Summary
Background
In the STEP-HFpEF (NCT04788511) and STEP-HFpEF DM (NCT04916470) trials, the GLP-1 receptor agonist semaglutide improved symptoms, physical limitations, bodyweight, and exercise function in people with obesity-related heart failure with preserved ejection fraction. In this prespecified pooled analysis of the STEP-HFpEF and STEP-HFpEF DM trials, we aimed to provide a more definitive assessment of the effects of semaglutide across a range of outcomes and to test whether these effects were consistent across key patient subgroups.
Methods
We conducted a prespecified pooled analysis of individual patient data from STEP-HFpEF and STEP-HFpEF DM, randomised, double-blind, placebo-controlled trials at 129 clinical research sites in 18 countries. In both trials, eligible participants were aged 18 years or older, had heart failure with a left ventricular ejection fraction of at least 45%, a BMI of at least 30 kg/m², New York Heart Association class II–IV symptoms, and a Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS; a measure of heart failure-related symptoms and physical limitations) of less than 90 points. In STEP-HFpEF, people with diabetes or glycated haemoglobin A1c concentrations of at least 6·5% were excluded, whereas for inclusion in STEP-HFpEF DM participants had to have been diagnosed with type 2 diabetes at least 90 days before screening and to have an HbA1c of 10% or lower. In both trials, participants were randomly assigned to either 2·4 mg semaglutide once weekly or matched placebo for 52 weeks. The dual primary endpoints were change from baseline to week 52 in KCCQ-CSS and bodyweight in all randomly assigned participants. Confirmatory secondary endpoints included change from baseline to week 52 in 6-min walk distance, a hierarchical composite endpoint (all-cause death, heart failure events, and differences in changes in KCCQ-CSS and 6-min walk distance); and C-reactive protein (CRP) concentrations. Heterogeneity in treatment effects was assessed across subgroups of interest. We assessed safety in all participants who received at least one dose of study drug.
Findings
Between March 19, 2021 and March 9, 2022, 529 people were randomly assigned in STEP-HFpEF, and between June 27, 2021 and Sept 2, 2022, 616 were randomly assigned in STEP-HFpEF DM. Overall, 1145 were included in our pooled analysis, 573 in the semaglutide group and 572 in the placebo group. Improvements in KCCQ-CSS and reductions in bodyweight between baseline and week 52 were significantly greater in the semaglutide group than in the placebo group (mean between-group difference for the change from baseline to week 52 in KCCQ-CSS 7·5 points [95% CI 5·3 to 9·8]; p<0·0001; mean between-group difference in bodyweight at week 52 −8·4% [−9·2 to −7·5]; p<0·0001). For the confirmatory secondary endpoints, 6-min walk distance (mean between-group difference at week 52 17·1 metres [9·2 to 25·0]) and the hierarchical composite endpoint (win ratio 1·65 [1·42 to 1·91]) were significantly improved, and CRP concentrations (treatment ratio 0·64 [0·56 to 0·72]) were significantly reduced, in the semaglutide group compared with the placebo group (p<0·0001 for all comparisons). For the dual primary endpoints, the efficacy of semaglutide was largely consistent across multiple subgroups, including those defined by age, race, sex, BMI, systolic blood pressure, baseline CRP, and left ventricular ejection fraction. 161 serious adverse events were reported in the semaglutide group compared with 301 in the placebo group.
Interpretation
In this prespecified pooled analysis of the STEP-HFpEF and STEP-HFpEF DM trials, semaglutide was superior to placebo in improving heart failure-related symptoms and physical limitations, and reducing bodyweight in participants with obesity-related heart failure with preserved ejection fraction. These effects were largely consistent across patient demographic and clinical characteristics. Semaglutide was well tolerated.
Funding
Novo Nordisk.
Introduction
Obesity is a major risk factor for heart failure with preserved ejection fraction.1,2 Although many people who have heart failure with preserved ejection fraction also have obesity, individuals with obesity are under-represented in clinical trials of heart failure. Trial exclusion criteria often prevent participation of people with very high BMIs (eg, >40 kg/m²) or with insufficient natriuretic peptide concentrations to meet eligibility thresholds (which is frequently the case in people with obesity).3 Type 2 diabetes is also common (prevalence roughly 45%) in people with heart failure with preserved ejection fraction.4–6 Obesity and diabetes create a pro-inflammatory state that can promote endothelial and coronary microvascular dysfunction in heart failure with preserved ejection fraction.1,7,8 Obesity also causes increased epicardial and chest wall adiposity, amplifying ventricular interdependence,9 which is further exacerbated by plasma and blood volume expansion and excessive vasoconstriction, resulting in further worsening of heart failure with preserved ejection fraction.10–12
The STEP-HFpEF trial13 aimed to assess the effect of the GLP-1 receptor agonist semaglutide in people with heart failure with preserved ejection fraction and a BMI of 30 kg/m² or higher who did not have type 2 diabetes. To our knowledge, the trial was the first to test a GLP1 receptor agonist in people with obesity-related heart failure with preserved ejection fraction. Significant improvements in symptoms, physical limitations, and exercise function, and reductions in bodyweight, were recorded in the semaglutide group compared with the placebo group.13 In previous trials of treatments for obesity (including studies of GLP-1 receptor agonists), people with diabetes lost less weight than those without diabetes.14–17 In the STEP-HFpEF DM trial, patients with obesity-related heart failure with preserved ejection fraction and type 2 diabetes were randomly assigned to either weekly semaglutide or placebo. Significant improvements in heart failure outcomes were recorded in the semaglutide group compared with the placebo group, findings that were consistent with those noted in STEP-HFpEF, although participants in STEP-HFpEF DM lost less weight.
In view of the relatively modest size of both trials, we conducted a prespecified pooled analysis of the STEP-HFpEF and STEP-HfpEF DM trial populations to provide a more definitive assessment of the effects of semaglutide across a broad range of outcomes in people with obesity-related heart failure with preserved ejection fraction with and without diabetes, and to assess whether these effects are consistent across key patient subgroups.
Methods
Study design and participants
In this study, we conducted a pooled analysis of the populations of STEP-HFpEF13 and STEP-HFpEF DM, randomised, double-blind, placebo-controlled trials at 129 clinical research site sites in 18 countries in Asia, Europe, North America, and South America. The steering committee, which included academic members and representatives from the study sponsor, designed both trials and was responsible for the academic publications. A global expert panel provided academic, medical, and operational input in each country. The methods and outcomes for this pooled analysis were prespecified before database lock and unblinding of the two trials. The design and baseline characteristics of participants in both trials have been published.18
In both STEP-HFpEF and STEP-HFpEF DM, eligible participants were aged 18 years or older, had a left ventricular ejection fraction (LVEF) of at least 45%, a BMI of at least 30 kg/m², New York Heart Association (NYHA) class II–IV symptoms, a Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (KCCQ-CSS) of less than 90 points, a 6-min walk distance (6MWD) of at least 100 m, and at least one of elevated left ventricular filling pressures (based on invasive measurements), increased natriuretic peptide concentrations (with thresholds stratified based on BMI) plus echocardiographic abnormalities, or a hospitalisation for heart failure within 12 months of screening plus ongoing treatment with diuretics or echocardiographic abnormalities. In STEP-HFpEF, people with diabetes or glycated haemoglobin A1c (HbA1c) concentrations of at least 6·5% were excluded, whereas for inclusion in STEP-HFpEF DM participants had to have been diagnosed with type 2 diabetes at least 90 days before screening and to have an HbA1c of 10% or lower. Key exclusion criteria for both trials included self-reported change in bodyweight of greater than 5 kg within 90 days of screening, type 1 diabetes, and treatment with a GLP-1 receptor agonist within 90 days of screening. In STEP-HFpEF DM, people with uncontrolled diabetic retinopathy or maculopathy were also excluded. The full eligibility criteria for both trials are in the appendix (pp 5–8).
Both STEP-HFpEF and STEP-HFpEF DM were done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocols for both trials were approved by the ethics committees or institutional review boards at each site, and all participants signed written informed consent. The protocol for STEP-HFpEF has been previously published.13 The STEP-HFpEF DM protocol can be accessed online.19
Randomisation and procedures
In both trials, eligible participants were randomly assigned (1:1), using an interactive web-based response system, to either semaglutide given subcutaneously once weekly or matching placebo for 52 weeks, followed by a 5-week follow-up period. Randomisation was stratified by baseline BMI (<35 kg/m² vs ≥35 kg/m²). Both participants and investigators were masked to treatment assignment.
Semaglutide or placebo treatment was initiated at a dose of 0·25 mg weekly for the first 4 weeks, with dose escalation every 4 weeks to reach a dose of 2·4 mg weekly by week 16, which was maintained for the rest of the trial. Participants who discontinued treatment prematurely remained in the trial. In the STEP-HFpEF DM trial, semaglutide or placebo were added to background glucose-lowering drugs that participants were taking to manage their type 2 diabetes. Any drugs other than GLP-1 receptor agonists were permitted. Modification of glucose-lowering treatment was at the discretion of investigators. Specific guidance regarding the adjustment of sulfonylurea and insulin doses was provided to investigators by the study sponsor to mitigate the risk of hypoglycaemia.
Outcomes
Endpoints were harmonised across STEP-HFpEF and STEP-HFpEF DM. The dual primary endpoints were change in the KCCQ-CSS and percentage change in bodyweight from baseline to week 52. The KCCQ is a standardised 23-item instrument that quantifies heart failure-related symptoms, physical function, quality of life, and social function.20–22 The KCCQ-CSS includes the symptom and physical function domains. Scores range from 0 to 100; higher scores reflect better health status. Confirmatory secondary endpoints included the change from baseline to week 52 in 6MWD; change in C-reactive protein (CRP) concentrations from baseline to week 52; and a hierarchical composite endpoint comprising all-cause mortality, number and timing of heart failure events (adjudicated hospitalisation for heart failure or urgent hospital visit requiring intravenous therapy), differences in KCCQ-CSS change from baseline to week 52 of at least 15, at least 10, or at least 5 points, and a difference of at least 30 m in change in 6MWD from baseline to week 52. Select exploratory and other pre-specified endpoints included change in NT-proBNP concentrations from baseline to week 52, time to first adjudicated heart failure event, time to cardiovascular death or first heart failure event, and time to cardiovascular death or total (ie, first and recurrent) heart failure events (appendix pp 2–4, 9–11). Undetermined causes of death were classified as cardiovascular deaths.
Safety endpoints included serious adverse events and adverse events of special interest, which comprised adverse events leading to permanent treatment discontinuation, adverse events related to COVID-19, acute pancreatitis and medication errors, and, in STEP-HFpEF DM, clinically significant episodes of hypoglycaemia and new or worsening diabetic retinopathy. An independent blinded external committee adjudicated heart failure hospitalisation events, urgent heart failure hospital visits requiring intravenous therapy, and all deaths. All laboratory assays were done by ICON Laboratory Services (Farmingdale, NY, USA, and Dublin, Ireland).
Statistical analysis
Details of the statistical methods for both trials are further detailed in the appendix and have been previously reported.13,18,19 Both trials included sample sizes that provided greater than 90% power to detect a between-group difference of 4·1 points for the change in KCCQ-CSS from baseline to week 52 (α 0·04) and greater than 99% to detect a between-group difference of 5·9–9·9% in change in bodyweight from baseline to week 52 (α 0·01). Efficacy endpoints were analysed in the full analysis set, which included all randomly assigned participants (except one person who was randomly assigned in error to the placebo group of STEP-HFpEF DM) according to the intention-to-treat principle. In both trials, the results of the primary and confirmatory secondary efficacy endpoints in the testing hierarchy were validated by a sponsor-independent statistician (Statogen Consulting, Durham, NC, USA), who had access to all relevant datasets.
Safety endpoints were analysed in the safety analysis set, which included all randomly assigned participants who received at least one dose of assigned study treatment. We distinguished between the in-trial period (which included temporary treatment discontinuations or rescue intervention) and the on-treatment period (which specifically referred only to when participants were actively receiving treatment). We used two estimands (a treatment policy estimand consistent with the intention-to-treat principle and a hypothetical trial product estimand for if treatment was taken as intended [ie, on-treatment analysis]) to assess treatment efficacy and to account for intercurrent events (including discontinuation of treatment [including due to death], initiation of other weight-management agents, or bariatric surgery). The appendix (pp 2–3) provides further details on estimands, statistical analyses, and imputation methods to account for missing data.
The dual primary endpoints were analysed using ANCOVA, with change in the corresponding endpoint at week 52 as the dependent variable and randomly assigned treatment (semaglutide vs placebo), trial (STEP-HFpEF vs STEP-HFpEF DM), and BMI (<35 kg/m² vs ≥35 kg/m²) as fixed factors, with adjustment for the baseline value of the corresponding endpoint as a continuous variable for each imputation dataset. Treatment effects and SEs were combined using Rubin’s rule. Analyses of continuous confirmatory secondary and supportive secondary or exploratory endpoints followed an approach similar to that used for the dual primary endpoints. For analyses of CRP and NTproBNP, values were log-transformed. Analysis of the hierarchical composite endpoint (win ratio) was based on comparisons of each participant randomly assigned to semaglutide versus each participant assigned to placebo, stratified according to trial and baseline BMI group. For each of these participant pairs, a so-called treatment winner, based on similar observation time, was declared on the basis of the endpoint hierarchy as previously reported.18 The win ratio (ie, the number of winners randomly assigned to semaglutide divided by the number of winners assigned to placebo) was estimated using 1000 imputations for the continuous endpoints.
The effects of semaglutide on the dual primary endpoints were examined across 14 prespecified subgroups defined by age (<64 years vs ≥65 to <75 years vs ≥75 years), sex (male vs female), race (White vs not White), geographical region (North America vs Europe vs other), BMI (<35 kg/m² vs ≥35 kg/m² to <40 kg/m² vs ≥40 kg/m²), LVEF (45–49% vs 50–59% vs ≥60%), systolic blood pressure (<135 mmHg vs ≥135 mmHg), NYHA class (II vs III or IV), median NT-proBNP and CRP concentrations, baseline use of a loop diuretic (yes vs no), baseline use of renin–angiotensin system inhibitors (yes vs no), history of atrial fibrillation (yes vs no), and history of coronary artery disease (yes vs no). Subgroup analysis in which participants were stratified by median heart rate was done post-hoc. Subgroup analyses were done using ANCOVA models (1000 imputations), with an interaction term between treatment and the relevant subgroup variables, adjusted for the baseline value of the relevant continuous outcome variable, trial, and BMI subgroup (<35 kg/m² vs ≥35 kg/m²). Estimates from the multiple imputations were derived using Rubin’s rule. Interaction p values were derived from an F-test of equality between the treatment differences across the relevant subgroups.
Time to event for clinical outcomes was plotted by treatment group using the Aalen–Johansen method for first event (the cumulative incidence) and the Ghosh–Lin method for first and recurrent event (the expected number of events per participants), with all-cause or non-cardiovascular death as competing events. We used Cox regression, with treatment as a fixed factor stratified by trial and BMI subgroup (<35 kg/m² vs ≥35 kg/m²), for analyses of time to first event, and the Ghosh–Lin regression model for analyses of time to first and recurrent event, with treatment as a fixed factor and adjusted for trial and BMI subgroup using data in a counting process format.
For KCCQ-CSS, pre-specified responder analyses with thresholds of improvement of at least 5, 10, 15, and 20 points, and deterioration thresholds of 5 points and 10 points, were performed with logistic regression adjusted for baseline KCCQ-CSS, with treatment, BMI subgroup (<35 kg/m² vs ≥35 kg/m²), and trial as fixed factors. For responder analysis of 6MWD, we used a threshold of 30 m. Anchor-based responder analyses based on patient global impression of severity were also done for KCCQ-CSS and 6MWD. Missing values were imputed (appendix pp 2–3). Numbers needed to treat for each responder threshold were calculated as the reciprocal of the estimated absolute difference between the treatment groups.
To assess potential heterogeneity across the two trials for the treatment effects on the dual primary and confirmatory secondary endpoints (using the treatment policy estimand), we used the I² criterion,23 which we calculated from the individual treatment effects for each trial and for each endpoint. We also used an ANCOVA model, with the effects of randomized treatment (semaglutide vs placebo) by trial (STEP-HFpEF vs STEP-HFpEF DM) adjusted for randomised treatment, trial, and BMI (<35 kg/m² vs ≥35 kg/m²) as fixed factors, and with adjustment for the baseline value of the corresponding endpoint as a continuous variable assessed using the imputed dataset. For the win ratio, the test for equality of the two trials was performed using a Cochran’s Q-test.
On-treatment safety events were pooled across the two trials and were summarised as numbers of participants with an event and event rates. No adjustment for multiplicity was done in the pooled analyses,24 and a p value <0·05 was considered significant. All results from statistical analyses are presented with two-sided 95% CIs and two-sided p values. We used SAS (version 9.4) for all analyses. STEP-HFpEF and STEP-HFpEF DM are registered with ClinicalTrials.gov, NCT04788511 and NCT04916470.
Role of the funding source
The funder of the study had roles in study design, data collection, data analysis, data interpretation, and writing of the report.
Results
1869 people were screened and 1145 were enrolled and randomly assigned: 529 in STEP-HFpEF between March 19, 2021 and March 9, 2022, and 616 in STEP-HFpEF DM between June 27, 2021 and Sept 2, 2022. The pooled trial population included 573 participants assigned to semaglutide and 572 assigned to placebo. Pooled baseline characteristics were balanced between groups (table 1). Median age was 69 years (IQR 62–75). 570 (50%) participants were female and 575 (50%) were male; 1026 (90%) were White, 76 (7%) were Asian, and 39 (3%) were Black or African American (table 1). 745 (65%) had a BMI of 35 kg/m2 or higher, and 785 (69%) had NYHA class II symptoms. Comorbidities were common, and KCCQ-CSS and 6MWD were consistent with poor health status and exercise function in both groups (table 1). At least 75% of participants in each group were taking β blockers, diuretics, and renin–angiotensin system inhibitors (table 1). Baseline characteristics in each trial are in the appendix (pp 14–15).
Table 1:
Baseline characteristics of the pooled STEP-HFpEF and STEP-HFpEF DM populations
| Semaglutide group (n=573) | Placebo group (n=572) | |
|---|---|---|
| Age, years | 70 (62–74) | 69·0 (62–75) |
| Sex | ||
| Female | 277 (48%) | 293 (51%) |
| Male | 296 (52%) | 279 (49%) |
| Race* | ||
| White | 506 (88%) | 520 (91%) |
| Asian | 45 (8%) | 31 (5%) |
| Black or African American | 21 (4%) | 18 (3%) |
| Other | 1 (<1%) | 3 (1%) |
| Ethnicity* | ||
| Hispanic or Latino | 53 (9%) | 59 (10%) |
| Not Hispanic or Latino | 520 (91%) | 513 (90%) |
| Weight, kg | 104·4 (92·0–119·0) | 103·1 (90·5–118·5) |
| BMI | ||
| Median, kg/m² | 37·0 (33·6–41·3) | 36·9 (33·4–41·5) |
| <35 kg/m²† | 197 (34%) | 203 (35%) |
| ≥35 kg/m² | 376 (66%) | 369 (65%) |
| Waist circumference, cm | 120·2 (111·8–129·5) | 119·0 (111·0–129·0) |
| Blood pressure, mm Hg | ||
| Systolic | 133·0 (123·0–144·0) | 134·5 (123·0–144·0) |
| Diastolic | 78·0 (71·0–85·0) | 78·0 (70·0–84·0) |
| NT-proBNP, pg/mL | 443·6 (237·6–997·9) | 500·7 (232·6–1059·7) |
| C-reactive protein, mg/L | 3·8 (1·8–7·6) | 3·4 (1·7–8·4) |
| Left ventricular ejection fraction | ||
| Median | 57% (50–60) | 56% (50–60) |
| ≥45% to <50%‡ | 94 (16%) | 97 (17%) |
| ≥50% to <60% | 234 (41%) | 240 (42%) |
| ≥60% | 245 (43%) | 235 (41%) |
| KCCQ-CSS | 59·9 (44·3–72·9) | 58·3 (40·6–71·9) |
| 6-min walk distance, m | 295·0 (230·0–368·1) | 294·3 (215·0–367·0) |
| Hospitalisation for heart failure within previous year | 91 (16%) | 102 (18%) |
| Comorbidities at screening | ||
| Atrial fibrillation | 252 (44%) | 266 (47%) |
| Hypertension | 471 (82%) | 488 (85%) |
| Coronary artery disease | 132 (23%) | 114 (20%) |
| Obstructive sleep apnoea | 58 (10%) | 61 (11%) |
| New York Heart Association functional class | ||
| Class II | 406 (71%) | 379 (66%) |
| Class III | 166 (29%) | 192 (34%) |
| Class IV | 1 (<1%) | 1 (<1%) |
| Concomitant medications | ||
| β blockers | 458 (80%) | 470 (82%) |
| Any diuretic | 453 (79%) | 472 (83%) |
| Loop diuretics | 344 (60%) | 358 (63%) |
| Mineralocorticoid receptor antagonists | 194 (34%) | 190 (33%) |
| Thiazides | 82 (14%) | 93 (16%) |
| ACE inhibitors, ARBs, or ARNIs | 445 (78%) | 454 (79%) |
| ARNIs | 32 (6%) | 26 (5%) |
| Biguanides (ie, metformin) | 240 (42%) | 212 (37%) |
| Sulfonylureas | 57 (10%) | 51 (9%) |
| SGLT2 inhibitors | 115 (20%) | 106 (19%) |
| DPP-4 inhibitors | 55 (10%) | 37 (6%) |
| Insulins | 53 (9%) | 75 (13%) |
Data are n (%) or median (IQR) and are for the full analysis set, which included 1145 of the 1146 randomly assigned participants (the set excludes one participant who was randomly assigned in error). Percentages might not total to 100% because of rounding. ACE=angiotensin-converting enzyme. ARBs=angiotensin II receptor blockers. ARNIs=angiotensin receptor–neprilysin inhibitors. KCCQ-CSS=Kansas City Cardiomyopathy Questionnaire Clinical Summary Score.
Race or ethnic group were reported by the investigators based on the participants’ perception of their race or ethnicity
All participants had a BMI of at least 30 kg/m².
Includes one participant with a left ventricular ejection fraction of 33%.
The median duration of follow-up was 401 days (IQR 400 to 404). In the pooled population, the improvement from baseline to week 52 in KCCQ-CSS was significantly larger in the semaglutide group than in the placebo group (15·0 vs 7·5; mean difference 7·5 points [95% CI 5·3 to 9·8]; p<0·0001), and the percentage reduction in bodyweight was significantly greater (−8·4% [−9·2 to −7·5]; p<0·0001; figure 1). The corresponding mean reduction in bodyweight from baseline to week 52 was 12·0 kg (SD 7·7) in the semaglutide group and 3·1 kg (7·7) in the placebo group (between-group difference –8·9 [95% CI –9·9 to –8·0]).
Figure 1: Change in KCCQ-CSS (A) and percentage reduction in bodyweight (B) from baseline to week 52 in the pooled STEP-HFpEF and STEP-HFpEF DM population.
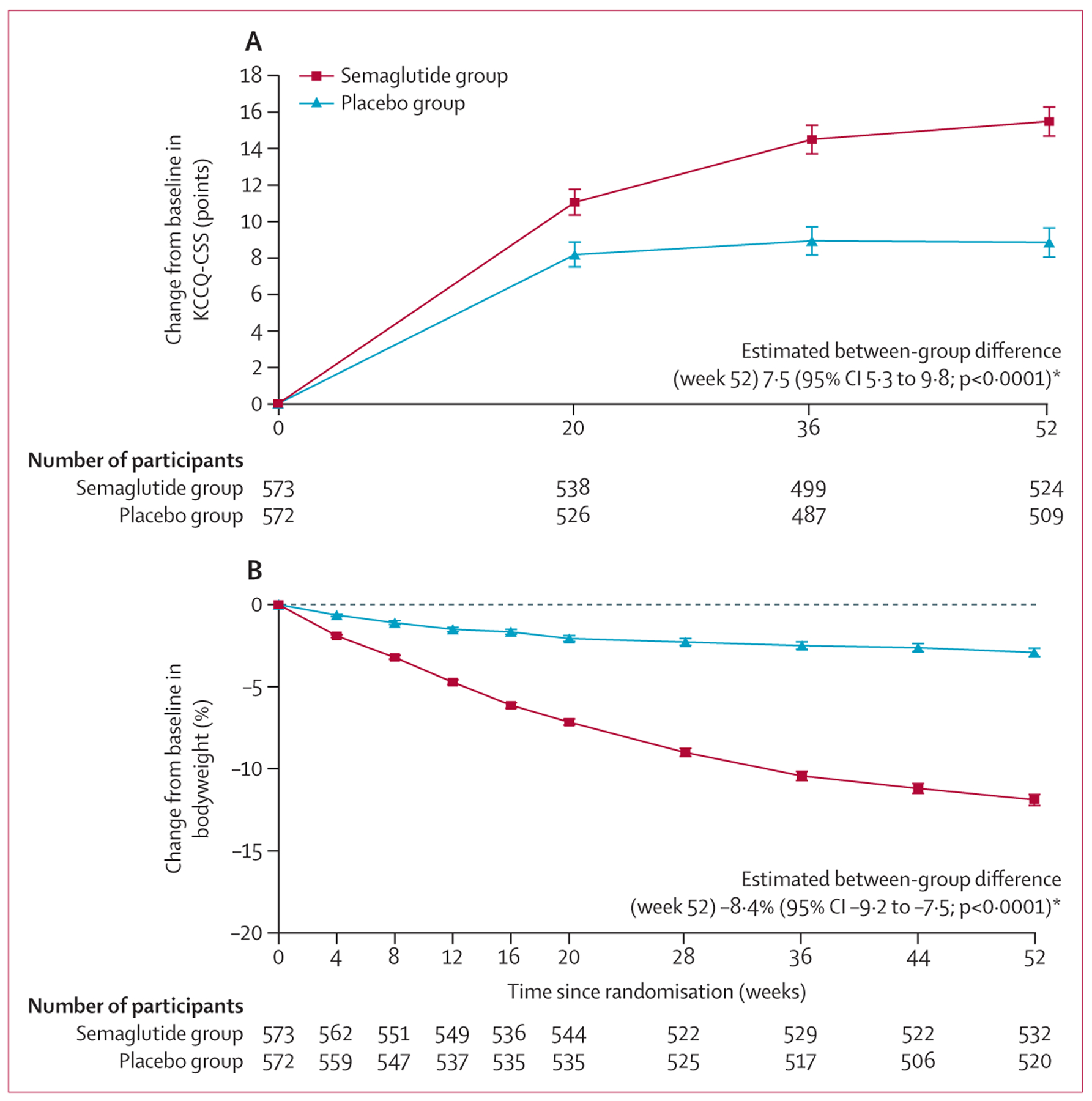
Analyses of between-group treatment differences were by ANCOVA and based on the treatment policy estimand, with missing data imputed. Data are observed (ie, as measured) mean changes. Error bars represent SEs.
The fluctuating numbers of participants at risk is a result of variations in participants attending study visits.
KCCQ-CSS=Kansas City Cardiomyopathy Questionnaire Clinical Summary Score. *The between-group comparisons at week 52 are for all participants in the full analysis set (n=573 in the semaglutide group and n=572 in the placebo group); missing data were imputed for these analyses.
Significant improvements in 6MWD (between-group difference 17·1 m [95% CI 9·2–25·0] for the change from baseline to week 52; p<0·0001), a significantly greater number of wins for the hierarchical composite endpoint (win ratio 1·65 [1·42–1·91]; p<0·0001) and significant reductions in CRP (estimated treatment ratio 0·64 [0·56–0·72]; p<0·0001) and NT-proBNP (estimated treatment ratio 0·82 [0·74–0·91]; p=0·0002) concentrations were noted in the semaglutide group compared with the placebo group (table 2; appendix pp 19–20). Data for other supportive secondary and select exploratory endpoints are in table 2. The corresponding results of the pooled trial data using the trial product estimand (ie, on-treatment analysis) for the dual primary and confirmatory secondary endpoints are in the appendix (pp 12–13).
Table 2:
Efficacy analysis of the pooled STEP-HFpEF and STEP-HFpEF DM populations
| Semaglutide group (n=573) | Placebo group (n=572) | Estimated between-group difference (95% CI) | Odds ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Dual primary endpoints | |||||
| KCCQ-CSS, points | 15·0 (18·0) | 7·5 (18·0) | 7·5 (5·3 to 9·8) | ·· | <0·0001 |
| Percentage bodyweight | −11·4% (7·1) | −3·0% (7·1) | −8·4% (−9·2 to −7·5) | ·· | <0·0001 |
| Confirmatory secondary endpoints | |||||
| 6-min walk distance, m | 16·7 (63·2) | −0·3 (63·2) | 17·1 (9·2 to 25·0) | ·· | <0·0001 |
| Hierarchical composite endpoint* | ·· | ·· | ·· | 1·65 (1·42 to 1·91) | <0·0001 |
| C-reactive protein ratio (week 52 to baseline) | 0·57 (0·55) | 0·90 (0·86) | ·· | 0·64 (0·56 to 0·72)† | <0·0001 |
| Supportive secondary endpoints | |||||
| Systolic blood pressure, mm Hg | −4·6 (15·7) | −1·7 (15·7) | −2·9 (−4·9 to −0·9) | ·· | 0·0052 |
| Waist circumference, cm | −10·3 (8·1) | −2·6 (8·1) | −7·6 (−8·7 to −6·6) | ·· | <0·0001 |
| KCCQ-OSS, points | 14·9 (18·1) | 7·5 (18·1) | 7·4 (5·2 to 9·6) | ·· | <0·0001 |
| ≥10% weight reduction | 55·8% (51·5–59·9) | 10·9% (8·5–14·0) | ·· | 10·3 (7·4 to 14·3) | <0·0001 |
| ≥15% weight reduction | 29·9% (26·1–33·9) | 3·4% (2·1–5·4) | ·· | 12·1 (7·2 to 20·2) | <0·0001 |
| ≥20% weight reduction | 12·2% (9·6–15·4) | 1·2% (0·6–2·6) | ·· | 11·2 (5·1 to 24·8) | <0·0001 |
| ≥5-point improvement in KCCQ-CSS | 73·7% (69·7–77·4) | 57·3% (52·9–61·5) | ·· | 2·1 (1·6 to 2·7) | <0·0001 |
| ≥10-point improvement in KCCQ-CSS | 61·1% (56·7–65·4) | 43·2% (38·8–47·6) | ·· | 2·1 (1·6 to 2·7) | <0·0001 |
| Met anchor-based threshold for change in KCCQ-CSS‡ | 43·0% (38·6–47·6) | 28·1% (24·3–32·4) | ·· | 1·9 (1·5 to 2·5) | <0·0001 |
| Met anchor-based threshold for change in 6-min walk distance§ | 47·2% (42·9–51·5) | 32·7% (28·7–37·0) | ·· | 1·8 (1·4 to 2·3) | <0·0001 |
| Exploratory endpoints | |||||
| NTproBNP ratio (week 52 to baseline) | 0·78 (0·65) | 0·95 (0·80) | ·· | 0·82 (0·74 to 0·91)† | 0·0002 |
| ≥15-point improvement in KCCQ-CSS | 47·6% (43·2–52·1) | 31·0% (26·9–35·3) | ·· | 2·0 (1·6 to 2·7) | <0·0001 |
| ≥20-point improvement in KCCQ-CSS | 36·5% (32·3–41·0) | 19·6% (16·3–23·4) | ·· | 2·4 (1·8 to 3·2) | <0·0001 |
| Heart failure events, per 100 patient-years | 1·3 | 4·9 | ·· | 0·27 (0·12 to 0·56)¶ | 0·0004 |
Data are the mean change from baseline to week 52 (SD) in the variable, or the estimated proportions of participants in each group meeting the specified criteria at week 52 (95% CI), unless otherwise specified. SDs were calculated as the square root of the residual variance. Analyses are based on the treatment policy estimand, which assessed the treatment effect irrespective of treatment discontinuation or rescue intervention. Continuous endpoint analyses at week 52 were conducted using ANCOVA models, with treatment group (ie, semaglutide vs placebo), trial (ie, STEP-HFpEF vs STEP-HfpEF DM), and BMI stratum (<35 kg/m² vs ≥35 kg/m²) as fixed factors and the baseline value for the variable as a covariate, using the in-trial period (ie, the time from randomisation to last contact with a trial site, irrespective of treatment discontinuation or rescue intervention). We imputed missing data (appendix p 18). For binary endpoints, proportions and odds ratios were estimated from a logistic regression model with treatment group, trial, and BMI stratum as fixed factors and the baseline value for the variable as a covariate, using the in-trial period. We imputed missing data (appendix p 18). Data showing rates or heart failure events were observed during the in-trial period. For supportive secondary and exploratory endpoints, the widths of CIs have not been adjusted for multiplicity and should not be used to infer treatment effects. KCCQ-CSS=Kansas City Cardiomyopathy Questionnaire Clinical Summary Score. KCCQ-OSS=Kansas City Cardiomyopathy Questionnaire Overall Summary Score.
A composite of death from any cause, number of heart failure events, number and timing of heart failure events (from baseline to week 57) using the in-trial period, a difference in the change in KCCQ-CSS from baseline to week 52 of at least 15, 10 or 5 points, and a difference of at least 30 m in the change in 6-min walk distance from baseline to week 52 using the in-trial period; this composite endpoint was assessed using a win-ratio approach, with all participants in the semaglutide group compared with all participants in the placebo group within each BMI stratum (<35 kg/m² vs ≥35 kg/m²) and missing data imputed.
This is an estimated treatment ratio rather than an odds ratio; the ratio to baseline and the corresponding baseline value were log-transformed before analysis; SDs for this endpoint were calculated using the delta method—the SD on the log scale was multiplied with the mean ratio.
The threshold was 16·7 points, which was based on the anchor using the change from baseline to week 52 in perception of heart failure symptoms measured with the patient global impression of severity; the mean change in KCCQ-CSS was calculated (using pooled data across treatment groups) in the participants with one-category improvement in the patient global impression of severity (n=358) to establish the threshold.
The threshold was 21·9 m, which was based on the anchor using the change from baseline to week 52 in perception of ability to walk quickly (one-category improvement) on the patient global impression of severity; the mean change in 6-min walk distance was calculated (using pooled data across treatment groups) in the participants with one-category improvement (n=293) to establish the threshold (appendix pp 3–4).
This ratio is a hazard ratio; time to first heart failure event (during the in-trial period) was analysed using a Cox regression model.
Treatment effects were consistent across STEP-HFpEF and STEP-HFpEF DM for KCCQ-CSS, 6MWD, hierarchical composite endpoint, and CRP concentrations (appendix pp 16–17). There was substantial heterogeneity across the trials for the mean percentage reduction in bodyweight (−13·3% in the semaglutide group vs −2·6% in the placebo group [estimated difference −10·7%, 95% CI −11·9 to −9·4] in STEP HFpEF; −9·8% in the semaglutide group vs −3·4% in the placebo group [−6·4%, −7·6 to −5·2%]; pinteraction<0·0001; I² 95·77%; appendix pp 16–17).
Changes in KCCQ-CSS and bodyweight from baseline to week 52 across patient subgroups are shown in figure 2 and the appendix (pp 21–22). Overall, the improvements in KCCQ-CSS and reductions in bodyweight with semaglutide compared with placebo were generally consistent across subgroups defined by sex, age, race, geographical region, and baseline BMI, LVEF, systolic blood pressure, CRP concentrations, and heart rate (figure 2; appendix pp 21–22). We noted significant interactions for the treatment effects of semaglutide on KCCQ-CSS by median NT-proBNP concentrations, use of loop diuretics, use of renin–angiotensin–aldosterone system inhibitors, and history of atrial fibrillation (figure 2). For weight reduction, there were significant interactions by sex (with greater reductions in women than in men) and race, but the numbers of non-White participants were small, resulting in wide CIs (appendix pp 21–22).
Figure 2: Change from baseline to week 52 in KCCQ-CSS across prespecified subgroups in the pooled STEP-HFpEF and STEP-HFpEF DM population, by treatment group.
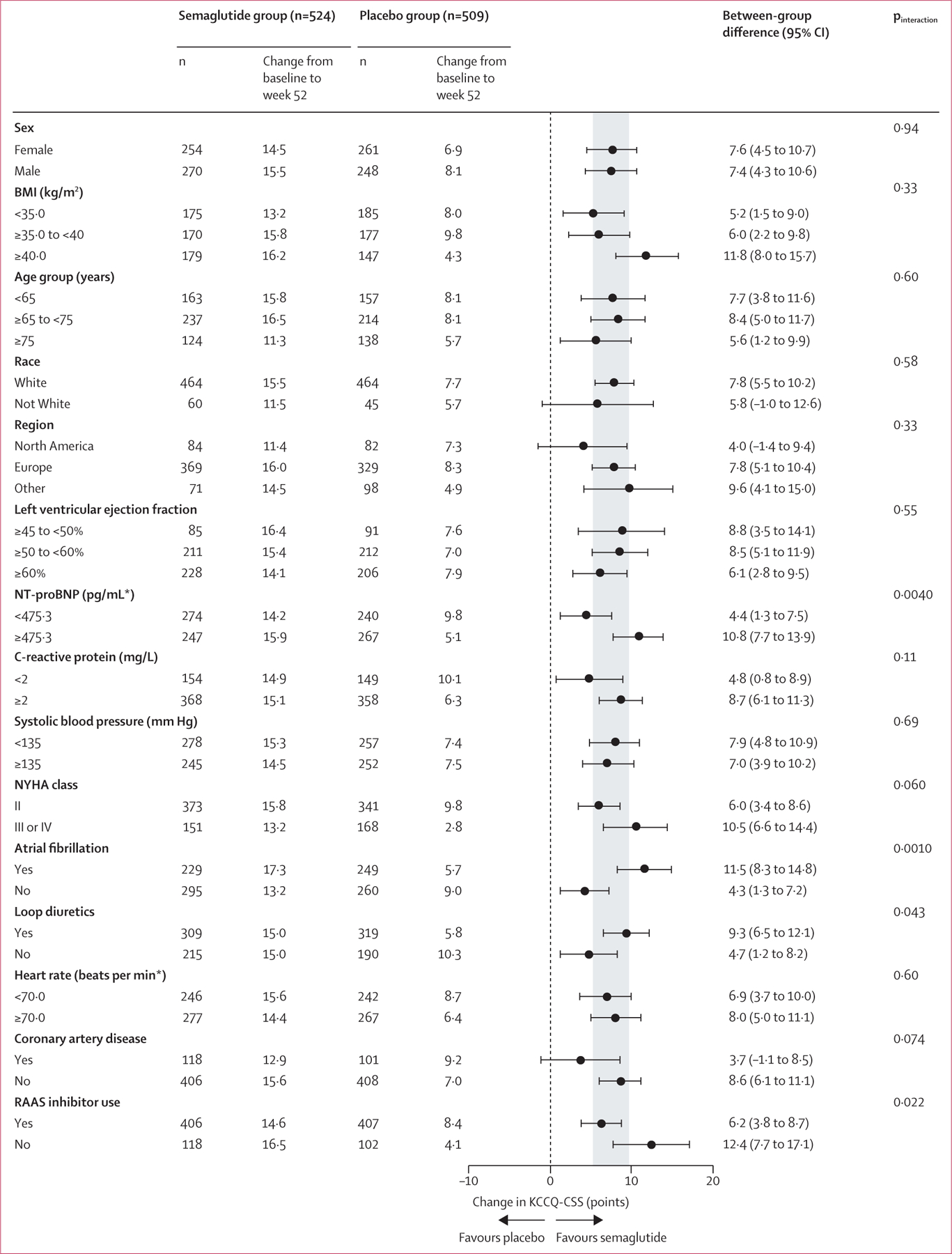
Analyses were by ANCOVA and based on the treatment policy estimand in the full analysis set for the in-trial period, with missing data imputed. KCCQ-CSS=Kansas City Cardiomyopathy Questionnaire Clinical Summary Score. NYHA=New York Heart Association. RAAS=renin–angiotensin–aldosterone system inhibitors.
*Subgroups defined on the basis of pooled median values.
Eight (1%) of 573 participants in the semaglutide group and 30 (5%) of 572 in the placebo group had adjudicated events of hospitalisation or an urgent visit for heart failure (hazard ratio 0·27 [95% CI 0·12–0·56]; figure 3A). The risk of an adjudicated cardiovascular death or heart failure event was lower in the semaglutide group than in the placebo group (hazard ratio 0·31 [0·15–0·62]; figure 3B). In the total event analysis (ie, first and recurrent events), 12 adjudicated events of cardiovascular death and heart failure-related hospitalisations or urgent visits occurred in the semaglutide group and 41 occurred in the placebo group (mean ratio 0·30 [0·14–0·64]).
Figure 3: Time to first heart failure event (A) and time to first heart failure event or cardiovascular death (B) in the pooled STEP-HFpEF and STEP-HFpEF DM population.
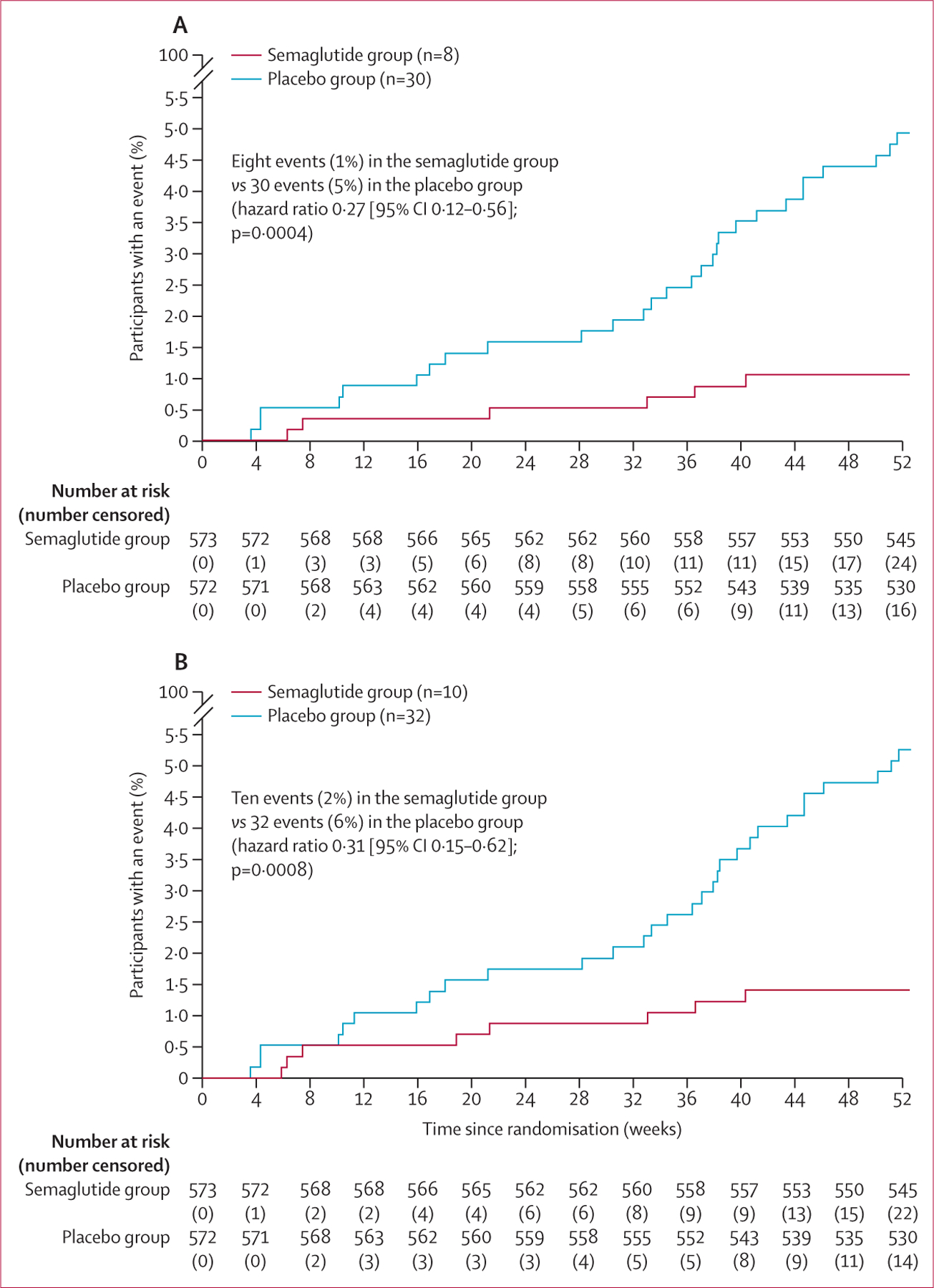
The adjusted cumulative incidence rate was calculated using the Aalen-Johansen method for first event, with all-cause death (A) and non-cardiovascular death (B) as a competing risks. Analyses were done in the full analysis set. Hazard ratios and 95% CIs were calculated using a Cox proportional hazards model, with treatment and BMI stratum (<35 kg/m² vs ≥35 kg/m²) as fixed factors and stratified by study.
A significantly higher proportion of participants in the semaglutide group than in the placebo group met predefined thresholds for KCCQ-CSS and 6MWD (table 2). The odds of achieving at least 5-point, 10-point, 15-point, and 20-point improvements in KCCQ-CSS from baseline to week 52 all favoured semaglutide compared with placebo (all p<0·0001; table 2), translating into an number needed to treat of 6 (95% CI 4–9) for each threshold in favour of semaglutide. For the anchor-based threshold of the patient global impression of severity (pre-defined as an improvement of 16·7 points in KCCQ-CSS from baseline to week 52), the corresponding odds ratio was 1·9 (1·5–2·5; p<0·0001), corresponding to a number needed to treat of 7 (5–11) in favour of semaglutide.
Similarly, the odds of deterioration in KCCQ-CSS from baseline to week 52 were lower in the semaglutide group than in the placebo group for at least a 5-point (0·5 [0·4–0·7]; p<0·0001) and 10-point (0·5 [0·3–0·7]; p=0·0002) worsening. The odds of achieving a 30-m improvement in 6MWD from baseline to week 52 were also higher with semaglutide than with placebo, and similar results were noted when assessed based on the anchor-based threshold of 21·9 m (table 2).
Safety data for the pooled population of STEP-HFpEF and STEP-HFpEF DM are in table 3. Overall, there were 161 serious adverse events reported in the semaglutide group (28·7 per 100 person-years) compared with 301 in the placebo group (52·7 per 100 person-years). There were fewer serious adverse cardiac disorders and infections during follow-up in the semaglutide group than in the placebo group (table 3). There were 60 gastrointestinal events leading to discontinuation of study treatment (10·7 per 100 person-years) in the semaglutide group versus 19 (3·3 per 100 person-years) in the placebo group. One serious hypoglycaemic event occurred in the semaglutide group in STEP-HFpEF in a participant who did not have type 2 diabetes. The affected participant recovered and the event was judged not to be related to semaglutide treatment.
Table 3:
Adverse events during the on-treatment period in the pooled STEP-HFpEF and STEP-HFpEF DM populations
| Semaglutide group (n=573) |
Placebo group (n=572) |
|||||
|---|---|---|---|---|---|---|
| n (%) | Events (n) | Events (per 100 person-years) | n (%) | Events (n) | Events (per 100 person-years) | |
| Serious adverse events | 90 (16%) | 161 | 28·7 | 159 (28%) | 301 | 52·7 |
| Serious adverse events leading to treatment discontinuation | 12 (2%) | 13 | 2·3 | 17 (3%) | 21 | 3·7 |
| Serious gastrointestinal disorders leading to treatment discontinuation | 2 (<1%) | 2 | 0·4 | 1 (<1%) | 1 | 0·2 |
| Adverse events leading to treatment discontinuation | 68 (12%) | 92 | 16·4 | 39 (7%) | 51 | 8·9 |
| Gastrointestinal disorders leading to treatment discontinuation | 45 (8%) | 60 | 10·7 | 16 (3%) | 19 | 3·3 |
| Fatal events | 7 (1%) | 7 | 1·25 | 12 (2%) | 17 | 3·0 |
| Most frequent serious adverse by system organ class* | ||||||
| Cardiac disorders | 26 (5%) | 31 | 5·5 | 70 (12%) | 101 | 17·7 |
| Atrial fibrillation | 8 (1%) | 8 | 1·4 | 15 (3%) | 20 | 3·5 |
| Cardiac failure | 3 (1%) | 3 | 0·5 | 34 (6%) | 43 | 7·5 |
| Cardiac failure congestive | 1 (<1%) | 1 | 0·2 | 6 (1%) | 6 | 1·1 |
| Infections and infestations | 16 (3%) | 22 | 3·9 | 44 (8%) | 60 | 10·5 |
| Gastrointestinal disorders | 12 (2%) | 14 | 2·5 | 12 (2%) | 13 | 2·3 |
| Nervous system disorders | 14 (2%) | 15 | 2·7 | 13 (2%) | 14 | 2·5 |
| Renal and urinary disorders | 8 (1%) | 9 | 1·6 | 12 (2%) | 14 | 2·5 |
| Vascular disorders | 7 (1%) | 7 | 1·3 | 7 (1%) | 7 | 1·2 |
| Respiratory, thoracic, and mediastinal | 6 (1%) | 6 | 1·1 | 16 (3%) | 18 | 3·2 |
| Musculoskeletal and connective tissue | 9 (2%) | 11 | 2·0 | 12 (2%) | 13 | 2·3 |
| Injury, poisoning, and procedural | 11 (2%) | 15 | 2·7 | 6 (1%) | 7 | 1·2 |
| Metabolism and nutrition disorders | 6 (1%) | 6 | 1·1 | 8 (1%) | 8 | 1·4 |
| Hepatobiliary disorders | 4 (1%) | 7 | 1·3 | 4 (1%) | 7 | 1·2 |
| General disorders and administration site | 2 (<1%) | 2 | 0·4 | 6 (1%) | 6 | 1·1 |
| Neoplasms benign, malignant, and unspecified | 9 (2%) | 9 | 1·6 | 10 (2%) | 10 | 1·8 |
| Safety focus areas† | ||||||
| COVID-19-related events‡ | 73 (13%) | 73 | 13·0 | 80 (14%) | 85 | 14·9 |
| Serious cardiovascular disorders | 45 (8%) | 55 | 9·8 | 92 (16%) | 135 | 23·6 |
| Serious gastrointestinal disorders | 12 (2%) | 14 | 2·5 | 12 (2%) | 13 | 2·3 |
| Medication errors‡ | 2 (<1%) | 2 | 0·4 | 6 (1%) | 7 | 1·2 |
| Serious neoplasms | 11 (2%) | 11 | 2·0 | 15 (3%) | 15 | 2·6 |
| Serious acute gallstone disease | 4 (1%) | 7 | 1·3 | 5 (1%) | 8 | 1·4 |
| Serious acute renal failure | 7 (1%) | 8 | 1·4 | 8 (1%) | 9 | 1·6 |
| Serious malignant neoplasms | 8 (1%) | 8 | 1·4 | 10 (2%) | 10 | 1·8 |
| Serious hepatic disorders | 0 | 0 | ·· | 1 (<1%) | 1 | 0·2 |
| Serious allergic reactions | 0 | 0 | ·· | 2 (<1%) | 2 | 0·4 |
| Acute pancreatitis‡ | 1 (<1%) | 1 | 0·2 | 1 (<1%) | 1 | 0·2 |
| Serious rare events | 1 (<1%) | 1 | 0·2 | 1 (<1%) | 1 | 0·2 |
| Serious hypoglycaemia | 1 (<1%) | 1 | 0·2 | 0 | 0 | ·· |
| COVID-19-related deaths | 1 (<1%) | 1 | 0·2 | 1 (<1%) | 1 | 0·2 |
| Adjudicated events§ | ||||||
| All-cause death | 8 (1%) | 8 | 1·3 | 14 (2%) | 14 | 2·3 |
| Cardiovascular death | 2 (<1%) | 2¶ | 0·3 | 5 (1%) | 5 | 0·8 |
| Heart failure events | 8 (1%) | 10 | 1·6 | 30 (5%) | 36 | 5·8 |
Data are adverse events during the on-treatment period in the safety analysis set, which included all randomly assigned participants who received at least one dose of semaglutide or placebo (all participants received at least one dose, and thus the safety analysis set was the same as the full analysis set). The on-treatment period spans from the date of first administration of semaglutide or placebo to the date of the last administration of semaglutide or placebo (excluding potential off-treatment time if two or more consecutive doses were missed). For the assessment of adverse events, each on-treatment period extends for 35 days from the date of most recent drug administration, unless otherwise stated. Investigators could report more than one event with a fatal outcome for the same participant. No treatment misuse was reported in either trial.
As per the Medical Dictionary for Regulatory Activity; adverse events occurring in ≥1% of participants in any treatment group are shown.
On the basis of therapeutic experience with GLP-1 receptor agonists and regulatory feedback and requirements, several safety focus areas (both serious and non-serious adverse events) were prespecified as being of special interest in the safety assessment; these preferred terms, identified through predefined searches of the Medical Dictionary for Regulatory Activity, were judged relevant for each of the safety focus areas.
Safety focus areas in STEP-HFpEF DM only.
Data are for the in-trial period (ie, the time from random assignment to last contact with a trial site, irrespective of treatment discontinuation or rescue intervention).
Includes one death of undetermined cause.
Discussion
In this prespecified, pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised placebo-controlled trials, a once-weekly subcutaneous dose of 2·4 mg semaglutide produced significant improvements in heart failure-related symptoms and physical limitations, and exercise function, and significant reductions in bodyweight in people with obesity-related heart failure with preserved ejection fraction with and without type 2 diabetes. The beneficial effects of semaglutide were consistent across various subgroups of study participants. Furthermore, semaglutide was well tolerated. Collectively, these data provide the most comprehensive evidence to date supporting semaglutide as an efficacious therapy for patients with obesity-related heart failure with preserved ejection fraction, who have few available treatment options.
Previous evidence suggested that semaglutide produced improvements in cardiometabolic risk factors and reductions in major adverse cardiovascular events compared with placebo in people in with diabetes or obesity, or both.25–27 Individually, the STEP-HFpEF and STEP-HFpEF DM trials showed improvements in heart failure-related health status and exercise function with semaglutide in people with obesity-related heart failure with preserved ejection fraction without and with type 2 diabetes.13 Our pooled analysis of the two trials is an important addition to this evidence base for several reasons. First, it provides a more definitive and precise assessment of the effects of semaglutide on the primary and confirmatory secondary endpoints, as a result of the increased sample size, the inclusion of a more representative patient population that better reflects the obesity phenotype of heart failure with preserved ejection fraction than either trial individually (given the inclusion of people with no diabetes, pre-diabetes, and overt type 2 diabetes), and the greater geographical diversity of the combined population.
Second, because of the larger sample size of the pooled analysis, we were better able to assess several endpoints (most importantly heart failure events) than was possible in either trial individually. Our pooled analysis showed narrower 95% CIs and lower rates of adjudicated heart failure events with semaglutide versus placebo. These results are important because they provide a strong rationale for future dedicated outcome trials of incretin-based therapies in patients with obesity-related heart failure with preserved ejection fraction, irrespective of diabetes status.
Third, the pooled analysis allowed for assessment of the dual primary outcomes stratified by clinically relevant subgroups, which was not possible in the individual STEP-HFpEF trials because of their modest size and limited power for subgroups analyses. Therefore, we prespecified that subgroup analyses were to be done only in the pooled dataset. The results across subgroups were largely consistent (including across age groups, BMI strata, and LVEF groups) with those of the overall analysis, although we observed some differences in the magnitude of beneficial treatment effects. Participants with more severe heart failure with preserved ejection fraction (as indicated by higher NT-proBNP concentration, and loop diuretic use) experienced larger improvements in KCCQ-CSS with semaglutide versus placebo than did those with less severe disease. The effects of semaglutide versus placebo on KCCQ-CSS were also more pronounced in participants with a history of atrial fibrillation, another marker of advanced heart failure with preserved ejection fraction, than in those without such a history. In addition, percentage reduction in bodyweight was greater in women than in men, a finding that has been reported in weight management trials with semaglutide and other incretin-based therapies.28 Although the exact reason for this sex difference is not known, a possible explanation is that women tend to have lower BMIs than men. Given that the semaglutide dose is the same in both sexes, women might thus in effect receive slightly higher doses proportionally than men, which could produce a greater response. Importantly, for each of these significant interactions, the between-subgroup differences were in magnitude only (ie, semaglutide consistently had favourable effects in all patient subgroups). Overall, the results of our subgroup analyses provide additional support for the use of semaglutide in various practice settings and across specific patient populations.
Fourth, the pooled analysis allowed for a robust safety assessment. Fewer serious adverse events, cardiac disorders, and infectious disease disorders were recorded in the semaglutide group than in the placebo group. Gastrointestinal events leading to treatment discontinuation were more common in the semaglutide group than in the placebo group, although the frequency of serious gastrointestinal adverse events, including pancreatitis, was similar in both groups. Only one serious event of hypoglycaemia in the entire cohort throughout follow-up was reported, and was judged not to be related to semaglutide treatment. These results are reassuring given previous findings of potential adverse safety signals with earlier-generation GLP-1 receptor agonists in people with heart failure and reduced ejection fraction in two small studies.29,30 Neither of these studies were powered for clinical events, were of sufficient duration to support definitive conclusions (each had a follow-up period of 180 days or less), or focused on patients with obesity. Nonetheless, it is important to note that the STEP-HFpEF findings cannot be extrapolated to people with heart failure and reduced ejection fraction. The lower frequency of infectious disease disorders in the semaglutide group compared with the placebo is noteworthy given similar findings in a trial of semaglutide verus placebo in patients with obesity and established atherosclerotic cardiovascular disease.26 Obesity, diabetes, and heart failure result in pro-inflammatory states,7,31–34 and each are risk factors for infectious disease complications, which are an important cause of morbidity and mortality in these patient groups. Thus, the reduced frequency of infectious disease complications in the semaglutide group compared with the placebo group in our pooled analysis further adds to the overall favourable risk–benefit balance for the use of semaglutide in people with heart failure with preserved ejection fraction, particularly given the high risk for non-cardiac morbidity and mortality in this patient population.35
Whether the effects of semaglutide in heart failure with preserved ejection fraction are related simply to weight reduction or also directly mediated by weight loss-independent effects on cardiovascular structure and function and haemodynamics is debated.36 Although this distinction might not be important to patients, several lines of data support that the effects of semaglutide extend beyond promotion of weight loss. Reductions in CRP concentrations associated with semaglutide are particularly noteworthy, because inflammation has been strongly and centrally implicated in the development and progression of heart failure with preserved ejection fraction,37 but favourable changes in CRP concentrations could be at least partly attributed to weight loss. However, obesity is associated with decreased production and increased clearance of natriuretic peptides, and weight loss resulted in an increase in natriuretic peptide concentrations among individuals with overweight and obesity in the LOOK-AHEAD trial.38
In the STEP-HFpEF and STEP-HFpEF DM trials, semaglutide significantly lowered NT-proBNP concentrations compared with placebo alongside substantial reductions in bodyweight, suggesting that the beneficial effects of semaglutide in heart failure extend beyond weight loss. Furthermore, the magnitude of improvements in KCCQ-CSS was similar across the two trials, despite roughly 40% less weight loss with semaglutide in people with diabetes (compared with those without diabetes), again suggesting potential effects beyond weight reduction. Further strengthening this argument is the finding that participants with a more severe phenotype of heart failure with preserved ejection fraction (ie, with high NT-proBNP concentrations, on loop diuretics, or with a history of atrial fibrillation) experienced greater improvements in KCCQ-CSS with semaglutide despite similar weight loss compared with those who had less severe disease. GLP-1 receptor agonists improve microvascular function39 and myocardial structure,40 increase insulin sensitivity,41 provide vascular protection,42,43 and reduce inflammation,41,44 oxidative stress,45 and neurohormonal activation46—all of which could have roles in the noted effects of semaglutide. The planned echocardiography substudy of the STEP-HFpEF trials could provide further insights into mechanisms of action.
Improvements in heart failure-related symptoms and physical function have been broadly recognised as key goals of care in people with heart failure with preserved ejection fraction by clinicians, professional societies, and regulators.47 Previous studies suggest that patients with heart failure value these outcomes at least as much as survival.48,49 Additionally, the US Food and Drug Administration has indicated that a treatment for heart failure is potentially approvable on the basis of effects on symptoms and physical function alone.50 To date, there has been a dearth of treatments with meaningful beneficial effects on these outcomes in patients with heart failure with preserved ejection fraction, which further highlights the clinical value of the STEP-HFpEF and STEP-HFpEF DM trials.
Our study has several strengths, including the prespecified pooling of individual, patient-level data from two moderately sized, multicentre, international trials that were purposefully harmonised to facilitate data amalgamation, a well characterised patient population for whom few effective treatments are available, and a broad spectrum of clinically meaningful outcomes. The study also has some limitations. The overall number of clinical events, although greater than those in the individual trials, was probably still not large enough to definitively show the efficacy of semaglutide in reducing heart failure events. There were too few non-White patients in the trial, and too few patients enrolled outside North America and Europe to do a detailed analysis by race beyond White versus non-White participants, or across multiple geographical regions, limiting the potential generalisability of results across all demographic groups. STEP-HFpEF and STEP-HFpEF DM were designed to have the appropriate statistical power for the analyses of the key endpoints in the overall patient population, rather than within specific subgroups. Our subgroup analyses should thus be interpreted within the context of this limitation. Finally, data for baseline kidney function were not collected in STEP-HFpEF, precluding an opportunity for pooled analysis of kidney function subgroups across both trials.
In conclusion, this prespecified pooled analysis of the STEP-HFpEF and STEP-HFpEF DM trials produces the most convincing evidence to date for the use of semaglutide to improve symptoms, physical limitations, and exercise function, in addition to producing weight loss, in people with obesity-related heart failure with preserved ejection fraction with and without type 2 diabetes, and across various clinically relevant subgroups. Semaglutide also had a favourable safety profile. Findings of a lower risk of heart failure-related hospitalisations and urgent visits with semaglutide compared with placebo (albeit with few events overall) provide a sound rationale for further assessment in dedicated outcome trials of incretin-based therapies in patients with heart failure with preserved ejection fraction.
Supplementary Material
Research in context.
Evidence before this study
Obesity is a major risk factor for the development and progression of heart failure with preserved ejection fraction. There are no approved therapies specifically targeting obesity-related heart failure with preserved ejection fraction. We searched PubMed with the terms (obesity-related HFpEF) AND (treatment) for papers published in any language from database inception to Feb 20, 2024. This search yielded two results: a review article summarising the diagnostic and therapeutic challenges associated with obesity-related heart failure with preserved ejection fraction, and a study comparing the clinical characteristics of heart failure with preserved ejection fraction in participants with and without obesity. Our search did not identify any studies or randomised controlled trials systematically assessing treatments for obesity-related heart failure with preserved ejection fraction. In the STEP-HFpEF and STEP-HFpEF DM trials, a weekly dose of semaglutide, a GLP-1 receptor agonist, significantly reduced heart failure-related symptoms and physical limitations, improved exercise function, and led to greater weight loss compared with placebo in patients with heart failure with preserved ejection fraction and a BMI of at least 30 kg/m² with and without type 2 diabetes.
Added value of this study
In this prespecified pooled assessment of individual patient-level data from STEP-HFpEF and STEP-HFpEF DM, we noted significant improvements in heart failure-related symptoms, physical limitations, and exercise function, and significant reductions in bodyweight in the semaglutide group compared with the placebo group. The beneficial effects of semaglutide were consistent across various participant subgroups. Additionally, semaglutide was well tolerated, with fewer serious adverse events in the semaglutide group than in the placebo group.
Implications of all the available evidence
Collectively, data from this pooled analysis and from the individual STEP-HFpEF and STEP-HFpEF DM trials support the use of semaglutide to improve heart failure-related symptoms, physical limitations, and exercise function across a broad population of patients with obesity-related heart failure with preserved ejection fraction, irrespective of patient demographic and clinical characteristics.
Acknowledgments
STEP-HFpEF and STEP-HFpEF DM were funded by Novo Nordisk. SJS was supported by grants from the US National Heart, Lung, and Blood Institute (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423). MCP was supported by a British Heart Foundation Centre of Research Excellence Grant (RE/18/6/34217). BAB receives research support from the US National Institutes of Health and Department of Defense. DWK was supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and US National Institutes of Health grants (U01AG076928, R01AG078153, P30AG021332, U24AG059624, and U01HL160272). VM has received research support from the National Institute for Metabolic and Cardiovascular Disease Research (Programme EXCELES; LX22NPO5104), funded by the EU–Next Generation EU. DW is a member of SFB1425, which is funded by the Deutsche Forschungsgemeinschaft, the German Research Foundation. We thank the participants, investigators, and site staff of STEP-HFpEF and STEP-HFpEF DM, and Isabella Goldsbrough Alves (Apollo, OPEN Health Communications, London, UK) for administrative support and development of the figures and tables, which was funded by Novo Nordisk.
Footnotes
Declaration of interests
JB is a paid consultant to Abbott, American Regent, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimension, Cardior, CVRx, Cytokinetics, Daxor Edwards, Element Science, Innolife, Impulse Dynamics, Imbria, Inventiva, Lexicon, Lilly, LivaNova, Janssen, Medtronics, Merck, Occlutech, Owkin, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Prolaio, Roche, Secretome, Sequana, SQ Innovation, Tenex, and Vifor. SJS has received research grants from AstraZeneca, Corvia, and Pfizer, and consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, ReCor, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, and Ultromics. MCP has received research funding from AstraZeneca, Boehringer Ingelheim, Boston Scientific, Medtronic, Novo Nordisk, Novartis, Pharmacosmos, Roche, and SQ Innovations, and has served on committees or consulted for AbbVie, Akero, AnaCardio, Applied Therapeutics, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Cardiorentis, Corvia, Eli Lilly, Horizon Therapeutics, LIB Therapeutics, Moderna, New Amsterdam, Novartis, Novo Nordisk, Pharmacosmos, Siemens, SQ Innovations, Takeda, Teikoku, and Vifor. BAB has received research funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, Rivus, and Tenax Therapeutics, has served as a paid consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, NGM, Novo Nordisk, NXT, and VADovations, and is named inventor (US patent number 10 307 179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. SZA, GKH, DVM, MNE, MLL, and SR are employees of, and shareholders in, Novo Nordisk. MJD has acted as paid consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi, a paid advisory board member and speaker for AstraZeneca, a paid advisory board member for Medtronic, Pfizer, and ShouTi Pharma, and a paid speaker for Amgen, Novartis, and Sanofi, and has received grants as an investigator in support of investigator-initiated trials from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi-Aventis. DWK reports receiving honoraria as a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Corvia Medical, Ketyo, Novartis, Novo Nordisk, Pfizer, and Rivus, has received grant funding from AstraZeneca, Bayer, Novartis, Novo Nordisk, Pfizer, and Rivus, and owns stock in Gilead. SV reports speaking honoraria or consulting fees from Abbott, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge Translation Research Group, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, and TIMI. WA reports honoraria or consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk. FZA reports honoraria or consulting fees from Abbott, AstraZeneca, Medtronic, Novo Nordisk, Occlutech, Pharmacosmos, and Vifor. VC reports speaker fees from AstraZeneca, Boehringer Ingelheim, Cipla, Dr Reddy’s, Lupin, Novartis, Novo Nordisk, Mankind, Pfizer, Sanofi, Sun Pharma, and Torrent. JAE reports research support for trial leadership from American Regent, Applied Therapeutics, Bayer, Cytokinetics, Merck, and Novo Nordisk, reports honoraria for consultancy from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, and Otsuka, and serves as an advisor to US2.ai. HI reports honoraria or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Mochida, Novartis, and Novo Nordisk. ML reports honoraria or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Ewopharma, Gedeon Richter, Novartis, Novo Nordisk, Roche, and Servier. VM reports consulting fees from Bayer, Merck Sharp & Dohme, and Novo Nordisk and research grants from Regeneron. BM reports speaker fees or research payments from Abbott, AstraZeneca, Biotronik, Boehringer Ingelheim, CSL Behring, Daiichi-Sankyo, DUKE Clinical Institute, Medtronic, and Novartis, and institutional grants from Abbott, AstraZeneca, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo, DUKE Clinical Institute, Eli Lilly, Medtronic, Novartis, Terumo, and Vifor. JN reports honoraria or consulting fees from Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Pfizer, Novartis, Novo Nordisk, Rovi, and Vifor. EP reports honoraria from Novo Nordisk. MSc reports speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, and Novo Nordisk. MSe reports honoraria or consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Vifor. KS received honoraria for serving as an advisory board member and consultant for Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Janssen, Novartis, Novo Nordisk, and Rivus. PvdM reports institutional payments for consultancy fees or grants from AstraZeneca, Boehringer Ingelheim, BridgeBio, Ionis, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharma Nord, and Vifor. DVL reports honoraria or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Novo Nordisk, Recardio, Sanofi, Sanova, and Vaxxinity. DW reports consultancy fees from Novo Nordisk. MNK served as a paid consultant or advisory board member for 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria Pharmaceuticals, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor, and Youngene Therapeutics, has received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, holds stocks in Artera Health and Saghmos Therapeutics, has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, and has received other research support from AstraZeneca. TB-G and MF report no competing interests.
Contributor Information
Javed Butler, Baylor Scott & White Research Institute, Dallas, TX, USA; Department of Medicine, University of Mississippi, Jackson, MS, USA.
Sanjiv J Shah, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Mark C Petrie, School of Cardiovascular and Metabolic Health, University of Glasgow, Glasgow, UK.
Barry A Borlaug, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Steen Z Abildstrøm, Novo Nordisk, Søborg, Denmark.
Melanie J Davies, Diabetes Research Centre, University of Leicester, Leicester, UK; National Institute for Health and Care Research Leicester Biomedical Research Centre, Leicester, UK.
G Kees Hovingh, Novo Nordisk, Søborg, Denmark.
Dalane W Kitzman, Department of Cardiovascular Medicine and Section on Geriatrics and Gerontology, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Daniél Vega Møller, Novo Nordisk, Søborg, Denmark.
Subodh Verma, Division of Cardiac Surgery, Li Ka Shing Knowledge Institute of St Michael’s Hospital, Unity Health Toronto, University of Toronto, Toronto, ON, Canada.
Mette Nygaard Einfeldt, Novo Nordisk, Søborg, Denmark.
Marie L Lindegaard, Novo Nordisk, Søborg, Denmark.
Søren Rasmussen, Novo Nordisk, Søborg, Denmark.
Walter Abhayaratna, College of Health and Medicine, Australian National University, Canberra, ACT, Australia.
Fozia Z Ahmed, Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Tuvia Ben-Gal, Heart Failure Unit, Department of Cardiology, Rabin Medical Center, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Vijay Chopra, Max Super Speciality Hospital, Saket, New Delhi, India.
Justin A Ezekowitz, Canadian VIGOUR Centre, University of Alberta, Edmonton, AB, Canada.
Michael Fu, Section of Cardiology, Department of Medicine, Sahlgrenska University Hospital-Ostra, Gothenburg, Sweden.
Hiroshi Ito, Department of General Internal Medicine 3, Kawasaki Medical School, Okayama, Japan.
Małgorzata Lelonek, Department of Noninvasive Cardiology, Medical University of Lodz, Lodz, Poland.
Vojtěch Melenovský, Institute for Clinical and Experimental Medicine—IKEM, Prague, Czech Republic.
Bela Merkely, Heart and Vascular Centre, Semmelweis University, Budapest, Hungary.
Julio Núñez, Hospital Clínico Universitario de Valencia, INCLIVA, Universidad de Valencia, Valencia, Spain; Centro de Investigación Biomédica en Red Cardiovascular, Valencia, Spain.
Eduardo Perna, Instituto de Cardiologia J F Cabral, Corrientes, Argentina.
Morten Schou, Department of Cardiology, Herlev-Gentofte Hospital, Hellerup, Denmark; Department of Clinical Medicine, University of Copenhagen, Herlev, Denmark.
Michele Senni, Azienda Socio Sanitaria Territorial Papa Giovanni XXIII, Bergamo, Italy.
Kavita Sharma, Heart Failure & Cardiac Transplantation, Johns Hopkins University Heart Failure with Preserved Ejection Fraction Program, Johns Hopkins Hospital, Baltimore, MD, USA.
Peter van der Meer, Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Dirk Von Lewinski, Medical University of Graz, Graz, Austria.
Dennis Wolf, Cardiology and Angiology, Medical Center—University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany.
Mikhail N Kosiborod, Department of Cardiovascular Disease, Saint Luke’s Mid America Heart Institute, Kansas City, MO, USA; University of Missouri–Kansas City School of Medicine, Kansas City, MO, USA.
Data sharing
Data will be shared with researchers who submit a valid research proposal approved by the independent review board. Access-request proposals can be found on the Novo Norodisk trials website. Data will be shared with researchers who submit valid requests after research completion and approval of the product and product use in the EU and the USA. Individual participant data will be shared in data sets in a de-identified, anonymised format.
References
- 1.Shah SJ, Borlaug BA, Kitzman DW, et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group summary. Circulation 2020; 141: 1001–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 2017; 69: 1129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SJ. BNP: biomarker not perfect in heart failure with preserved ejection fraction. Eur Heart J 2022; 43: 1952–54. [DOI] [PubMed] [Google Scholar]
- 4.Lindman BR, Davila-Roman VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 2014; 64: 541–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McHugh K, DeVore AD, Wu J, et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 602–11. [DOI] [PubMed] [Google Scholar]
- 6.De Marco C, Claggett BL, de Denus S, et al. Impact of diabetes on serum biomarkers in heart failure with preserved ejection fraction: insights from the TOPCAT trial. ESC Heart Fail 2021; 8: 1130–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–71. [DOI] [PubMed] [Google Scholar]
- 8.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018; 39: 3439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer M Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018; 71: 2360–72. [DOI] [PubMed] [Google Scholar]
- 11.Miller WL, Borlaug BA. Impact of obesity on volume status in patients with ambulatory chronic heart failure. J Card Fail 2020; 26: 112–17. [DOI] [PubMed] [Google Scholar]
- 12.Sorimachi H, Burkhoff D, Verbrugge FH, et al. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail 2021; 23: 1648–58. [DOI] [PubMed] [Google Scholar]
- 13.Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069–84. [DOI] [PubMed] [Google Scholar]
- 14.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989–1002. [DOI] [PubMed] [Google Scholar]
- 15.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021; 397: 971–84. [DOI] [PubMed] [Google Scholar]
- 16.Jastreboff AM, Aronne LJ, Ahmad NM, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205–16. [DOI] [PubMed] [Google Scholar]
- 17.Garvey WG, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023; 402: 613–26. [DOI] [PubMed] [Google Scholar]
- 18.Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Design and baseline characteristics of STEP-HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail 2023; 11: 1000–10. [DOI] [PubMed] [Google Scholar]
- 19.Kosiborod MN, Petrie MC, Borlaug BA, et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med 2024; published online April 6. DOI: 10.1056/NEJMoa2313917. [DOI] [PubMed] [Google Scholar]
- 20.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–55. [DOI] [PubMed] [Google Scholar]
- 21.Joseph SM, Novak E, Arnold SV, et al. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail 2013; 6: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol 2020; 76: 2379–90. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski AF, Bate A, Bousquet C, et al. Good signal detection practices: evidence from IMI PROTECT. Drug Saf 2016; 39: 469–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosiborod MN, Bhatta M, Davies M, et al. Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes Obes Metab 2023; 25: 468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221–32. [DOI] [PubMed] [Google Scholar]
- 27.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–44. [DOI] [PubMed] [Google Scholar]
- 28.Rentzeperi E, Pegiou S, Koufakis T, Grammatiki M, Kotsa K. Sex differences in response to treatment with glucagon-like peptide 1 receptor agonists: opportunities for a tailored approach to diabetes and obesity care. J Pers Med 2022; 12: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016; 316: 500–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail 2017; 19: 69–77. [DOI] [PubMed] [Google Scholar]
- 31.Altara R, Giordano M, Nordén ES, et al. Targeting obesity and diabetes to treat heart failure with preserved ejection fraction. Front Endocrinol 2017; 8: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaar D, Dumont BL, Vulesevic B, et al. Neutrophils and circulating inflammatory biomarkers in diabetes mellitus and heart failure with preserved ejection fraction. Am J Cardiol 2022; 178: 80–88. [DOI] [PubMed] [Google Scholar]
- 33.Kitzman DW, Nicklas BJ. Pivotal role of excess intra-abdominal adipose in the pathogenesis of metabolic/obese HFpEF. JACC Heart Fail 2018; 6: 1008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvatore T, Galiero R, Caturano A, et al. Dysregulated epicardial adipose tissue as a risk factor and potential therapeutic target of heart failure with preserved ejection fraction in diabetes. Biomolecules 2022; 12: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol 2023; 81: 1810–34. [DOI] [PubMed] [Google Scholar]
- 36.Kosiborod MN, Borlaug BA, Petrie MC. Semaglutide and heart failure with preserved ejection fraction and obesity. Reply. N Engl J Med 2023; 389: 2398–99. [DOI] [PubMed] [Google Scholar]
- 37.Verma R, Dhingra NK, Connelly KA. Obesity/cardiometabolic phenotype of heart failure with preserved ejection fraction: mechanisms to recent trials. Curr Opin Cardiol 2024; 39: 92–97. [DOI] [PubMed] [Google Scholar]
- 38.Bertoni AG, Wagenknecht LE, Kitzman DW, et al. Impact of the look AHEAD intervention on NT-pro brain natriuretic peptide in overweight and obese adults with diabetes. Obesity 2012; 20: 1511–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldney J, Sargeant JA, Davies MJ. Incretins and microvascular complications of diabetes: neuropathy, nephropathy, retinopathy and microangiopathy. Diabetologia 2023; 66: 1832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Withaar C, Meems LMG, Nollet EE, et al. The cardioprotective effects of semaglutide exceed those of dietary weight loss in mice with HFpEF. JACC Basic Transl Sci 2023; 8: 1298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 2022; 28: 2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi L, Ji Y, Jiang X, et al. Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc Diabetol 2015; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma X, Liu Z, Ilyas I, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci 2021; 17: 2050–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications 2017; 31: 1237–42. [DOI] [PubMed] [Google Scholar]
- 45.Oh YS, Jun HS. Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci 2017; 19: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puglisi S, Rossini A, Poli R, et al. Effects of SGLT2 inhibitors and GLP-1 receptor agonists on renin-angiotensin-aldosterone system. Front Endocrinol 2021; 12: 738848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forman DE, Arena R, Boxer R, et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017; 135: e894–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed SD, Fairchild AO, Johnson FR, et al. Patients’ willingness to accept mitral valve procedure-associated risks varies across severity of heart failure symptoms. Circ Cardiovasc Interv 2019; 12: e008051. [DOI] [PubMed] [Google Scholar]
- 49.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001; 20: 1016–24. [DOI] [PubMed] [Google Scholar]
- 50.US Food and Drug Administration. Treatment for heart failure: endpoints for drug development guidance for industry June 2019. Silver Spring, MD: Center for Drug Evaluation and Research, US Food and Drug Administration, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared with researchers who submit a valid research proposal approved by the independent review board. Access-request proposals can be found on the Novo Norodisk trials website. Data will be shared with researchers who submit valid requests after research completion and approval of the product and product use in the EU and the USA. Individual participant data will be shared in data sets in a de-identified, anonymised format.


