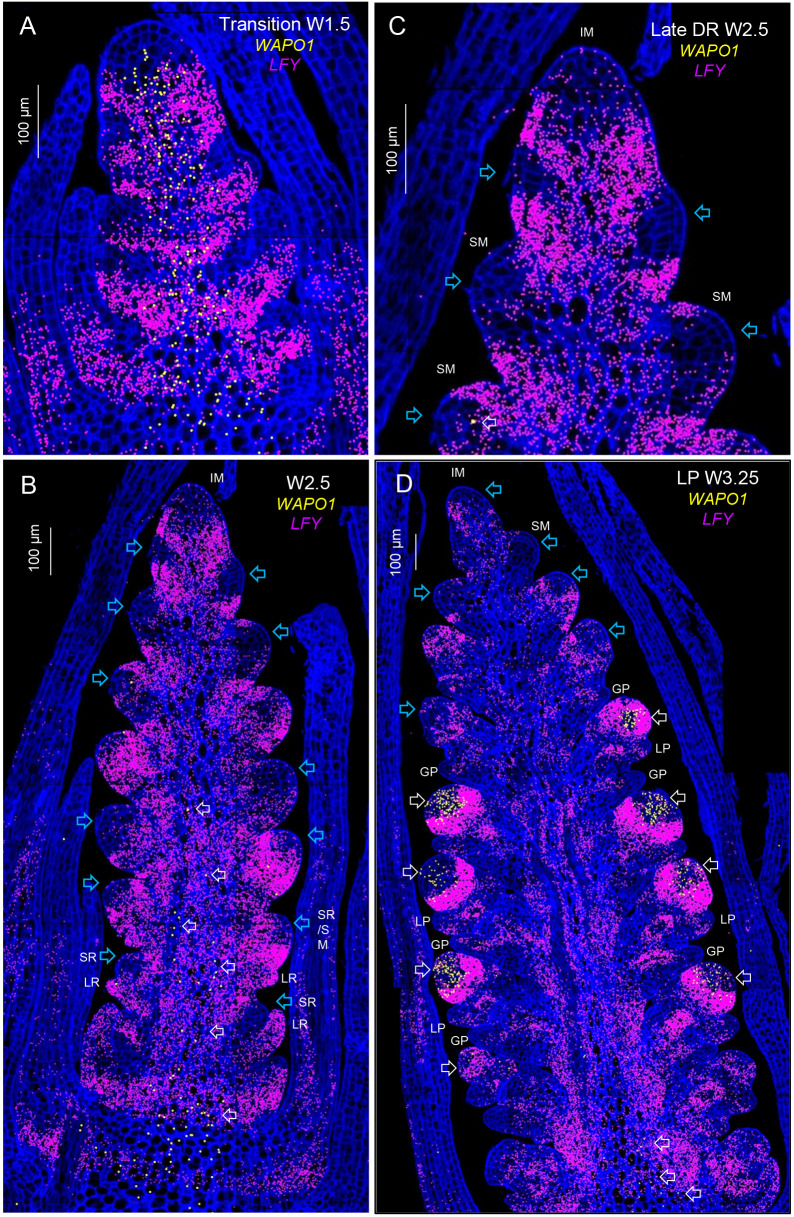
Fig. 4.
Single-molecule fluorescence in-situ hybridization (smFISH) of LFY and WAPO1 during spike development. Cell walls stained with calcofluor are presented in dark blue. (A) Elongated shoot apical meristem transitioning from a vegetative to an inflorescence meristem (IM, W1.5). (B) Late double-ridge stage (W2.5). (C) Detail of the IM region from B. (D) Lemma primordia stage (W3.25). Blue arrows indicate regions of the SM where LFY expression is lower and white arrows show regions of WAPO1 expression. GP, glume primordium; LP, lemma primordium; LR, leaf ridge; SR, spikelet ridge. Scale bars: 100 μm. W, Waddington scale (Waddington et al., 1983).
During the transition between the vegetative and reproductive phases (W1.5 in Waddington scale; Waddington et al., 1983), LFY transcripts were concentrated in bands radiating from the axis of the elongating shoot apical meristem towards the lateral primordia (Fig. 4A). Only a few LFY transcripts were detected at the tip of the IM at this or later spike developmental stages (Fig. 4C,D). At the late double-ridge stage (W2.5), in the less-developed lateral meristems present at the bottom (Fig. 4B) and top (Fig. 4C) of the developing spike, LFY expression was stronger at the leaf ridge (also known as the lower ridge) than at the spikelet or upper ridge (Fig. 4B,C, blue arrows). In the more mature SMs located in the central part of the developing spike, LFY expression was abundant in the basal region but low in central-distal regions of the SMs (Fig. 4B, blue arrows), suggesting that low LFY levels may favor spikelet development. Some SMs showed a more uniform distribution of LFY, but those may be the result of off-centered sections.
We used the gene FRIZZY PANICLE (FZP, TraesCS2A02G116900) as an early marker for the IM→TS transition. At W2.5, FZP was not detected in the distal part of the wheat spike and was present only at the axils of the developing glumes in the more mature central spikelets, similar to previously reported results in rice (Komatsu et al., 2003). However, at W3.25, when the developing spikes reach the final SNS, FZP was detected in the youngest lateral meristems immediately below the IM (Fig. S4, Table S8), indicating their transition to glumes and serving as a marker of the IM→TS transition.
At the W3.25 stage, the more-developed spikelets at the center of the spike showed glume and lemma primordia (Fig. 4D). In these spikelets, LFY transcripts were highly expressed within a narrow band that, in a tridimensional space, may appear similar to a bird's nest located distal to the lemma primordia. This high-expression LFY band delimited a distal region of the developing spikelet that has lower LFY and higher WAPO1 hybridization signals (Fig. 4D).
WAPO1 transcripts were detected at the axis of the developing spike at W1.5, in a region that overlapped with LFY (Fig. 4A, Fig. S5). However, at later stages (W2.5 and W3.25) WAPO1 expression was restricted to the base and center of the developing spike, likely in the differentiating vascular tissue (Fig. 4B,D). This distribution is easier to visualize in Fig. S5, which presents WAPO1 expression alone. At the lemma primordia stage (W3.25), WAPO1 expression was also detected in the distal part of the more developed spikelets (Fig. 4D, Fig. S5C), in agreement with previous in situ hybridization results (Kuzay et al., 2022). A detail of the WAPO1 expression domain shows colocalization with LFY in multiple cells within the distal region of the developing spikelet, which extends to one or two cell layers into the area of high LFY expression (Fig. S6).
In summary, the distinct but partially overlapping expression domains of LFY and WAPO1 in the developing spikelets generate an area of overlap, which likely favors interactions between their encoded proteins and provides important spatial information for normal floral development.
Spatiotemporal expression profiles of LFY and SQUAMOSA MADS-box genes
In Arabidopsis, LFY activates the meristem identity genes AP1 (Parcy et al., 1998; Wagner et al., 1999) and CAL (William et al., 2004), so we first investigated whether the homologous wheat VRN1 and FUL2 genes (Table S9) were also regulated by LFY using qRT-PCR (Fig. S7, Table S10). We found no significant differences between lfy and the WT control for VRN1 or FUL2 transcript levels at W2.0, W3.0 or W4.0 (Fig. S7). Analyses of previously published RNAseq data for Kronos spike development (VanGessel et al., 2022) showed that VRN1 is induced earlier and is expressed at higher levels than the other two SQUAMOSA genes, with FUL2 expressed at higher levels than FUL3 (Fig. S8A). In the same RNAseq study, LFY was expressed at low levels in the vegetative meristem (W1.0) and increased rapidly during W2.0 and W3.0 (Figs S3B, S8A).
We then compared the smFISH spatial and temporal expression profiles of VRN1 and FUL2 during spike development. At the late vegetative stage (W1.0), the hybridization signal of VRN1 was relatively low and FUL2 was not detected in the apical meristem (Fig. 5A). The signal for both genes increased during the early transition to the reproductive stage, although FUL2 remained low (Fig. 5B; W1.5). These results were consistent with the RNAseq data (Fig. S8A). At later stages (W2.5 and W3.25), VRN1 and FUL2 were both highly expressed in the IM and young lateral SMs (Fig. 5C,D). In the more developed spikelets, located at the center of the developing spike, VRN1 and FUL2 expression was stronger at the glume and lemma primordia than in the distal region (Fig. 5D; W3.25), which overlapped with the WAPO1 expression domain (Fig. 4D). FUL3 showed a similar spatial expression profile as FUL2 and it is presented separately (Fig. S9) because of its lower expression levels in the RNAseq data (Fig. S8A) and limited impact on SNS (Li et al., 2019).
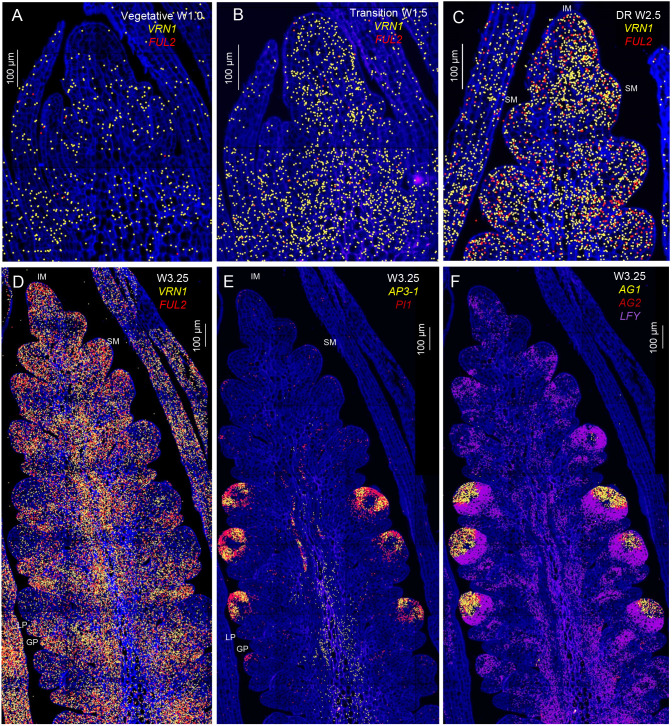
Fig. 5.
Transcription profiles of MADS-box floral genes during wheat spike development. (A-C) Relative distribution of VRN1 and FUL2 at late vegetative shoot apical meristem (A), transitioning shoot apical meristem (B) and late double-ridge (C) stages. (D-F) Expression of VRN1 and FUL2 (D), the class-B MADS-box genes AP3-1 and PI1 (E) and the class-C MADS-box genes AG1 and AG2 (F) at lemma primordia stage. DR, double-ridge; GP, glume primordium; IM, inflorescence meristem; LP, lemma primordium; SM, spikelet meristem. Gene identifications and rice orthologs are in Table S9.
To quantify VRN1, FUL2 and LFY expression changes in the distal part of the developing spike between the late double-ridge stage (W2.5) and the start of the transition to a terminal spikelet (W3.25), we calculated their signal density (hybridization signal per 100 μm2). In both the IM and the IM plus the two youngest lateral meristems (IM+2LM), we observed a three- to fourfold increase in FUL2 signal density (Fig. S8B) and a 53-69% increase in VRN1 between W2.5 and W3.25, although only the differences for FUL2 were significant (Fig. S8C, Table S8). Similar results were obtained when the VRN1 and FUL2 hybridization signals were normalized using the CDC20 signal (Table S8). In the same tissue sections, we detected a 78% decrease in LFY signal density (Fig. S8D), and these changes were significant or highly significant depending on the normalization method used (Table S8).
Analyses of the ratios between the SQUAMOSA and LFY signals showed that the FUL2/LFY ratio increased more than 20-fold between W2.5 and W3.25 in both the IM and IM+2LM (Fig. S8E, Table S8). Similarly, VRN1/LFY ratios increased eightfold between the same developmental stages (Fig. S8E, Table S8). Because the SQUAMOSA/LFY ratios are independent of the normalization method used, and they are determined in the same tissue sections, they provide the best evidence of a significant increase in the expression of the SQUAMOSA genes relative to LFY in the distal part of the developing spike at the time of the IM→TS transition.
Spatial expression profiles of floral organ identity genes
We also characterized the spatial distribution of MADS-box genes involved in floral organ development (Table S9). The hybridization signals of class-B (AP3-1 and PI1; Fig. 5E), class-C (AG1and AG2; Fig. 5F) and class-E (SEP3-1 and SEP3-2; Fig. S10) floral organ identity genes were concentrated in a distal region of the developing spikelets, where they overlapped with the expression of WAPO1 (Figs 4D and 5D). SEP1-2, SEP1-4 and SEP1-6 were expressed outside of the region where the two SEP3 genes were expressed (Fig. S10), suggesting functional divergence between the SEP1 and SEP3 genes in wheat.
Finally, we used qRT-PCR to characterize the effect of the lfy mutation on the expression of the floral organ identity genes in the wheat developing spike at W4.0, when these genes are highly expressed (Kuzay et al., 2022). The lfy mutant showed a significant downregulation of AP3-1 and PI1 (Fig. S11A), AG1 (Fig. S11B), SEP3-1 and SEP3-2 (Fig. S11C) relative to the WT (Table S11). Taken together, these results indicate that LFY plays an important role in the direct or indirect regulation of the floral organ identity genes.
Genetic interactions between LFY and class-A MADS-box genes
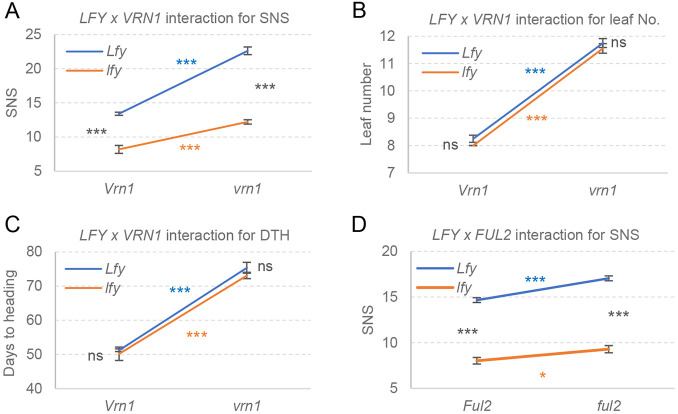
Given the opposite effects of LFY and the MADS-box genes VRN1 and FUL2 on SNS and their opposite expression changes during the IM→TS transition, we examined their genetic interactions for this trait. We crossed a plant homozygous for lfy-A and heterozygous for lfy-B with mutants homozygous for vrn1 and ful2-A but heterozygous for ful2-B and, in the progeny, selected sister plants homozygous for the four gene combinations for each gene (WT, lfy, vrn1, lfy vrn1 and WT, lfy, ful2, lfy ful2).
A factorial ANOVA including the four homozygous VRN1-LFY combinations showed highly significant effects on SNS for both VRN1 and LFY, and a highly significant interaction between these two genes (P<0.0001; Fig. 6A, Table S12). The effect of LFY on SNS was stronger in the vrn1 mutant (10.3 spikelets) than in the presence of the functional Vrn1 allele (5.2 spikelets). In contrast, the effect of VRN1 on SNS was stronger in the presence of the functional LFY allele (9.1 spikelets) than in the presence of the lfy combined mutant (4.0 spikelets; Fig. 6A). VRN1 also showed highly significant effects on the number of leaves and heading time, similar to previous studies (Li et al., 2019), whereas LFY showed no significant differences for these traits in the presence of the Vrn1 or vrn1 alleles. No significant interactions between these two genes were detected for these two traits (Fig. 6B,C, Table S12).
Fig. 6.
Genetic interactions between LFY and the SQUAMOSA MADS-box genes VRN1 and FUL2. (A-C) Interaction graphs between LFY and VRN1 for SNS (total n=34) (A), leaf number (total n=34) (B) and days to heading (DTH) (total n=34) (C). (D) Interaction between LFY and FUL2 for SNS (total n=54). In the interaction graphs, parallel lines indicate additive effects and non-parallel lines reflect interactions. 2×2 factorial ANOVA with P-values indicating the four simple effects (Table S12). *P<0.05, **P<0.01, ***P<0.001. ns, not significant. Error bars are s.e.m. See Table S12 for raw data.
The effect of FUL2 on SNS was smaller than the effect of VRN1 (Fig. 6D; adjusted means from two experiments), but the main effects of both LFY and FUL2 were still highly significant in both experiments. The contrasts for the four single effects were also consistent between the two FUL2 experiments (Table S12) and also with the interactions between LFY and VRN1: a stronger effect of LFY on SNS in the presence of the mutant ful2 allele than in the presence of the WT Ful2 allele, and a stronger effect of FUL2 in the presence of the WT LFY allele than in the presence of lfy (Table S12). The FUL2×LFY interaction was significant only in the second experiment with a higher number of replications (P=0.0175). In summary, these interactions suggest the existence of a cross-talk between the SQUAMOSA and LFY genes in the regulation of SNS.
DISCUSSION
Similarities and differences in LFY function between Arabidopsis and wheat
The most conserved functions of LFY across the flowering plants are those associated with the regulation of organ identity in the three inner floral whorls, which include pistils, stamens and petals in eudicot or lodicules in grass species (Yoshida, 2012). LFY mutations have more limited effects on bracts (lemmas in grasses) or on the outermost floral whorls, including sepals in Arabidopsis and paleas in grasses. However, the first floret of the basal spikelet frequently showed a bifurcated palea (Fig. 1F), suggesting that interactions between LFY and genes expressed in the base of the wheat spike regulate palea development.
In the grass species, similar defects in the inner floral organs have been observed for wheat lfy mutants (Fig. 1F, Table S2), rice apo2 mutants (Ikeda-Kawakatsu et al., 2012), barley multiovary 5 mutants (Selva et al., 2021), and maize zfl1 zfl2 mutants (Bomblies et al., 2003). These defects include fused organs, reduced number and altered morphology of lodicules (including transformation into bracts and fusions with stamens), reduced number of stamens and increased number of pistils. Fused lodicules with stamens and homeotic conversions of stamens into pistils were observed frequently in the wheat lfy mutant (Fig. 1F). The Arabidopsis strong lfy mutants fail to develop flowers, but weak lfy mutants show petals transformed into small sepals or mosaic organs, reduced stamen numbers and increased pistil numbers (Huala and Sussex, 1992; Weigel et al., 1992), similar to the grass species.
Despite the conserved roles of LFY in floral organ development, there are also important differences in LFY functions between Arabidopsis and grasses. First, strong lfy mutations in Arabidopsis result in the replacement of most flowers by shoots subtended by cauline leaves and the few observed late flowers exhibit intermediate inflorescence characteristics (Schultz and Haughn, 1991; Weigel et al., 1992). In contrast, floret meristems initiate normally in lfy in wheat (Fig. 1F) and other grasses (Bomblies et al., 2003; Ikeda-Kawakatsu et al., 2012), and defects appear only later at the inner floral whorls. These results indicate that LFY is required to confer the initial floral meristem identity in Arabidopsis but not in the grass species.
This difference likely contributes to the opposite functions of LFY in inflorescence development in these species. In Arabidopsis, constitutive expression of LFY (35S:LFY) results in conversion of both apical and axillary meristems into terminal flowers, demonstrating that LFY is a limiting factor defining when and where flowers are produced (Weigel and Nilsson, 1995). In contrast, constitutive expression of LFY in wheat increases the number of lateral spikelets (Fig. 2). Mutations in LFY also result in contrasting effects in eudicots and grasses inflorescence development. Weak lfy mutants delay the formation of flowers and increase the number of secondary branches in Arabidopsis and other eudicot species (Coen et al., 1990; Schultz and Haughn, 1991; Weigel et al., 1992; Souer et al., 1998; Molinero-Rosales et al., 1999). In contrast, LFY loss-of-function mutations result in significant reductions in SNS in wheat (Fig. 1B-E), and in the number of branches in rice panicles (Ikeda-Kawakatsu et al., 2012) and maize male inflorescences (Bomblies et al., 2003).
In summary, LFY has a similar role on floral organ development in Arabidopsis and grasses, but plays different roles in floral meristem identity and inflorescence development.
LFY and WAPO1 jointly regulate floret and spike development
LFY and WAPO1 proteins physically interact with each other in wheat (Fig. 3A), barley (Selva et al., 2021), rice (Ikeda-Kawakatsu et al., 2012) and Arabidopsis (Chae et al., 2008). In Arabidopsis, the LFY-UFO complex binds to a different set of genes than LFY alone (Rieu et al., 2023b), including class-B genes AP3-1 and PI. In addition to the class-B genes, ufo mutations in other eudicot species show altered expression of class-C genes (snapdragon), class-E genes (cucumber) and both class-C and -E genes (petunia; reviewed by Rieu et al., 2023a). The simultaneous regulation of class-B, -C and -E MADS-box genes seems to be conserved in the temperate grasses. lfy mutations in wheat (Fig. S11) and barley (Selva et al., 2021), and wapo1 mutants in wheat (Kuzay et al., 2022) have all been associated with the downregulation of class-B (AP3-1 and PI1), class-C (AG1) and class-E (SEP3) floral organ identity genes. This result explains the similar floral defects observed in the wheat lfy (Fig. 1F) and wapo1 mutants (Kuzay et al., 2022), and indicates that both LFY and WAPO1 are required for the proper regulation of these floral organ identity genes.
In addition to their roles in floral development, LFY and WAPO1 jointly regulate SNS. The wapo1, lfy and combined lfy wapo1 mutants all show similar reductions in SNS (Fig. 3B). Similar reductions in the number of panicle branches were also observed in the apo1, apo2 and combined apo1 apo2 mutants in rice (Ikeda-Kawakatsu et al., 2012). In this study, we show that the reduction in wheat SNS in the lfy and wapo1 mutants is the result of a reduction in the rate of SM formation per day rather than a change in the timing of the IM→TS transition. Interestingly, overexpression of either LFY (FALSIFLORA) or UFO (ANANTHA) in tomato can transform multiflowered inflorescences into solitary flowers (MacAlister et al., 2012). By contrast, heterochronic shifts during meristem maturation of UFO and few other flowering regulators can modulate inflorescence complexity (Lemmon et al., 2016). These results suggest that LFY and UFO homologs can affect meristem maturation rates in both tomato and wheat.
In summary, LFY and WAPO1 act jointly to regulate both inflorescence architecture and floral organ development in wheat, and likely in other plant species.
Interactions between LFY and SQUAMOSA genes modulate their opposite effects on the IM development
MADS-box transcription factors act as master regulators of developmental switches and organ specification, with meristem identity genes from the SQUAMOSA clade playing essential roles in the initiation of flower development. Among them, AP1 can modulate chromatin accessibility and facilitate access of other transcriptional regulators to their target gene, suggesting that it acts as a pioneer transcription factor (Pajoro et al., 2014). In the wheat vrn1 ful2 ful3 combined mutant, lateral spikelets are completely transformed into vegetative tillers subtended by leaves (Li et al., 2019), whereas the flowers in the Arabidopsis ap1 cal ful triple mutant are transformed into leafy shoots (Ferrándiz et al., 2000). LFY is also a pioneer transcription factor that can bind nucleosomes in closed chromatin, displace H1 linker histones and recruit the SWI/SNF chromatin-remodeling complex, permitting the binding of other transcription factors (Jin et al., 2021; Lai et al., 2021; Yamaguchi, 2021).
In Arabidopsis, LFY positively regulates the expression of AP1 and CAL (Parcy et al., 1998; Wagner et al., 1999; William et al., 2004), thereby indirectly regulating their downstream targets. LFY also directly regulates hundreds of genes independently of AP1 and CAL (Goslin et al., 2017). However, induction of LFY in ap1 cal mutants is insufficient to rescue the limited and late formation of flowers observed in this mutant and, instead, results in a lower proportion of plants with flowers than in the control (Goslin et al., 2017). This last result indicates that, in the absence of AP1 and CAL, LFY can inhibit flower formation in Arabidopsis, similar to its function in the grass species. These results also suggest that the ability of LFY to directly regulate the SQUAMOSA genes in Arabidopsis but not in wheat (Fig. S7) likely contributes to the opposite functions of LFY in the regulation of IM development between these species.
Approximately 200 genes have been identified in Arabidopsis as high-confidence direct targets of both LFY and AP1 (Winter et al., 2015). Many of the shared genes directly regulated by the induction of AP1 or LFY show changes in expression with identical directionality, but some of them are regulated in opposite directions, including several key regulators of floral initiation (Goslin et al., 2017). These common gene targets can contribute to the epistatic genetic interactions between LFY and AP1 and to their ability to coordinate the transcriptional programs required for flower initiation and early flower development in Arabidopsis.
Genes that are directly regulated by both LFY and SQUAMOSA are likely to also exist in wheat, and may contribute to the significant genetic interaction for SNS observed in this study between LFY and both VRN1 (Fig. 6A) and FUL2 (Fig. 6D). The net effect of the LFY×VRN1 interaction, 5.2 spikelets per spike, was smaller than the maximum differences of 14.4 spikelets observed between the plants carrying the vrn1 Lfy combination (22.6 spikelets/spike) and those carrying the Vrn1 lfy combination (8.2 spikelets/spike) (Table S12). These results indicate that the interaction between LFY and SQUAMOSA explains only part of the variation in SNS.
In summary, lfy mutations have opposite effects on SNS than vrn1 or ful2 mutations, and those effects are mostly additive. However, significant genetic interactions between LFY and the SQUAMOSA genes contribute to the observed differences in SNS in the different mutant combinations.
LFY and WAPO1 show dynamic spatiotemporal expression patterns during wheat spike and spikelet development
Spike development
From the beginning of the wheat spike development, LFY expression is not uniform, with higher expression levels at the lower or leaf ridge than at the upper or spikelet ridge (Fig. 4A-C), a pattern reported previously in wheat by in situ hybridization (Shitsukawa et al., 2006). This spatial differentiation continues in the early spike development (W2.5) when LFY is abundant in the proximal region of the young SMs but rare in their distal region (Fig. 4B,C). A similar pattern was also reported in the SMs of young barley spikes (Zhong et al., 2021) and in the primary and secondary branch meristems in the developing rice panicles (Kyozuka et al., 1998; Miao et al., 2022). These results suggest a conserved LFY spatial pattern in developing grass inflorescences and highlight the importance of reduced LFY levels in initiating spikelet development.
In wheat, the SM regions with low LFY expression (Fig. 4B,C) show high levels of VRN1 and FUL2 transcripts (Fig. 5C,D) and, therefore, high SQUAMOSA/LFY ratios. We hypothesize that the change in the balance between SM-promoting and -repressing pioneer transcription factors is crucial for marking the regions where the lateral spikelets will develop. This hypothesis is also supported by the drastic changes in the relative smFISH signal densities of these genes in the IM and two youngest lateral meristems between the early stages of spike development (W2.5) and the time of the IM→TS transition (determined by the presence of FZP, W3.25; Fig. S4). In the IM, the VRN1/LFY ratio increased more than eightfold and the FUL2/LFY ratio increased more than 25-fold between W2.5 and W3.25 at the initiation of the terminal spikelet (Fig. S8, Table S8).
Changes in gene dosage for LFY (Fig. S1) or the SQUAMOSA genes result in opposite changes in SNS, confirming the importance of their relative transcript levels on spike development. The single lfy-A and lfy-B mutants show intermediate reductions in SNS relative to the combined lfy mutant (Fig. S1), whereas mutations in FUL2 result in smaller increases in SNS than mutations in VRN1, which is expressed at higher levels than FUL2 in the IM (Fig. S8A, Table S8) (Li et al., 2019). Interestingly, four recently cloned genes affecting SNS in wheat – WAPO1 (Kuzay et al., 2022), FT-A2 (Shaw et al., 2019; Glenn et al., 2022), bZIPC1 (Glenn et al., 2023) and SPL17 (Liu et al., 2023) – show potential connections with the regulation of VRN1 or LFY (Fig. S12). In summary, we propose that the modulation of VRN1 or LFY transcript levels in the IM plays an important role in the determination of SNS in wheat.
LFY was co-expressed with WAPO1 in the IM at the early stages of spike development (Fig. 4A), but not at later stages (Fig. 4B,C, Fig. S5B,C). This early colocalization in the IM seems to be sufficient to explain the similar reduction in SNS observed in the lfy and wapo1 mutants. Both mutants were associated with similar reductions in the rate of SM formation relative to the WT, rather than by a change in the timing of the IM→TS transition (Fig. 3C,D). Given that these differences were evident from the earliest stages of spikelet development, when both LFY and WAPO1 were co-expressed in the IM (Fig. 4A), we hypothesize that the transient formation of the LFY-WAPO1 complex is sufficient to activate the gene expression networks that accelerate the rate of SM initiation.
Spikelet development
The smFISH studies also provided insights into the dynamic spatial distribution of LFY, WAPO1, and the floral organ identity genes during spikelet development. In the more-developed central spikelets at W3.25, LFY was highly expressed in a narrow nest-shaped region distal to the lemma primordia, delimiting a distal spikelet meristem region with lower LFY expression (Fig. 4D). This intense LFY expression band has been also observed distal to the lemma primordia of the second and third florets by in situ hybridization in more-developed wheat spikelets (Shitsukawa et al., 2006), highlighting its importance for normal floret development.
Within the distal region of the developing spikelets, WAPO1 was co-expressed with LFY in multiple cells, including one or two cell layers within the region of high LFY expression (Fig. 4D, Fig. S6). Within this overlapping region LFY and WAPO1 proteins may have a higher chance to interact with each other, providing valuable spatial information to the floral organ identity genes. This hypothesis is indirectly supported by similar reductions in the expression levels of the floral organ identity genes and similar floral abnormalities in both the wapo1 and lfy mutants (Fig. 1F, Fig. S11; Kuzay et al., 2022).
In Arabidopsis, the UFO-LFY complex regulates a different set of gene targets than LFY alone (Rieu et al., 2023b), and both genes are required for the correct regulation of the floral organ identity genes AP3 (Chae et al., 2008; Rieu et al., 2023b) and PI (Honma and Goto, 2000). These results are consistent with the downregulation of the wheat floral organ identity genes in the lfy and wapo1 mutants, and with the overlap between the expression domains of the wheat floral organ identity genes (Fig. 5, Fig. S10) and LFY-WAPO1 co-expression region in the distal part of the wheat developing spikelets.
In summary, this study shows that LFY plays important roles in both floral organ and spike development. The dynamic spatial and temporal expression profiles of LFY and WAPO1 in the developing spikelets correlate well with the function of these genes in the regulation of floral organ identity genes and normal floret development. In addition, these genes regulate the rate of SM formation, and interact with the SQUAMOSA genes in the regulation of SNS. Therefore, natural or induced variation in these genes can be used to improve this important agronomic trait in a crop that is central for global food security.
MATERIALS AND METHODS
Ethyl methanesulfonate-induced LFY mutants and their interactions with VRN1, FUL2 and WAPO1
We screened the sequenced tetraploid wheat variety Kronos population (Krasileva et al., 2017) by BLASTN to identify loss-of-function mutations in the LFY-A1 and LFY-B1 homeologs. To reduce background mutations, the lfy-B mutant was backcrossed twice to Kronos and then to the lfy-A mutant. The double mutant was backcrossed once to Kronos and, among the progeny, homozygous sister lines were selected for the four possible homozygous combinations, including the lfy-A lfy-B combined mutant (here termed lfy). This line is BC1 for lfy-A and BC2 for lfy-B so it is referred to as BC1-2. Genome-specific markers for the lfy-A and lfy-B mutations were designed and used to genotype plants during backcrossing and combination with other mutations described below. Primers are listed in Table S1.
To study the interactions between LFY and other spike development genes, we intercrossed the lfy combined mutant with previously developed Kronos lines homozygous for loss-of-function ethyl methanesulfonate or CRISPR mutations in both genomes of VRN1 (Chen and Dubcovsky, 2012), FUL2 (Li et al., 2019) or WAPO1 (Kuzay et al., 2022). These lines had at least two backcrosses to the parental line Kronos to reduce the number of background mutations. For these crosses, we used a line heterozygous for one of the LFY mutations to restore fertility, and molecular markers to select the four possible homozygous combinations in the F2 progeny of each cross.
Plant growth and phenotypic characterization
Plants were stratified for 2 days at 4°C in the dark and then planted in growth chambers (PGR15, Conviron, Manitoba, Canada). Lights were set to 350 μmol m−2 s−1 at canopy level. Plants were grown under inductive 16-h long days or non-inductive 8-h short days with temperatures set during the day to 22°C and during the night to 17°C. Relative humidity in growth chambers was maintained at 60-70% throughout the duration of the experiments. Heading time was recorded as the number of days from germination to full emergence of the spike from the leaf sheath. SNS was measured at maturity from the main tiller.
Generation of the wheat transgenic lines overexpressing LFY
We cloned the LFY-A coding regions by PCR from cDNA derived from Kronos developing spikes using primer LFY-A-GW-F combined with LFY-A-GW-R2 (listed in Table S1). We then recombined it into the pDONR/zeo entry vector using Life Technologies BP Clonase II following the manufacturer's protocol. The pDONR/zeo vector containing the LFY-A coding region was next recombined into the Japan Tobacco pLC41 vector downstream of the maize UBIQUITIN promoter using Life Technologies LR Clonase II to generate a construct expressing LFY with a C-terminal 3xHA tag, which was verified by Sanger sequencing at each cloning step. The T-DNA binary construct was transformed into the wheat variety Kronos using Agrobacterium-mediated transformation (EHA105) at the UC Davis Plant Transformation Facility as described previously (Debernardi et al., 2020b). The presence of the LFY transgene was determined with primers LFY-Genotyping-R5 and UBI-F2 (listed in Table S1).
LFY transcript levels were determined by qRT-PCR using primers LFY_qPCR_F and LFY_qPCR_R and ACTIN as endogenous control (Table S1). For qRT-PCR experiments, RNA was extracted using the Spectrum Plant Total RNA Kit (Sigma-Aldrich) or as previously described by Ream et al. (2014). One μg of RNA was treated with RQ1 RNase-Free DNase (Promega, M6101) first and then used for cDNA synthesis with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The qRT-PCR experiments were performed using Quantinova SYBR Green PCR kit (QIAGEN, 208052) in a 7500 Fast Real-Time PCR system (Applied Biosystems). Transcript levels for all genes are expressed as linearized fold-ACTIN levels calculated by the formula 2(ACTIN CT−TARGET CT)±s.e.m.
smFISH
We used the Molecular Cartography™ technology from Resolve BioSciences, which is based on combinatorial smFISH. Wheat shoot apical meristems were collected from the vegetative to the spike lemma primordia stage. The samples were immediately fixed in 4% paraformaldehyde after harvest, dehydrated, and embedded in paraffin. Sections from the central plane of the developing spikes (10 µm thick) were placed on the slides and dried overnight at 37°C, followed by a 10-min bake at 50°C. The sections were then deparaffinized, permeabilized, and refixed according to the Resolve BioSciences user guide. After complete dehydration, the sections were mounted using SlowFade-Gold Antifade reagent, covered with a thin glass coverslip, and sent to Resolve BioSciences on dry ice for analysis as described in our previous study (Glenn et al., 2023).
Probes were designed using Resolve BioSciences' proprietary design algorithm and gene annotations from Chinese Spring RefSeqv1.1. To identify potential off-target sites, searches were confined to the coding regions. Each target sequence underwent a single scan for all k-mers, favoring regions with rare k-mers as seeds for full probe design. For each of the wheat genes selected for smFISH probe design (Table S9), we selected the homoeolog expressed at higher levels in a Kronos transcriptome including different stages of spike development (VanGessel et al., 2022), and provided Resolve BioSciences with their respective homeologs to be excluded in their quality control for primer specificity performed against all the coding sequences of the wheat genome (Ref Seq v1.1). Therefore, probes are not genome specific and may detect both homeologs for each gene (catalog number PGGS; all these probes are part of kit number K7128).
Imaging and image processing was performed as described previously (Glenn et al., 2023). Final image analysis and quantification of dot density was performed in ImageJ using the Polylux tool plugin to examine specific Molecular Cartography™ signals.
Co-IP assay and western blotting
To examine the physical interaction between WAPO and LFY, we performed Co-IP experiments in wheat leaf protoplasts using a method described previously (Zhang et al., 2023), with minor modifications. The WAPO1 coding region was initially synthesized by GENEWIZ into the pUC57 vector, amplified with primers WAPO1_BP_F and WAPO1_BP_R (Table S1), cloned into pDONR/zeo vector using Life Technologies BP Clonase II, and recombined into the Japan Tobacco pLC41 vector downstream of the maize UBIQUITIN promoter with a C-terminal 4xMYC tag (for transgenic experiments). Next, we switched both UBI:WAPO1-MYC and UBI:LFY-HA from the pLC41 binary vector to the smaller pUC19 vector to enhance the transfection efficiency of the protoplasts. The UBI:WAPO-MYC and UBI-LFY-HA DNA fragments were cleaved using restriction enzymes HindIII and SpeI, gel purified, and then ligated with the HindIII-XbaI linearized pUC19 vector (SpeI and XbaI create compatible ends). Both constructs were verified by digestions with restriction enzymes and Sanger sequencing.
We transformed Kronos leaf protoplasts with 50 μg of each of the UBI:LFY-HA and UBI: WAPO1-MYC plasmids in 50 ml tubes containing 2 ml of protoplast (roughly 0.5×106 cell per ml). As negative controls, we performed separate transformations including only one of the two plasmids. After transformation, protoplasts were resuspended in 5 ml W5 buffer and incubated in a 6-well plate at room temperature overnight. Total protein was extracted with 1 ml of IP lysis buffer [25 mM Tris-HCl pH7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40 substitute, 5% glycerol and 1×protease inhibitors (Millipore Sigma, P9599)]. Part of the protein extract was set aside as input control (50 μl), and the rest was used for Co-IP using Pierce Anti-HA Magnetic Beads (Thermo Fisher Scientific, 88836) by gentle agitation on a tube rotator for 30 min at room temperature with additional 1× proteinase inhibitors (Millipore Sigma, P9599). Proteins were washed twice with 300 μl 1× TBS (25 mM Tris, 0.15 M NaCl at pH 7.5) with 0.05% Tween-20, and once with ultra-pure water before they were eluted by boiling the beads in 50 μl 1× Laemmli sample buffer for 10 min.
For western blotting, half of the Co-IP elution and 50 μg of input for each sample were loaded onto a 12% SDS-PAGE gel. After proteins were transferred to a PVDF membrane using the Bio-Rad Trans-Blot Turbo Transfer System (1704150), the membrane was blocked with 1× TBST buffer (20 mM Tris, 0.15 M NaCl, 0.1% Tween-20 at pH 7.5) containing 5% non-fat milk for 1 h at room temperature. Anti-cMyc-peroxidase monoclonal antibody (Roche, 11814150001) was added at a dilution of 1:10,000 and was incubated at room temperature for 1 h. After four 10 min washes using 1× TBST buffer, the signals were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, 34096). After imaging, the membrane was stripped with mild stripping buffer (1.5% glycine, 0.1% SDS, 1% Tween 20, pH 2.2), re-blocked for 1 h at room temperature, and then probed with anti-HA-peroxidase at a dilution of 1:2500 (Roche, 12013819001) for 1 h at room temperature.
Supplementary Material
Acknowledgements
We thank Stephen Pearce (Rothamsted Research) and Youngjun Mo (JeonBuk National University) for identifying the LFY-B mutant, developing markers and the initial backcrossing to Kronos. We thank Maria von Korff (Heinrich Heine University Düsseldorf) for a valuable revision of the manuscript and for her supervision of Tianyu Lan. We also thank Dr. Kun Li (UC Davis) for her help with multiple experiments, Dr Junli Zhang (UC Davis) for valuable suggestions, and the UC Davis transformation facility for the generation of the UBI:LFY-HA transgenic plants.
Footnotes
Author contributions
Conceptualization: J.D.; Methodology: F.P., H.L., C.L., A.J.; Formal analysis: J.D., C.L., J.M.D.; Investigation: F.P., H.L., C.L., T.L., C.T., J.M.D., A.J.; Resources: J.D., C.L., J.M.D.; Writing - original draft: F.P., J.D.; Writing - review & editing: F.P., H.L., C.L., D.P.W., T.L., C.T., J.M.D., A.J., J.D.; Visualization: F.P., H.L., C.L., J.D.; Supervision: C.L., D.P.W., J.M.D., J.D.; Project administration: J.D.; Funding acquisition: J.D.
Funding
This work was supported by the United States Department of Agriculture National Institute of Food and Agriculture (2022-67013-36209 and 2022-68013-36439 to J.D.), Howard Hughes Medical Institute Researcher Support (J.D.), the Life Sciences Research Foundation (D.P.W.) and the National Science Foundation (C.T.). Open Access funding provided by the University of California. Deposited in PMC for immediate release.
Data availability
All relevant data can be found within the article and its supplementary information. Kronos mutants K2613 for lfy-A and K350 for lfy-B are available from the authors upon request without any restrictions for use or from the Germplasm Resources Unit (GRU) at the John Innes Centre. Images for the spike sections used in the smFISH and hybridization coordinates for the genes presented in this study are available at https://dubcovskylab.ucdavis.edu/content/spatial-transcriptomic.
Peer review history
The peer review history is available online at https://journals.biologists.com/dev/lookup/doi/10.1242/dev.202803.reviewer-comments.pdf
References
- Alvarez, M. A., Tranquilli, G., Lewis, S., Kippes, N. and Dubcovsky, J. (2016). Genetic and physical mapping of the earliness per se locus Eps-Am1 in Triticum monococcum identifies EARLY FLOWERING 3 (ELF3) as a candidate gene. Funct. Integr. Genomic 16, 365-382. 10.1007/s10142-016-0490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies, K., Wang, R.-L., Ambrose, B. A., Schmidt, R. J., Meeley, R. B. and Doebley, J. (2003). Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130, 2385-2395. 10.1242/dev.00457 [DOI] [PubMed] [Google Scholar]
- Chae, E., Tan, Q. K.-G., Hill, T. A. and Irish, V. F. (2008). An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development 135, 1235-1245. 10.1242/dev.015842 [DOI] [PubMed] [Google Scholar]
- Chen, A. and Dubcovsky, J. (2012). Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. 8, e1003134. 10.1371/journal.pgen.1003134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choulet, F., Alberti, A., Theil, S., Glover, N., Barbe, V., Daron, J., Pingault, L., Sourdille, P., Couloux, A., Paux, E.et al. (2014). Structural and functional partitioning of bread wheat chromosome 3B. Science 345, 1249721. 10.1126/science.1249721 [DOI] [PubMed] [Google Scholar]
- Coen, E. S., Romero, J. M., Doyle, S., Elliott, R., Murphy, G. and Carpenter, R. (1990). Floricaula - a homeotic gene required for flower development in Antirrhinum majus. Cell 63, 1311-1322. 10.1016/0092-8674(90)90426-F [DOI] [PubMed] [Google Scholar]
- Debernardi, J. M., Greenwood, J. R., Jean Finnegan, E., Jernstedt, J. and Dubcovsky, J. (2020a). APETALA 2-like genes AP2L2 and Q specify lemma identity and axillary floral meristem development in wheat. Plant J. 101, 171-187. 10.1111/tpj.14528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi, J. M., Tricoli, D. M., Ercoli, M. F., Hayta, S., Ronald, P., Palatnik, J. F. and Dubcovsky, J. (2020b). A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274-1279. 10.1038/s41587-020-0703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz, C., Gu, Q., Martienssen, R. and Yanofsky, M. F. (2000). Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127, 725-734. 10.1242/dev.127.4.725 [DOI] [PubMed] [Google Scholar]
- Frank, A. B. and Bauer, A. (1982). Effect of temperature and fertilizer-N on apex development in spring wheat. Agron. J. 74, 504-509. 10.2134/agronj1982.00021962007400030024x [DOI] [Google Scholar]
- Frank, A. B., Bauer, A. and Black, A. L. (1987). Effects of air-temperature and water-stress on apex development in spring wheat. Crop Sci. 27, 113-116. 10.2135/cropsci1987.0011183X002700010028x [DOI] [Google Scholar]
- Glenn, P., Zhang, J., Brown-Guedira, G., DeWitt, N., Cook, J. P., Li, K., Akhunov, E. and Dubcovsky, J. (2022). Identification and characterization of a natural polymorphism in FT-A2 associated with increased number of grains per spike in wheat. Theor. Appl. Genet. 135, 679-692. 10.1007/s00122-021-03992-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn, P., Woods, D. P., Zhang, J., Gabay, G., Odle, N. and Dubcovsky, J. (2023). Wheat bZIPC1 interacts with FT2 and contributes to the regulation of spikelet number per spike. Theor. Appl. Genet. 136, 237. 10.1007/s00122-023-04484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin, K., Zheng, B., Serrano-Mislata, A., Rae, L., Ryan, P. T., Kwaśniewska, K., Thomson, B., Ó’Maoiléidigh, D. S., Madueño, F., Wellmer, F.et al. (2017). Transcription factor interplay between LEAFY and APETALA1/CAULIFLOWER during floral initiation. Plant Physiol. 174, 1097-1109. 10.1104/pp.17.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma, T. and Goto, K. (2000). The Arabidopsis floral homeotic gene PSTILLATA is regulated by discrete cis-elements responsive to induction and maintenance signals. Development 127, 2021-2030. 10.1242/dev.127.10.2021 [DOI] [PubMed] [Google Scholar]
- Huala, E. and Sussex, I. M. (1992). Leafy interacts with floral homeotic genes to regulate Arabidopsis floral development. Plant Cell 4, 901-913. 10.2307/3869458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, K., Nagasawa, N. and Nagato, Y. (2005). ABERRANT PANICLE ORGANIZATION 1 temporally regulates meristem identity in rice. Dev. Biol. 282, 349-360. 10.1016/j.ydbio.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Ikeda, K., Ito, M., Nagasawa, N., Kyozuka, J. and Nagato, Y. (2007). Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant J. 51, 1030-1040. 10.1111/j.1365-313X.2007.03200.x [DOI] [PubMed] [Google Scholar]
- Ikeda-Kawakatsu, K., Maekawa, M., Izawa, T., Itoh, J. I. and Nagato, Y. (2012). ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1. Plant J. 69, 168-180. 10.1111/j.1365-313X.2011.04781.x [DOI] [PubMed] [Google Scholar]
- Jin, R., Klasfeld, S., Zhu, Y., Garcia, M. F., Xiao, J., Han, S.-K., Konkol, A. and Wagner, D. (2021). LEAFY is a pioneer transcription factor and licenses cell reprogramming to floral fate. Nat. Commun. 12, 626. 10.1038/s41467-020-20883-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu, M., Chujo, A., Nagato, Y., Shimamoto, K. and Kyozuka, J. (2003). FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130, 3841-3850. 10.1242/dev.00564 [DOI] [PubMed] [Google Scholar]
- Krasileva, K. V., Vasquez-Gross, H. A., Howell, T., Bailey, P., Paraiso, F., Clissold, L., Simmonds, J., Ramirez-Gonzalez, R. H., Wang, X., Borrill, P.et al. (2017). Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. USA 114, E913-E921. 10.1073/pnas.1619268114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzay, S., Xu, Y., Zhang, J., Katz, A., Pearce, S., Su, Z., Fraser, M., Anderson, J. A., Brown-Guedira, G., DeWitt, N.et al. (2019). Identification of a candidate gene for a QTL for spikelet number per spike on wheat chromosome arm 7AL by high-resolution genetic mapping. Theor. Appl. Genet. 132, 2689-2705. 10.1007/s00122-019-03382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzay, S., Lin, H., Li, C., Chen, S., Woods, D. P., Zhang, J., Lan, T., von Korff, M. and Dubcovsky, J. (2022). WAPO-A1 is the causal gene of the 7AL QTL for spikelet number per spike in wheat. PLoS Genet. 18, e1009747. 10.1371/journal.pgen.1009747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyozuka, J., Konishi, S., Nemoto, K., Izawa, T. and Shimamoto, K. (1998). Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proc. Natl. Acad. Sci. USA 95, 1979-1982. 10.1073/pnas.95.5.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, X. L., Blanc-Mathieu, R., GrandVuillemin, L., Huang, Y., Stigliani, A., Lucas, J., Thévenon, E., Loue-Manifel, J., Turchi, L., Daher, H.et al. (2021). The LEAFY floral regulator displays pioneer transcription factor properties. Mol. Plant 14, 829-837. 10.1016/j.molp.2021.03.004 [DOI] [PubMed] [Google Scholar]
- Lee, I., Wolfe, D. S., Nilsson, O. and Weigel, D. (1997). A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr. Biol. 7, 95-104. 10.1016/S0960-9822(06)00053-4 [DOI] [PubMed] [Google Scholar]
- Lemmon, Z. H., Park, S. J., Jiang, K., Van Eck, J., Schatz, M. C. and Lippman, Z. B. (2016). The evolution of inflorescence diversity in the nightshades and heterochrony during meristem maturation. Genome Res. 26, 1676-1686. 10.1101/gr.207837.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, J. Z. and Meyerowitz, E. M. (1995). Ufo – an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7, 529-548. 10.1105/tpc.7.5.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. and Dubcovsky, J. (2008). Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J. 55, 543-554. 10.1111/j.1365-313X.2008.03526.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Lin, H. and Dubcovsky, J. (2015). Factorial combinations of protein interactions generate a multiplicity of florigen activation complexes in wheat and barley. Plant J. 84, 70-82. 10.1111/tpj.12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Lin, H., Chen, A., Lau, M., Jernstedt, J. and Dubcovsky, J. (2019). Wheat VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet development and spike determinacy. Development 146, dev175398. 10.1242/dev.175398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. Y., Chen, J., Yin, C. B., Wang, Z. Y., Wu, H., Shen, K. C., Zhang, Z. L., Kang, L. P., Xu, S., Bi, A. Y.et al. (2023). A high-resolution genotype-phenotype map identifies the TaSPL17 controlling grain number and size in wheat. Genome Biol. 24, 196. 10.1186/s13059-023-03044-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, B., Nitcher, R., Han, X., Wang, S., Ni, F., Li, K., Pearce, S., Wu, J., Dubcovsky, J. and Fu, D. (2014). Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS ONE 9, e94171. 10.1371/journal.pone.0094171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, E. V. and Grieve, C. M. (1990). Spike and leaf development in salt-stressed wheat. Crop. Sci., 30, 1309-1313. 10.2135/cropsci1990.0011183X003000060031x [DOI] [Google Scholar]
- MacAlister, C. A., Park, S. J., Jiang, K., Marcel, F., Bendahmane, A., Izkovich, Y., Eshed, Y. and Lippman, Z. B. (2012). Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat. Genet. 44, 1393-1398. 10.1038/ng.2465 [DOI] [PubMed] [Google Scholar]
- Maizel, A., Busch, M. A., Tanahashi, T., Perkovic, J., Kato, M., Hasebe, M. and Weigel, D. (2005). The floral regulator LEAFY evolves by substitutions in the DNA binding domain. Science 308, 260-263. 10.1126/science.1108229 [DOI] [PubMed] [Google Scholar]
- Miao, Y. L., Xun, Q., Taji, T., Tanaka, K., Yasuno, N., Ding, C. Q. and Kyozuka, J. (2022). ABERRANT PANICLE ORGANIZATION2 controls multiple steps in panicle formation through common direct-target genes. Plant Physiol. 189, 2210-2226. 10.1093/plphys/kiac216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinero-Rosales, N., Jamilena, M., Zurita, S., Gómez, P., Capel, J. and Lozano, R. (1999). FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. Plant J. 20, 685-693. 10.1046/j.1365-313X.1999.00641.x [DOI] [PubMed] [Google Scholar]
- Pajoro, A., Madrigal, P., Muiño, J. M., Matus, J. T., Jin, J., Mecchia, M. A., Debernardi, J. M., Palatnik, J. F., Balazadeh, S., Arif, M.et al. (2014). Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol. 15, R41. 10.1186/gb-2014-15-3-r41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy, F., Nilsson, O., Busch, M. A., Lee, I. and Weigel, D. (1998). A genetic framework for floral patterning. Nature 395, 561-566. 10.1038/26903 [DOI] [PubMed] [Google Scholar]
- Preston, J. C., Christensen, A., Malcomber, S. T. and Kellogg, E. A. (2009). MADS-box gene expression and implications for developmental origins of the grass spikelet. Am. J. Bot. 96, 1419-1429. 10.3732/ajb.0900062 [DOI] [PubMed] [Google Scholar]
- Ream, T. S., Woods, D. P., Schwartz, C. J., Sanabria, C. P., Mahoy, J. A., Walters, E. M., Kaeppler, H. F. and Amasino, R. M. (2014). Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 164, 694-709. 10.1104/pp.113.232678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu, P., Arnoux-Courseaux, M., Tichtinsky, G. and Parcy, F. (2023a). Thinking outside the F-box: how UFO controls angiosperm development. New Phytol. 240, 945-959. 10.1111/nph.19234 [DOI] [PubMed] [Google Scholar]
- Rieu, P., Turchi, L., Thévenon, E., Zarkadas, E., Nanao, M., Chahtane, H., Tichtinsky, G., Lucas, J., Blanc-Mathieu, R., Zubieta, C.et al. (2023b). The F-box protein UFO controls flower development by redirecting the master transcription factor LEAFY to new cis-elements. Nat. Plants 9, 315-329. 10.1038/s41477-022-01336-2 [DOI] [PubMed] [Google Scholar]
- Schultz, E. A. and Haughn, G. W. (1991). LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell 3, 771-781. 10.2307/3869271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva, C., Shirley, N. J., Houston, K., Whitford, R., Baumann, U., Li, G. and Tucker, M. R. (2021). HvLEAFY controls the early stages of floral organ specification and inhibits the formation of multiple ovaries in barley. Plant J. 108, 509-527. 10.1111/tpj.15457 [DOI] [PubMed] [Google Scholar]
- Shaw, L. M., Turner, A. S., Herry, L., Griffiths, S. and Laurie, D. A. (2013). Mutant alleles of Photoperiod-1 in wheat (Triticum aestivum L.) that confer a late flowering phenotype in long days. PLoS ONE 8, e79459. 10.1371/journal.pone.0079459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, L. M., Lyu, B., Turner, R., Li, C., Chen, F., Han, X., Fu, D. and Dubcovsky, J. (2019). FLOWERING LOCUS T2 regulates spike development and fertility in temperate cereals. J. Exp. Bot. 70, 193-204. 10.1093/jxb/ery350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitsukawa, N., Takagishi, A., Ikari, C., Takumi, S. and Murai, K. (2006). WFL, a wheat FLORICAULA/LEAFY ortholog, is associated with spikelet formation as lateral branch of the inflorescence meristem. Genes Genet. Syst. 81, 13-20. 10.1266/ggs.81.13 [DOI] [PubMed] [Google Scholar]
- Souer, E., van der Krol, A., Kloos, D., Spelt, C., Bliek, M., Mol, J. and Koes, R. (1998). Genetic control of branching pattern and floral identity during inflorescence development. Development 125, 733-742. 10.1242/dev.125.4.733 [DOI] [PubMed] [Google Scholar]
- VanGessel, C., Hamilton, J., Tabbita, F., Dubcovsky, J. and Pearce, S. (2022). Transcriptional signatures of wheat inflorescence development. Sci. Rep. 12, 17224. 10.1038/s41598-022-21571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington, S. R., Cartwright, P. M. and Wall, P. C. (1983). A quantitative scale of spike initial and pistil development in barley and wheat. Ann. Bot. 51, 119-130. 10.1093/oxfordjournals.aob.a086434 [DOI] [Google Scholar]
- Wagner, D., Sablowski, R. W. M. and Meyerowitz, E. M. (1999). Transcriptional activation of APETALA1 by LEAFY. Science 285, 582-584. 10.1126/science.285.5427.582 [DOI] [PubMed] [Google Scholar]
- Weigel, D. and Nilsson, O. (1995). A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495-500. 10.1038/377495a0 [DOI] [PubMed] [Google Scholar]
- Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. and Meyerowitz, E. M. (1992). Leafy controls floral meristem identity in Arabidopsis. Cell 69, 843-859. 10.1016/0092-8674(92)90295-N [DOI] [PubMed] [Google Scholar]
- William, D. A., Su, Y. H., Smith, M. R., Lu, M., Baldwin, D. A. and Wagner, D. (2004). Genomic identification of direct target genes of LEAFY. Proc. Natl. Acad. Sci. USA 101, 1775-1780. 10.1073/pnas.0307842100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, C. M., Yamaguchi, N., Wu, M. F. and Wagner, D. (2015). Transcriptional programs regulated by both LEAFY and APETALA1 at the time of flower formation. Physiol. Plantarum 155, 55-73. 10.1111/ppl.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, N. (2021). LEAFY, a pioneer transcription factor in plants: a mini-review. Front. Plant. Sci. 12, 701406. 10.3389/fpls.2021.701406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H. (2012). Is the lodicule a petal: molecular evidence? Plant Sci. 184, 121-128. 10.1016/j.plantsci.2011.12.016 [DOI] [PubMed] [Google Scholar]
- Zhang, J. L., Gizaw, S. A., Bossolini, E., Hegarty, J., Howell, T., Carter, A. H., Akhunov, E. and Dubcovsky, J. (2018). Identification and validation of QTL for grain yield and plant water status under contrasting water treatments in fall-sown spring wheats. Theor. Appl. Genet. 131, 1741-1759. 10.1007/s00122-018-3111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Li, C., Zhang, W., Zhang, X., Mo, Y., Tranquilli, G. E., Vanzetti, L. S. and Dubcovsky, J. (2023). Wheat plant height locus RHT25 encodes a PLATZ transcription factor that interacts with DELLA (RHT1). Proc. Natl. Acad. Sci. USA 120, e2300203120. 10.1073/pnas.2300203120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, J., van Esse, G. W., Bi, X., Lan, T., Walla, A., Sang, Q., Franzen, R. and von Korff, M. (2021). INTERMEDIUM-M encodes an HvAP2L-H5 ortholog and is required for inflorescence indeterminacy and spikelet determinacy in barley. Proc. Natl. Acad. Sci. USA 118, e2011779118. 10.1073/pnas.2011779118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.