Abstract
The surge in electronic cigarette (e-cig) use in recent years has raised questions on chemical exposures that may result from vaping. Previous studies have focused on measuring known toxicants, particularly those present in traditional cigarettes, while fewer have investigated unknown compounds and transformation products formed during the vaping process in these diverse and constantly evolving products. The primary aim of this work was to apply liquid chromatography–high-resolution mass spectrometry (LC–HRMS) and chemical fingerprinting techniques for the characterization of e-liquids and aerosols from a selection of popular e-cig products. We conducted non-target and quantitative analyses of tobacco-flavored e-liquids and aerosols generated using four popular e-cig products: one disposable, two pod, and one tank/mod. Aerosols were collected using a condensation device and analyzed in solution alongside e-liquids by LC–HRMS. The number of compounds detected increased from e-liquids to aerosols in three of four commercial products, as did the proportion of condensed hydrocarbon-like compounds, associated with combustion. Kendrick mass defect analysis suggested that some of the additional compounds detected in aerosols belong to homologous series resulting from decomposition of high-molecular weight compounds during vaping. Lipids (e.g., Vitamin E acetate) in inhalable aerosols have been associated with severe respiratory effects, and lipid-like compounds were observed in aerosols as well as e-liquids analyzed. Six potentially hazardous additives and contaminants, such as the industrial chemical tributylphosphine oxide and the stimulant caffeine, were identified and quantified in the e-cig liquids and aerosols analyzed. These findings demonstrate the potential of non-target HRMS-based analysis to identify compounds and compound classes that were previously unidentified in e-cig aerosols as a novel tool to assess chemical exposures resulting from vaping.
Keywords: electronic cigarettes, non-target analysis, chemical fingerprinting, quantitative analysis
Graphical Abstract
1. Introduction
While the past 50 years have seen a decline in combustible cigarette smoking in the United States, nearly 30% of US high school students reported use of electronic cigarettes (e-cig) in 20191. Increasing e-cig use, or vaping, has raised concerns about toxicant exposures, especially as popularity grows among youth and never-smokers.2,3 E-cig devices work by heating a liquid mixture (e-liquid) with a metallic coil to generate a fine aerosol that is inhaled by the user. E-liquid typically contains propylene glycol (PG), vegetable glycerin (VG), nicotine, flavorings, and other chemicals, although information about chemical additives is not typically disclosed by manufacturers.4,5 Thus, the aerosols inhaled during e-cig use are complex mixtures of solvents and additives as well as compounds formed during the vaping process.6 Five generations of e-cig devices and a wide variety of e-liquid formulations are commercially available, and new e-cig devices and liquids are rapidly proliferating1, including over eight thousand e-liquid flavors.7,8 While the FDA enacted some sales bans in the US in 2020, the policies only restrict a subset of flavors and products.9–11 As a result of the heterogeneity, complexity, and lack of transparency associated with e-cig products, exposures from e-cig use are poorly understood and their health effects difficult to predict, as exemplified by the “e-cigarette, or vaping, product use associated lung injury” (EVALI) outbreak of 2019.12 Thus, there is a need for the development of new analytical approaches that can identify a wide spectrum of organic chemicals that are present in e-cig liquids and aerosols.
Numerous known toxicants have been identified and measured in e-cig e-liquids and aerosols to date, including metals, carbonyls, free radicals, and phthalates.2,13,14 Existing studies investigating e-cig exposures have almost exclusively focused on quantifying specific, known constituents in e-liquids, especially those present at high levels in combustible cigarettes such as tobacco alkaloids, polycyclic aromatic hydrocarbons, and formaldehyde.2,4,15 A growing number of studies have investigated chemicals unique to e-cig aerosols as well as e-liquids, including compounds that are formed by thermal decomposition of e-liquid solvents (PG and VG),6,15,16 contaminants from e-liquid packaging and e-cig device components,5,14 and flavorants.8,17–19
In addition to known toxicants, the identification of unknown compounds in e-cig aerosols, including undisclosed additives, transformation products formed during the vaping process, and contaminants, is critical for the assessment of chemical exposures from vaping. A comprehensive understanding of chemical exposures is needed for appropriate regulatory action and to reduce involuntary risks to e-cig users. Previous non-target studies using gas chromatography (GC) coupled to quadrupole or time-of-flight mass spectrometry have focused on the identification of semi-volatile and volatile compounds in e-cig samples.20,21 Liquid chromatography (LC) coupled to high-resolution mass spectrometry (HRMS) with electrospray ionization (ESI) provides complementary information because of its ability to detect semi-volatile and non-volatile compounds, such as industrial chemicals, new-generation pesticides, food additives, and pharmaceuticals.22,23 In addition, non-target analysis based on LC–HRMS has excellent mass accuracy, sensitivity, and specificity due to high resolving power, which lend the technique to advanced chemical fingerprinting applications.24,25 This combined approach has previously been used for the identification of organic compounds in urine, blood, (waste)water, food, and plant material, but has not been applied for the analysis of e-cig samples.26–28
The objectives of this work were to (1) apply non-target LC–HRMS methods to the chemical characterization of e-liquids and aerosols from a selection of popular tobacco-flavored e-cig products, and (2) identify, confirm and quantify the presence of any unknown compounds of potential health concern in the analyzed e-cig samples. The use of a novel aerosol condensing device enabled the analysis of both aerosols and e-liquids in liquid phase, facilitating the investigation of chemical transformations due to vaping. Recognizing the rapidly changing market and regulatory environment, we included a representative selection of popular commercial e-cig products with diverse device and e-liquid characteristics.
2. Experimental Procedures
2.1. Reagents and Standards
High-purity propylene glycol (Amresco VWR, Solon, OH, USA) and ultra-pure glycerol (MP Biomedicals, Santa Ana, USA) were combined in-house to produce a 40/60 (w/w) PG/VG e-liquid base solution. HPLC-grade methanol (MeOH, Thermo Fisher Scientific, Waltham, MA, USA) and 18.2 MΩ-cm ultrapure water from a Millipore-Sigma system (Burlington, MA, USA) were used throughout this work. Acetic acid (Alfa Aesar, Haverhill, MA, USA) was used in the LC mobile phase.
A confirmation mix stock solution for quantitation of compounds of potential health concern in e-cig samples was prepared in MeOH from eleven analytical standards: caffeine, tributylphosphine oxide (Sigma Aldrich, St. Louis, MO); catechol, isophorone, vanillin, 2,4-diaminotoluene (Acros Organics, Fair Lawn, NJ); harmaline, skatole, triethyl citrate (Alfa Aesar); difenzoquat methyl sulfate, tributyl O-acetylcitrate (TCI America, Portland, OR). Working standards with concentrations of 1 and 100 μg/mL in ultrapure water were prepared from the stock solution and stored at −20° C. A quality control (QC) mix of 15 analytes was analyzed alongside e-cig samples in each analytical run. All standards were purchased at the highest available purity.
2.2. E-Cig Products
E-cig devices from four brands were included in this study: one 3rd-generation modifiable-power (“mod”) device (Smok ProColor 225W with TFV8 Big Baby Beast Tank, Shenzhen Ivps Co. Ltd, Shenzhen, PRC), two 4th-generation cartridge (“pod”) devices (Juul, Juul Labs, San Francisco, CA, USA and Vuse Alto, British American Tobacco, London, UK), and one 4th generation disposable device (Blu Disposable, Imperial Brands, Bristol, UK). E-liquids covered a range of nicotine concentrations (Table 1) but a single popular flavor (tobacco). According to the manufacturers, the Blu product contained freebase nicotine, while the Mi-Salt, Vuse, and Juul products contained nicotine salts. Mi-Salt e-liquid (Sv3, LLC, Phoenix, AZ, USA) was the refill liquid used with the Smok Mod device. All products were purchased from US internet vendors. The in-house e-liquid PG/VG base was vaped using a Juul device and a refillable cartridge (Blankz, Online retailer). Because of the electronic power regulation system of Juul batteries, pods with different coil resistances (1.6 ohm for Juul and 1.4 ohm for Blankz) reach approximately the same maximum power (8.1 W) when used with a Juul device.29 Relevant characteristics of e-cig devices and e-liquid products analyzed are summarized in Table 1.
Table 1:
Relevant characteristics of e-cig devices and e-liquids analyzed.
| E-liquid flavor brand, name | Device brand (type) | Maximum power (W) | Coil resistance (ohm) | Nicotine content (%)*, form | PG (%) / VG (%) |
|---|---|---|---|---|---|
| Blu, Classic tobacco | Blu (cigalike) | 5.430 | 3.031 | 2.4, Freebase32 | 0/10030,33,34 |
| Juul, Virginia tobacco | Juul (pod) | 8.129 | 1.629 | 3, Salt* | 30/7029 |
| Mi-Salt, Tobacco | Smok (mod) | 40* | 0.15* | 4, Salt* | 50/50* |
| Vuse, Golden tobacco | Vuse Alto (pod) | 6.535 | 1.135 | 5, Salt* | 45/5536 |
| PG/VG Base | Juul (pod), Blankz refillable pod | 8.1† | 1.437 | 0†, N/A† | 40/60† |
Information from product label or personal communication with manufacturer.
In-house solution vaped using Juul device and refillable pod.
2.3. Aerosol Generation and Collection
Unused pods and tanks were either prefilled or filled in-house with fresh e-liquid to manufacturer recommended volumes. The Blu disposable device, purchased within one month of analysis, was unused and pre-filled with e-liquid by the manufacturer. The mouthpiece of each device was inserted into a tube (16-cm long; 4.8 mm internal diameter, Masterflex L/S 15, Vernon Hills, USA) which was looped through a peristaltic pump (drive no. 07522-20 and head no. 77200-62, Cole-Parmer, Vernon Hills, IL, USA), operated at a rate of 1.1 L/min (Figure 1). The pod and disposable devices were activated by the pump, while the mod device was manually activated. Aerosol was pulled through the pump tubing and funneled into a series of tubes and pipette tips to generate an aerosol condensate, described previously.38,39 Aerosol collection efficiencies using this apparatus have been previously observed to be 72–83% and 78–83% for cigalike and mod devices, respectively.40 A different set of tubes and pipette tips was used for each product. While typical user puff topography may vary by device type,42 to ensure comparability with previous studies, a single standardized puff topography was used for all devices in this study.41 The standardized puff topography used was based on the International Organization for Standardization 20768:2018 method 1541 with 3-s puff duration and an inter-puff interval of 10 s rather than 30 s. A shorter inter-puff interval was found to be necessary to produce sufficient condensed aerosol volume for analysis of Blu, Juul, and the PG/VG base, corresponding to the devices with the lowest power (Table 1). Each product was vaped in three consecutive intervals of 100 puffs each to examine changes in aerosol composition with increased vaping, and masses of aerosols generated after each vaping interval were recorded. Devices were re-charged fully prior to each 100-puff interval and device activation was noted for each puff. A single pool of e-liquid was used for each product, and e-liquid was not replaced or replenished between consecutive intervals. Slightly higher aerosol mass was generated in the final 100-puff interval for Juul (Table 2), despite the stated maximum 200-puff limit of that product, consistent with our repeated observation that the maximum puff number for a given device is dependent on puff topography. Condensed aerosol from each 100-puff interval was collected into an amber glass LC vial (Agilent Technologies, Santa Clara, CA, USA) at the end of the condensation device and stored at 4°C until analysis.
Figure 1:
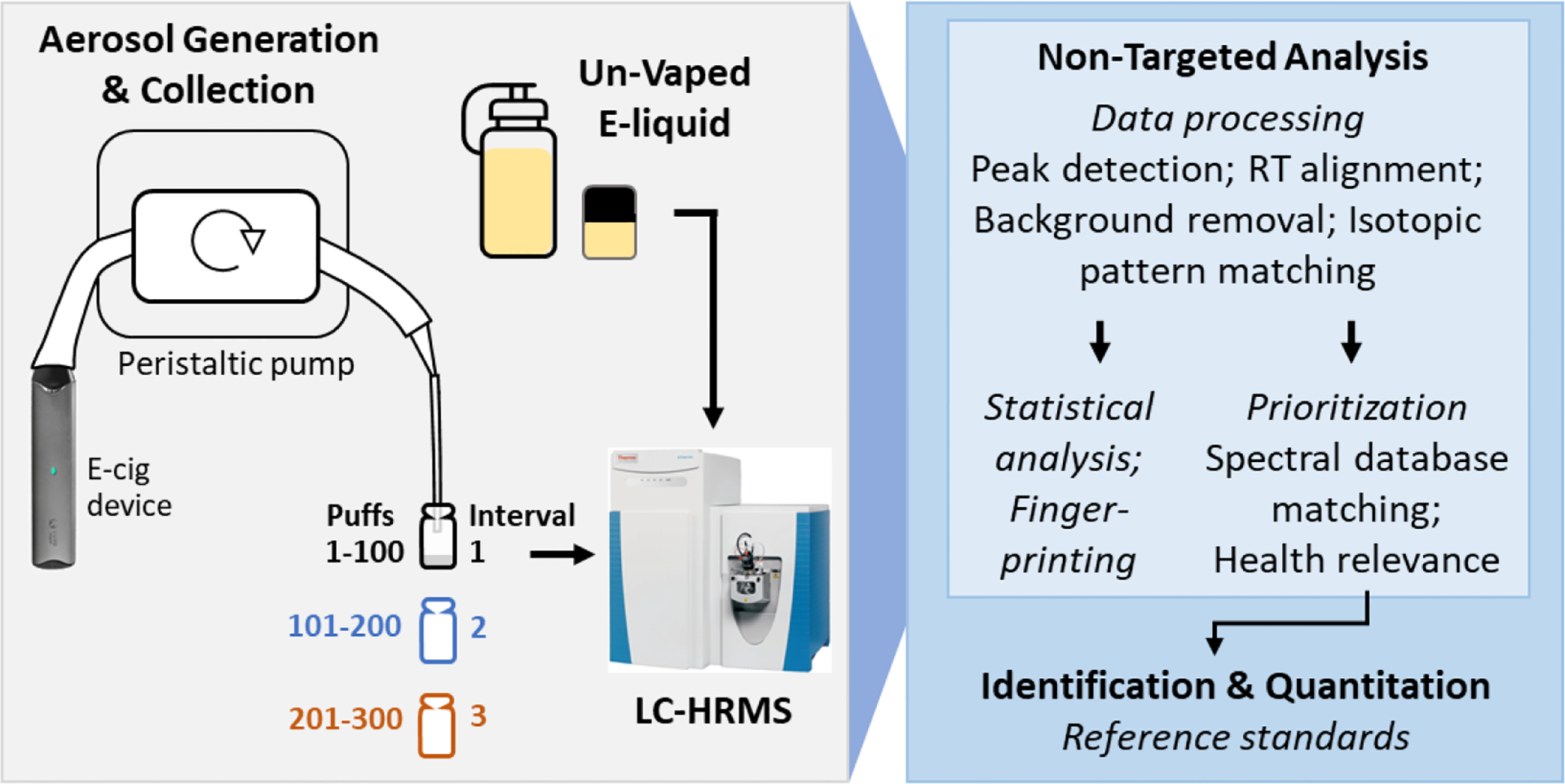
Summary of experimental and data analysis workflows for non-target and quantitative analysis of organic compounds in e-cig liquids and aerosols.
Table 2:
Mass of aerosols generated by vaping in three consecutive vaping intervals (100 puffs each) and corresponding numbers of compounds detected in LC-HRMS analysis of e-liquids and aerosols of analyzed e-cig products.
| Blu | Juul | Mi-Salt (Smok) | Vuse | PG/VG Base | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample type | Aerosol mass (mg) | Compound count | Aerosol mass (mg) | Compound count | Aerosol mass (mg) | Compound count | Aerosol mass (mg) | Compound count | Aerosol mass (mg) | Compound count |
| E-Liq. | N/A | 2,129 | N/A | 930 | N/A | 769 | N/A | 880 | N/A | 1,135 |
| Interval 1 | 92 | 1,694 | 42 | 1,003 | 218 | 920 | 336 | 956 | 234 | 1,895 |
| Interval 2 | 67 | 1,537 | 52 | 963 | 379 | 828 | 420 | 908 | 25 | 2,075 |
| Interval 3 | 26 | 1,455 | 72 | 941 | 306 | 906 | 448 | 940 | 28 | 2,140 |
2.4. Liquid Chromatography–High-Resolution Mass Spectrometry Analysis
Chromatographic separation for all analyses was achieved using a RSLC3000 nanoHPLC system (Thermo Fisher Scientific) on a Synergi Hydro-RP column (4μm, 150 × 3mm, Phenomenex, Torrance, CA, USA) and KrudKatcher guard column (Phenomenex, 2.0μm × 0.004 in ID) with an injection volume of 10 μL and flow rate of 75 μL/min. Mobile phase A was 0.1% acetic acid in ultrapure water and mobile phase B was MeOH. The LC gradient began with 100% A for 4 min, linearly increased to 98% B from minute 4 – 15, held at 98% B until minute 22, and returned to 100% A at minute 22.1; the column was then re-equilibrated for 4 minutes.
Organic compounds were detected using a Q-Exactive HF Orbitrap (Thermo Fisher Scientific) with a heated electrospray ionization (HESI) source. Mass calibrations in each ionization mode were performed weekly per manufacturer recommendations. For non-target analysis, data were acquired with a full scan/data-dependent MS2 method across a mass range m/z 80 – 1200 in polarity switching mode with resolutions 120,000 (full scan) and 60,000 (MS2) at m/z 200. After acquiring a MS scan (full scan), compounds surpassing the intensity threshold (1.6×105) were subsequently isolated and fragmented (data-dependent MS2) with normalized collision energies of 20, 30, and 40. The same instrumental methods were used for the two non-target and two quantitation runs. An additional compound confirmation analysis used a different method based on parallel reaction monitoring (PRM) using an extended range of collision energies, a narrower scan range and an inclusion list for the 11 compounds of interest (Table S1, Supporting Information).
For non-target analysis, a set of three consecutively-vaped aerosol samples and a fresh e-liquid sample from each e-cig product and the PG/VG base were analyzed by LC–HRMS in two runs one day apart. Opposite sample order was employed in the two runs to account for potential carryover and drift. For the quantitation runs, which used the non-target analytical method, sample groups (by brand) were separated by ultrapure water blanks to prevent carryover. Background subtraction for each sample group in the quantitation runs was achieved by subtracting the preceding blank. The first ultrapure water blank was used for background subtraction in the non-target runs. Accurately weighed aliquots of aerosols and e-liquids (25–30 mg) were each diluted with 1 mL of 5% MeOH in ultrapure water diluent.
Compounds of interest in e-cig samples were confirmed based on RT (<0.5 min window), accurate mass (<5 ppm), and MS/MS (min. 2 major fragments) information. Concentrations of the compounds in e-liquids and aerosols were determined using the standard addition method (example calibration curves are shown in Figure S1, Supporting Information). For Mi-Salt (Smok), Vuse, and the PG/VG base, the interval 1 aerosol sample was used, while Blu and Juul aerosol samples used for quantification represented multiple vaping intervals due to the relatively small volume of aerosol generated by those products. Standard addition calibrators contained 50-fold dilutions of e-liquid or aerosol in 5% MeOH diluent. Calibrators contained 0, 10, 100, 1,000 and 10,000 ng of the confirmation mix compounds in a total volume of 1 mL for e-liquids, and 0, 4, 40, 400 and 4,000 into 400 μL for aerosols. The smaller total volume of calibrator solutions for aerosols was due to the limited aerosol sample volumes available. Concentrations, as mass fractions (μg/g), were calculated using the mass of the analyzed sample aliquot.
A QC mix of 15 analytes was analyzed at the beginning and end of each run to monitor analytical performance of the instrument (Table S2, Supporting Information), as well as spiked into PG/VG and Juul to investigate matrix effects (Table S3, Supporting Information). QC mix analytes were selected to encompass a range of polarities (log Kow −3.0 – 6.8), retention times (RT) (2 – 23 min), molecular weights (122 – 821 Da), and ionizability in either positive or negative ESI mode. Variability among RTs of the QC mix inter-run replicates (n=8) was ≤5% (RSD) for all compounds; mass error was <2.8 ppm, and abundances exceeded 4.5×106 for all of the compounds (Table S2, Supporting Information).
2.5. Compound Identification and Data Analysis
Xcalibur Quan Browser software (v4.1, Thermo Fisher Scientific) was used for data processing for compound quantification and compound confirmation was achieved using FreeStyle software (v1.3, Thermo Fisher Scientific). Non-target data analysis was conducted using Compound Discoverer software (v3.1, Thermo Fisher Scientific). Compound Discoverer implements a node-based workflow that aligns RTs across samples, removes background signals, extracts features and groups them into compounds, predicts chemical formulas, searches spectral (MS1 and MS2) annotation sources, and assigns compound annotations. Data from inter-run replicates were combined by the Group Compounds and Differential Analysis Nodes in Compound Discoverer for all statistical analyses except principal component analysis (PCA). Details about the Compound Discoverer workflow are given in Figure S2 and Table S4, Supporting Information.
Only compounds with peak areas >5x background (ultrapure water blank) and isotope pattern similarity scores (SFit%) >40 were included in the statistical analyses. SFit% threshold was determined based on results from confirmation mix and QC mix compounds, described below. In cases of multiple possible molecular formulas, the top-matched molecular formula, i.e., the top hit in the Predicted Compositions table in Compound Discoverer, indicating the closest match between the measured and theoretical isotopic patterns, was selected for each compound. Compounds with probable structure assignments based on MS2 fragmentation pattern matches (mzCloud match score >70%) were selected for confirmation of the chemical structures using authentic reference standards.
Compound counts for each e-cig product analyzed represent the number of compounds with peak areas >5x background (ultrapure water blank) that were the top hit in the Predicted Compositions table in Compound Discoverer. Compound count fold change was calculated as the aerosol compound count (mean of three vaping intervals) divided by the e-liquid compound count. PCA and Kendrick mass defect analysis were employed using Compound Discoverer software. Chemical fingerprinting classifications were applied using RStudio (v1.3.959). The analytical and data processing workflow used in this study is summarized in Figure 1. In addition to unknown sample data, confirmation mix and QC mix analyte data were also analyzed by the non-target workflow. SFit >47% and mzCloud match score >72% was obtained for all analytes; the workflow proposed the correct molecular formula as the top match for all compounds (Table S5, Supporting Information).
Compounds detected in the analyzed e-cig products were classified into major organic compound categories using two classification tools: (1) the classical van Krevelen approach based on O/C and H/C ratios, and (2) a modified van Krevelen diagram approach, multidimensional stoichiometric compound classification (MSCC), which incorporates additional C/N/P ratio constraints in its six compound categories: aminosugar-, protein-, lipid-, carbohydrate-, nucleotide-, and phytochemical-like.43 In classical van Krevelen diagrams, compounds in the condensed hydrocarbon-like category were constrained to H/C ratios of 0.5 – 1.2 and O/C ratios of <0.25.26 The lipid-like category of MSCC is composed by the following constraints: H/C ≥1.32, O/C ≤0.6, N/C ≤0.126, P/C <0.35, and N/P ≤5. In a previous study by Rivas-Ubach et al., over 98% of the compounds were accurately classified using MSCC.43 Fingerprinting analysis based on stoichiometric ratios was employed as a proof of concept of the potential to identify compound classes of concern. A potential application of this approach is the identification of compound classes which can then be further investigated using e.g., suspect screening methods.
Kendrick Mass Defect analysis was conducted using Compound Discoverer software with ceiling rounding according to formula 1, where KM = Kendrick Mass, m = monoisotopic mass, and R=14.0157 Da (methylene homologous series) or 58.0419 Da (propylene glycol homologous series).44
| (1) |
Relationships between all the variables listed in Table 1 (maximum power, coil resistance, nicotine content and PG%) and two aerosol variables (generated aerosol mass and fold change in compound count in the aerosols) were investigated by non-parametric (Spearman) correlation analysis with two-tailed exact p-value with α = 0.05 in Prism v8.0 (GraphPad Software, San Diego, CA).
3. Results and Discussion
3.1. Characteristics of generated aerosols and influence of device and e-liquid formulation
The aerosol condensation approach enabled estimation of generated aerosol masses for each e-cig product analyzed. Collected aerosol mass increased over three consecutive vaping intervals in Juul and Vuse, decreased in Blu, and peaked in interval 2 for Mi-Salt (Smok) (Table 2). The PG/VG base showed a steep drop in mass in the second two vaping intervals, suggesting that, despite manual agitation between intervals, phase separation of PG and VG may have occurred with longer vaping times in the absence of an emulsifier due to the difference in density of the two reagents.45
Significant Spearman correlations were found between vaped aerosol mass and coil resistance (r = −0.73, p = 0.006), nicotine content (r = 0.71, p = 0.008), and PG% (r = 0.73, p = 0.006). Device power was not significantly correlated with aerosol mass, despite the inverse relationship between power and resistance according to (W=power, V=voltage, and R=resistance). The largest masses were collected for Mi-Salt (Smok) and Vuse, which have both the lowest coil resistances as well as the highest stated concentrations of nicotine salt (Table 1). Adjustment of compound counts to sample masses weighed before analysis did not affect statistical significance of any of the correlations. Consistent with our observations, e-liquid consumption has been inversely associated with coil resistance46 and nicotine yield with PG content.47 However, because device coil resistance and e-liquid PG content are perfectly inversely correlated in the products analyzed, the effects of coil resistance and e-liquid PG content on aerosol mass could not be distinguished. Total particulate matter and nicotine content have previously been shown to be highly correlated,47 but the effect of nicotine content on aerosol generation is unknown.
The total number of detected compounds increased from before to after vaping in all products except Blu, although Blu contained more than double the number of compounds in the other three commercial product e-liquids pre-vaping. Unexpectedly, despite the absence of any additives, the second-highest number of compounds was detected in the PG/VG base (Table 2; for further discussion see below). Compound count across products decreased with generated aerosol mass, suggesting the aerosol may undergo dilution with increased aerosol generation, although the correlation was not significant (Spearman r = −0.47, p = 0.10). Fold change of detected compound count in aerosols versus e-liquids was also not significantly correlated with device power, coil resistance, e-liquid PG/VG ratio, or nicotine content.
3.2. Chemical fingerprints of e-cig liquids and aerosols
The overall chemical compositions of e-liquid and aerosol samples between brands were compared using PCA. The e-liquid and aerosol samples from Blu, the first-generation product and the only product with freebase nicotine, were the most chemically distinct from the other commercial products and the PG/VG base (Figure 2). In e-liquids, the highest variance (along principal component (PC) 1) was between products, with a secondary source of variation (along PC 2) between Blu inter-run replicates likely due to volatilization of flavorant compounds during storage between the two runs. A comparison of aerosols showed PG/VG, Blu, and the other three commercial products in three distinct groups.
Figure 2:
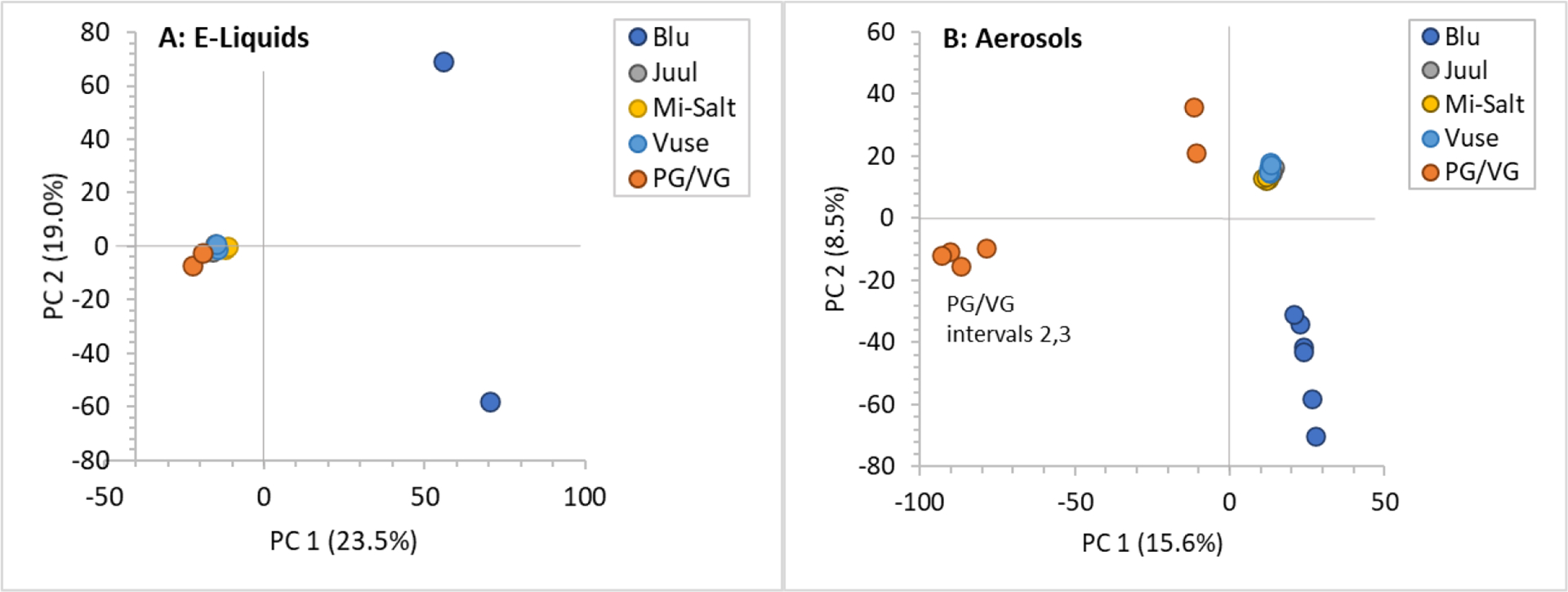
PCA score plots showing differences in chemical composition among (A) e-liquids and (B) aerosols of analyzed e-cig products. Two points for each e-liquid or aerosol are shown, each representing one analytical run.
The PCA score plots show PG/VG base aerosol samples from vaping intervals 2 and 3 separated from the interval 1 sample and the commercial samples. Excluding these two PG/VG aerosol samples from the PCA did not affect the grouping of the remaining samples relative to each other. PG/VG aerosol samples 2 and 3 were excluded from further data analyses in this study based on these results and the difference in aerosol masses (Table 2).
3.2.1. Characterizing e-liquids and aerosols using compound classification
For the PG/VG base and commercial products except Blu, condensed hydrocarbon-like compounds increased as a proportion of the total number of compounds after vaping (Table 3). The relative number of compounds in every other compound category generally decreased or remained constant. Between 60 and 90% of detected compounds were not matched to any category in the MSCC approach, with a higher proportion un-matched in aerosols (79–88%) than e-liquids (62–73%). These results, together with the increase in total compound count after vaping observed for most products, indicate that vaping may have transformed some compounds into condensed hydrocarbon-like compounds and compounds that do not fall within any of the existing categories. Overall, compounds that were not matched to any compound category were characterized by lower O/C ratios than matched compounds: O/C values of 39% of un-matched compounds were below 0.11, representing mainly hydrocarbons, compared to 16% in the matched compounds.
Table 3:
Compounds in three chemical categories* as absolute number detected (percentage of total) percentages of total numbers of detected compounds, for three consecutive vaping intervals, in analyzed e-cig products.
| Blu | Lipid-like, % | Condensed hydrocarbon-like, % | Un-matched, % |
|---|---|---|---|
| E-Liquid | 135 (6.3%) | 383 (18%) | 2123 (62%) |
| Aerosol 1 | 59 (3.5%) | 296 (17%) | 1687 (86%) |
| Aerosol 2 | 44 (2.9%) | 258 (17%) | 1532 (87%) |
| Aerosol 3 | 50 (3.4%) | 242 (17%) | 1450 (87%) |
| Juul | |||
| E-Liquid | 71 (7.6%) | 84 (9%) | 927 (70%) |
| Aerosol 1 | 40 (4.0%) | 115 (11%) | 1001 (88%) |
| Aerosol 2 | 50 (5.2%) | 96 (10%) | 960 (85%) |
| Aerosol 3 | 40 (4.3%) | 102 (11%) | 938 (87%) |
| Mi-Salt (Smok) | |||
| E-Liquid | 42 (5.5%) | 61 (8%) | 762 (73%) |
| Aerosol 1 | 39 (4.2%) | 97 (11%) | 913 (86%) |
| Aerosol 2 | 30 (3.6%) | 91 (11%) | 820 (86%) |
| Aerosol 3 | 35 (3.9%) | 93 (10%) | 896 (86%) |
| Vuse | |||
| E-Liquid | 48 (5.5%) | 98 (11%) | 873 (69%) |
| Aerosol 1 | 35 (3.7%) | 134 (14%) | 944 (83%) |
| Aerosol 2 | 38 (4.2%) | 131 (14%) | 900 (84%) |
| Aerosol 3 | 36 (3.8%) | 143 (15%) | 931 (86%) |
| PG/VG Base | |||
| Pre-Vaped | 127 (11.2%) | 53 (5%) | 1131 (62%) |
| Aerosol 1 | 145 (7.7%) | 49 (3%) | 1888 (79%) |
Lipid-like and un-matched % are based on MSCC compound category criteria. Condensed hydrocarbon-like category was defined by the classical van Krevelen diagram criteria. Because four MSCC categories are not shown, row sums do not equal 100%. Refer to text for information about classification approaches.
3.2.1.1. Condensed hydrocarbon-like compound content and H/C ratios vary between products
Condensed hydrocarbon-like compounds increased after vaping in Juul, Mi-Salt (Smok), Vuse and the PG/VG base (Table 3, Figure 3). Significant correlations were found between the fold change in condensed hydrocarbon-like content and coil resistance (r = −0.79, p = 0.002) and PG content (r = 0.79, p = 0.002). As noted, the effects of coil resistance and PG content could not be distinguished in this study due to inter-correlation.
Figure 3:
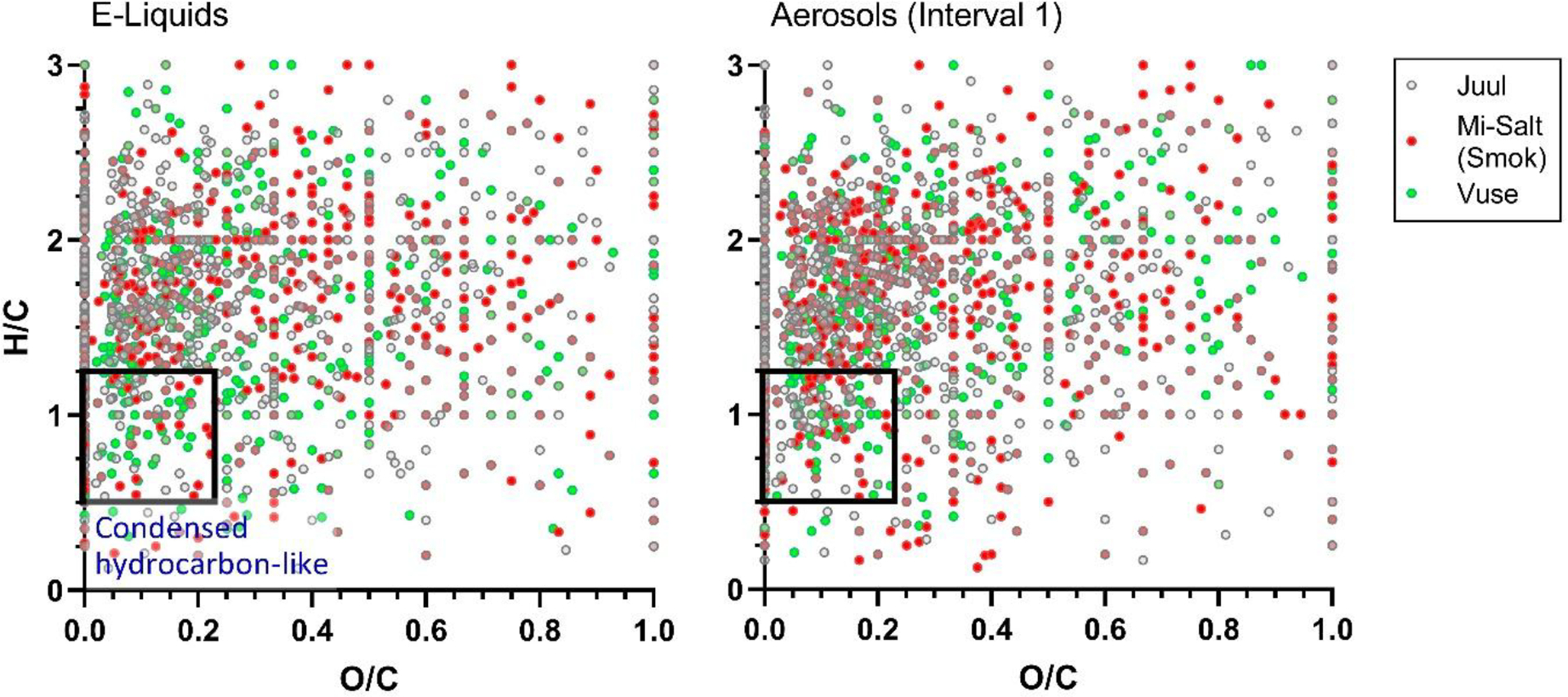
van Krevelen diagram of H/C and O/C elemental ratios for Juul, Mi-Salt (Smok) and Vuse (A) e-liquids and (B) aerosols from analyzed products, with the constraints for the condensed hydrocarbon-like compound category shown as a black rectangle.
In classical van Krevelen diagrams, compounds in the condensed hydrocarbon-like category are typically constrained to low H/C ratios of 0.5 to 1.2 and low O/C ratios of <0.25.26 In the e-liquid and aerosol samples analyzed here, the proportion of condensed hydrocarbon-like compounds in each sample were strongly inversely correlated to the H/C ratio (r = −0.91, p<0.001) as the majority of compounds were characterized by O/C <0.25 (Figure 3). Blu e-liquid and aerosols contained the highest proportion of condensed hydrocarbon-like compounds and the lowest mean H/C (1.54 ± 0.60), while the PG/VG base contained the lowest proportion of condensed hydrocarbon-like compounds and the highest mean H/C (1.92 ± 0.50). Juul, Mi-Salt (Smok), and Vuse were characterized by intermediate condensed-hydrocarbon-like compound content and H/C ratios of 1.67 ± 0.59, 1.70 ± 0.62, and 1.64 ± 0.60, respectively (Table 3, Figure 3). The high mean H/C ratios observed in the PG/VG base may be due to the high degree of saturation of its ingredients, PG and VG. Conversely, the high proportion of condensed hydrocarbon-like compounds and low mean H/C ratio in Blu both before and after vaping may be related to the presence of aromatic and other unsaturated e-liquid additives.
Previous studies have reported increased condensed hydrocarbon-like content with biomass combustion48 and dehydrogenation events in cigarette pyrolysis.49 Our observations of changes in H/C ratios and condensed hydrocarbon-like content after vaping suggest that combustion-like processes occur during vaping despite the lower temperatures than combustible cigarette smoking. Previous studies have also detected the combustion-related byproducts benzene and toluene in e-cig aerosols.50
3.2.1.2. Presence of lipid-like molecules in e-liquids and aerosols
Similar to other compound categories, the proportion of lipid-like compounds decreased with vaping in all products, from 6–11% of compounds in e-liquids to 3–8% in aerosols. The highest number of lipid-like compounds was observed in e-liquid and aerosol of the PG/VG base, supporting previous findings that e-liquid solvents may be a source of exposure to inhaled lipid compounds from vaping.51,52
A group of high molecular weight compounds with high RTs was detected in e-liquids and aerosols of Juul (only intervals 2–3), Mi-Salt (Smok) and the PG/VG base and in the Blu e-liquid (Figure 4). The majority of these compounds (53%, n=38) were identified as lipid-like using the MSCC scheme, compared to 5% in the compounds detected across all products’ e-liquids and aerosols overall (Table 3).
Figure 4:
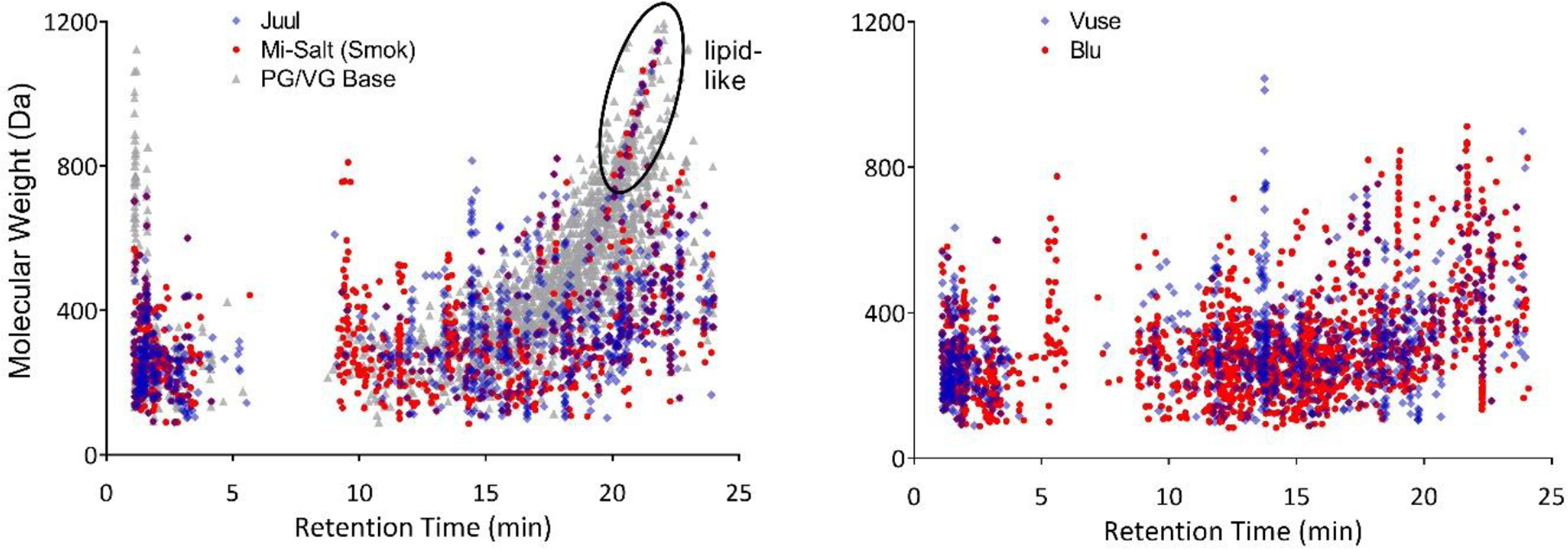
Molecular weight as a function of RT of detected compounds in aerosols of e-cig samples. Left: Juul (interval 2), Mi-Salt (Smok) (interval 1) and the PG/VG base (interval 1), highlighting predominantly lipid-like compounds determined based on multidimensional stoichiometric classification (circled). Right: Vuse (interval 1), Blu (interval 1).
Vitamin E acetate has been identified as a source of lipids in some e-cig aerosols,53 and incompletely-processed vegetable oils in VG have been suspected as an additional source.52 Inhalation of lipid molecules from vaping has been associated with lipoid pneumonia via the generation of lipid-laden macrophages, as seen in the 2019 EVALI outbreak as well as other cases.51,52
3.2.2. Appearance of homologous series after vaping
The presence of homologous series was investigated using Kendrick mass defect plots, identified as points along a horizontal line.54 Homologous series of compounds differing by methylene (CH2) units were detected in all e-liquids and aerosols (Figures 5,6, and S3, Supporting Information). Homologous series of four or more compounds differing by methylene units and containing nitrogen and phosphorous as part of the backbone were present in aerosols, but not e-liquids, of all products and the PG/VG base.
Figure 5:
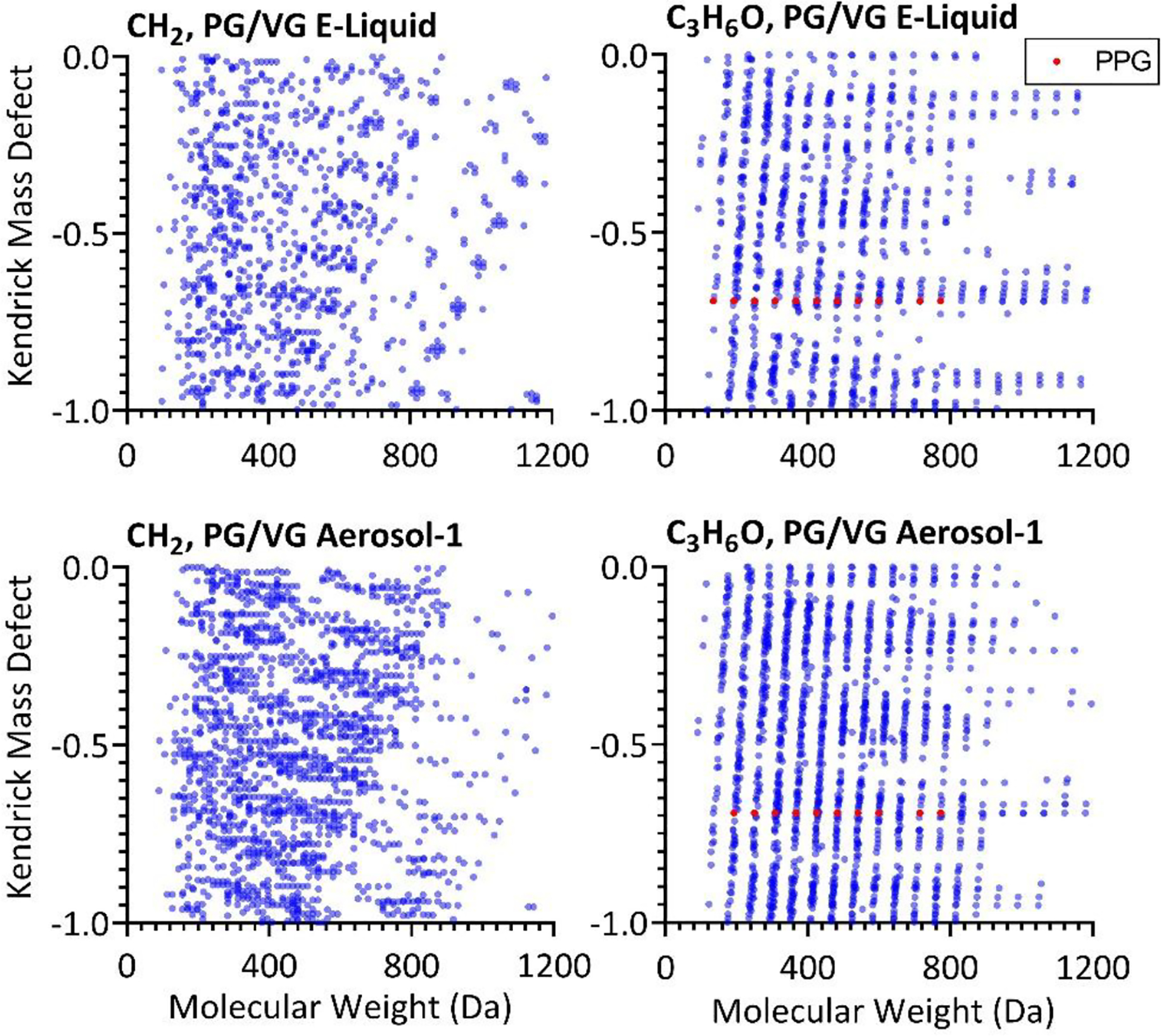
Plots of Kendrick mass defect as a function of molecular weight showing methylene (left) and propylene glycol (right, PPG compounds shown in red) homologous series in the PG/VG base e-liquid and aerosol.
Figure 6:
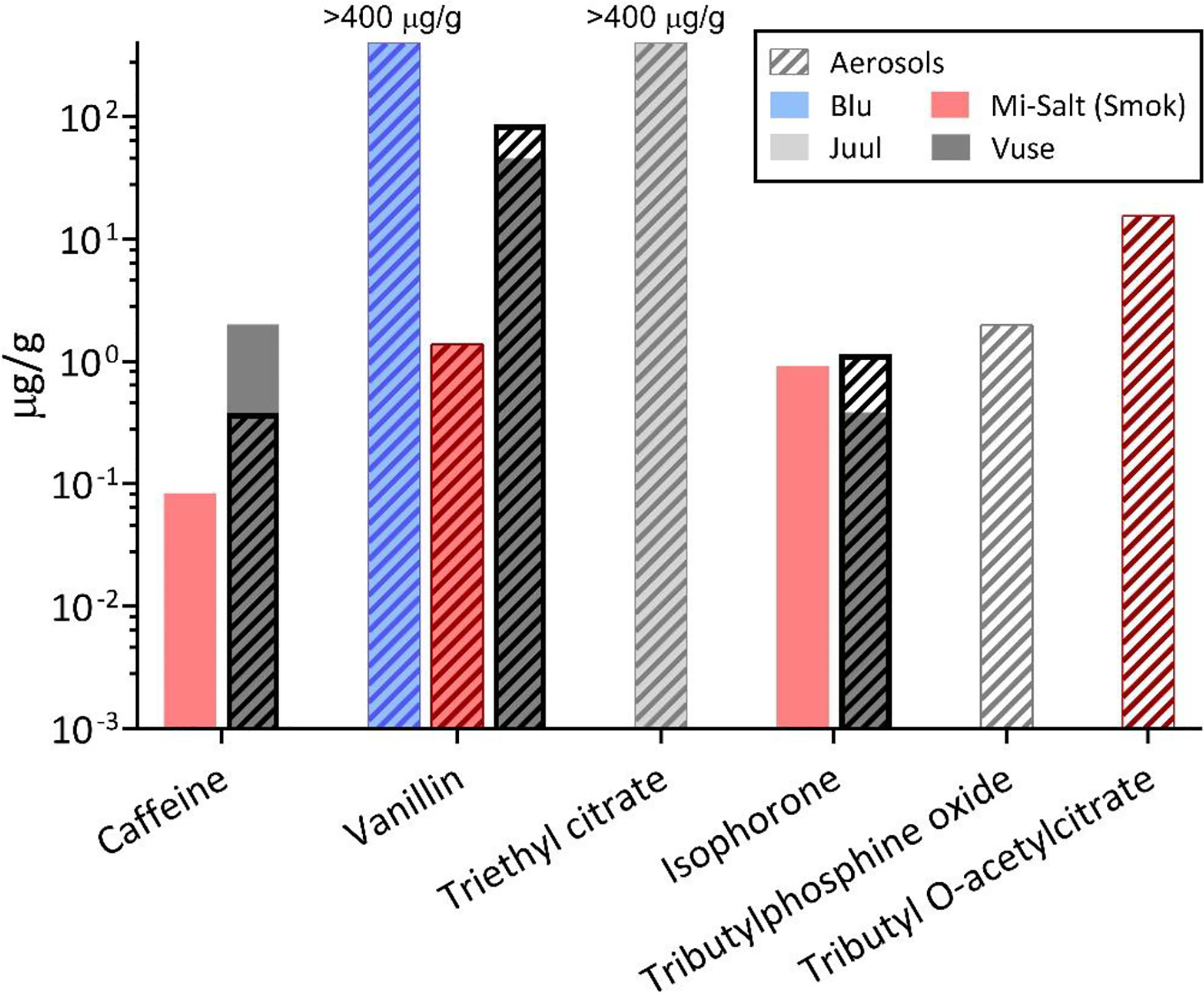
Concentrations of six compounds in commercial e-liquids and aerosols.
Three series of homologous compounds detected only after vaping in Blu, all classified as lipid-like, shared similar retention times of 22.29 ± 0.01 min. For example, C10H16O was tentatively identified as camphor at a Kendrick mass defect of −0.2 along with six other compounds with chemical formulas with successive additions of methylene units (Figure S4, Supporting Information). An increase in homologous series differing by both methylene and PG (C3H6O) units was also observed in the PG/VG base (Figure 6). Both before and after vaping, these compounds included polypropylene glycol (PPG) compounds of varying chain lengths (e.g., PPG-n4 through PPG-n8 had probable MS2 fragmentation pattern matches, with mzCloud match score >70%). In addition to the series of PPG compounds, homologous series of compounds increasing by C3H6O units were observed in the Kendrick mass defect plot for the PG/VG base (Figure 6). These results suggest that homologous series may explain the unexpectedly high number of compounds detected in the PG/VG base, higher than three of the four commercial products, despite its lack of any additives. The total ion chromatograms for PG/VG and Juul aerosols further demonstrate that PG/VG consists of polypropylene glycol (PPG) molecules at various chain lengths (e.g., PPG-n4 contains 4 PPG units) (Figure S5, Supporting Information).
Previous studies have identified important reaction pathways of PG and VG during vaping, focusing on relatively small compounds such as aldehydes and flavorant reaction products.6,55 Our observation of homologous series demonstrates the presence of compounds of varying alkyl chain lengths in commercial e-liquids and aerosols as well as a base consisting of only PG and VG solvents, possibly contributing to the higher number of compounds detected in most of the analyzed aerosols than e-liquids. Several of these series were present only after vaping, suggesting the possible decomposition of high-molecular weight compounds during vaping.
The finding that PG and VG are not each single chemical solutions, but rather consist of a large number of oligomers, is supported by two previous studies. Using GC/EI-MS and ESI-Q-TOF-MS, these series were observed in pure PG and VG before vaping.12 Escobar et al (2020) also observed oligomer formation in PG, VG and PG/VG aerosols using UPLC-ESI-Q-TOF-MS.56 The reason for the larger number of compounds in the PG/VG base compared to three of the commercial e-liquids analyzed is uncertain. The difference may be explained by the presence of emulsifiers in the commercial samples, which attract solvent and additive molecules to form larger compounds and droplets, unlike in the lab-made PG/VG solution. These emulsified compounds may have higher molecular weights outside of the scan range of our analytical methods. Further research to specifically address this aspect is needed.
3.3. Confirmation of compounds of potential health concern in commercial e-cig products
Six of eleven compounds of potential health concern that had probable structure assignments based on MS2 fragmentation pattern matches (mzCloud match score >70%) were confirmed using commercially available reference standards and their concentrations were determined in e-cig liquids and aerosols: one stimulant, three industrial chemicals, and two flavorants with possible toxic effects (Table 4 and Figure 6). Example MS2 spectra, RTs, and mass accuracies of the six compounds in the confirmation mix and e-cig samples are shown in Figure S6 and Table S6, Supporting Information.
Table 4:
Overview of 6 compounds quantified and detection frequencies for e-liquids and aerosols of analyzed products.
| Compound | Chemical class | Relevant use(s) | Hazard class* / health effects |
|---|---|---|---|
| Caffeine | Purine alkaloid | Central nervous system stimulant | Irritant† |
| Isophorone | α,β-unsaturated ketone | Solvent, flavorant | Irritant; Health Hazard |
| Citroflex-A4 (Tributyl O-acetylcitrate) | Citrate | Plasticizer | Induces CYP3A459 |
| Tributylphosphine oxide | Organophosphorous compound | Catalyst, flame retardant | Corrosive; Irritant†; generally regarded as toxic |
| Citroflex-2 (Triethyl citrate) | Citrate | Emulsifier | Irritant† |
| Vanillin | Phenol, benzaldehyde | Flavorant | Irritant† |
Globally Harmonized System (GHS) hazard classification is given unless otherwise indicated.
Irritant hazard class in GHS refers to dermal irritation
Caffeine, a central nervous system stimulant, was detected in samples from three of the commercial e-cig products analyzed. Caffeine was detected in Vuse and Mi-Salt (Smok) e-liquids and/or aerosols. The stimulant has previously been reported in coffee, tea, chocolate and energy drink-flavored e-liquids, some at levels up to 104 μg/g,57 but its presence in tobacco flavored products in the current study was unexpected.
Three industrial chemicals and a pesticide were identified at relatively low detection frequencies and concentrations. Tertiary phosphine oxides such as tributylphosphine oxide, which was detected in Juul aerosol at 2 μg/g, are commonly used as flame retardants for polymers58 and may be present as contaminants from the device. Tributyl O-acetylcitrate, detected in Mi-Salt (Smok) aerosol at 20 μg/g, is the most widely used phthalate substitute plasticizer and has been reported to induce cytochrome P450, associated with various adverse health effects.59 This compound was not detected in any of the e-liquid samples, suggesting that it may leach from device components during vaping. Triethyl citrate, reported to be used as an e-liquid emulsifier in Juul e-liquids,19 was detected at high concentrations, above the maximum quantifiable (400 μg/g), in Juul e-liquid and aerosol samples in this study.
Two flavorants, vanillin and isophorone, were found in multiple analyzed products. A previous study reported vanillin in e-liquids between 100 and 3000 mg/mL17 and higher toxicity was associated with vanillin-containing e-liquids using cell assays.7 Vanillin and other flavor aldehydes form acetals with PG in the e-liquid solvent which have been linked with respiratory irritation.55 Here we found the highest vanillin content in Blu e-liquid and aerosol, above the maximum quantifiable level (400 μg/g). Vuse e-liquid and aerosol also contained high vanillin levels of 50 and 90 μg/g, respectively. Mi-Salt (Smok) contained ≤1 μg/g, and levels were undetected in the remaining products. Isophorone was detected at ≤1 μg/g in Mi-Salt (Smok) and Vuse. It is an α,β-unsaturated ketone, also used as a solvent and present in combustible cigarettes60 and is classified as a health hazard. Confectionary flavor chemicals such as vanillin have been previously reported in tobacco flavor e-liquids,17 consistent with our observations. Unlike isophorone, vanillin is not naturally present in tobacco.
For harmaline in commercial samples (Blu (aerosol only), Juul, Mi-Salt, Vuse), exact mass and RT matches with the reference standard were observed, but MS2 spectra were not fully consistent with the standard (Figure S6D). These results suggest the presence of a harmaline isomer, but further research is needed for confirmation. Harmaline is a stimulant used recreationally as a hallucinogen as well as in pharmaceutical drugs.61,62 It is one of the harmala alkaloids, some of which are naturally found in tobacco, and which have been associated with the addictive effects of cigarettes as monoamine oxidase (MAO) inhibitors.62 In the e-cigs analyzed here, isophorone and harmaline could be derived from nicotine produced by extraction from tobacco, but further study is needed to confirm this hypothesis.
Two compounds (tributyl O-acetylcitrate and tributylphosphine oxide) were detected in the aerosols but not in the corresponding e-liquid from the same product. This may be due to an increase in concentration of the aerosol compared to the e-liquid, e.g., due to a loss of moisture during the vaping process. This observation would be consistent with the increase observed in the overall number of detected compounds in the aerosols vs. e-liquids analyzed (Table 2). Additional explanations include chemical transformations to form new compounds in the aerosols, or leaching of compounds from device components during vaping as has previously been shown to occur with the metallic coil.13
4. Conclusions
In this study, non-target analysis based on LC–HRMS, coupled with chemical fingerprinting techniques, enabled comparisons of chemical compositions of four commercial e-cig products and an investigation of chemical transformations due to vaping. The number of total compounds detected in the PG/VG base, particularly the aerosol, was higher than in the three nicotine salt products, emphasizing that the e-liquid base alone, without chemical additives, may be a source of toxicant exposure to vapers. Homologous series analysis suggested that this elevated compound count may be due to the degradation of high-molecular weight compounds into compounds with varying alkyl and polypropylene glycol chain lengths. Further research is needed to explain these differences between commercial product samples and the PG/VG base alone. The total number of compounds detected in the three nicotine salt-based commercial products was higher in aerosols, in which homologous compounds of varying alkyl chain lengths were observed, than in e-liquids. We also observed the presence of several compounds of potential health concern in tobacco-flavored e-liquid and aerosol samples, including the industrial chemical tributylphosphine oxide and the stimulant caffeine. Our results also suggested that combustion byproducts and lipid-like compounds may be present in the products analyzed, with levels of each affected by vaping. Further investigations into the identities of the specific compounds, formation processes, and associated exposure risks is required.
A challenge in non-target analysis for exposure science applications continues to be the relatively low mass spectral database coverage of the vast number of chemicals present.23 The focus of our analyses was therefore on accurate mass and molecular formula information, which can provide valuable insights into chemical composition. Four e-cig products and a single e-liquid flavor were evaluated in this study as a proof of concept of the analytical and data treatment methods applied. Future work featuring additional e-cig devices and flavors is needed to generalize the findings reported here and to investigate the influence of specific e-cig device and liquid characteristics on chemical composition. Further method development of to increase the range of detected compounds, including detection of both non-volatile and volatile compounds, is needed. The results of this study highlight the presence of unexpected, potentially hazardous compounds in e-cig products, and the accompanying need for toxicological assessments of compounds prioritized using a non-target approach.
Supplementary Material
Acknowledgments
MWT acknowledges support from R01ES030025, MNN is grateful for support from NIEHS (T32ES007141) and CP for support from Johns Hopkins University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge the assistance of Gayane Yenokyan and the Johns Hopkins Institute for Clinical and Translational Research (ICTR) funded in part by Grant Number UL1 TR003098 from NCATS and NIH Roadmap for Medical Research, with statistical analysis. The authors thank Christopher Brueck, Zhuoyue Zhang, and Steven Chow for helpful discussions on non-target methodology.
Footnotes
Supporting Information
Instrument settings and acquisitions parameters; data processing parameters; QC results; standard addition calibration curve example; PCA loading plots; Kendrick Mass Defect plots; PG/VG control sample TIC; MS2 spectra for confirmed compounds
References
- (1).Schmidt S Vaper, Beware : The Unique Toxicological Profile of Electronic Cigarettes. EHP. 2020, pp 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).National Academies of Sciences Engineering and Medicine. Public Health Consequences of E-Cigarettes; Stratton K, Kwan LY, Eaton DL, Eds.; The National Academies Press: Washington, D.C., 2018. 10.17226/24737. [DOI] [PubMed] [Google Scholar]
- (3).Miech R; Johnston L; O’Malley PM; Bachman JG; Patrick ME Trends in Adolescent Vaping, 2017–2019. N. Engl. J. Med 2019, 381 (15), 1490–1491. 10.1056/nejmc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cheng T Chemical Evaluation of Electronic Cigarettes. Tob. Control 2014, 23 (SUPPL. 2). 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wei B; Goniewicz M; O’Connor RJ Concurrent Quantification of Emerging Chemicals of Health Concern in E-Cigarette Liquids by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. ACS Omega 2019, 4 (13), 15364–15372. 10.1021/acsomega.9b01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jensen RP; Strongin RM; Peyton DH Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. Rep 2017, 7, 1–11. 10.1038/srep42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sassano MF; Davis ES; Keating JE; Zorn BT; Kochar TK; Wolfgang MC; Glish GL; Tarran R Evaluation of E-Liquid Toxicity Using an Open-Source High-Throughput Screening Assay. PLoS Biol. 2018, 16 (3), 1–24. 10.1371/journal.pbio.2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Salam S; Saliba NA; Shihadeh A; Eissenberg T; El-hellani A Flavor-Toxicant Correlation in E-cigarettes: A Meta-Analysis. 2020. 10.1021/acs.chemrestox.0c00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kelley A The vaping “flavor ban” goes into effect today. Here’s what it does https://thehill.com/changing-america/well-being/prevention-cures/481853-the-vaping-flavor-ban-goes-into-effect-today. [Google Scholar]
- (10).U.S. Food and Drug Administration. FDA Finalizes Enforcement Policy on Unauthorized Flavored Cartridge-Based e-Cigarettes That Appeal to Children, Including Fruit and Mint; 2020.
- (11).U.S. Food and Drug Administration. FDA Notifies Companies, Including Puff Bar, to Remove Flavored Disposable E-Cigarettes and Youth-Appealing E-Liquids from Market for Not Having Required Authorization; 2020.
- (12).Jiang H; Ahmed CMS; Martin TJ; Canchola A; Oswald IWH; Garcia JA; Chen JY; Koby K; Zhao Z; Zhang H; Chen K; Lin Y Chemical and Toxicological Characterization of Vaping Emission Products from Commonly Used Vape Juice Diluents. Chem. Res. Toxicol 2020. 10.1021/acs.chemrestox.0c00174. [DOI] [PubMed] [Google Scholar]
- (13).Olmedo P; Goessler W; Tanda S; Grau-Perez M; Jarmul S; Aherrera A; Chen R; Hilpert M; Cohen JE; Navas-Acien A; Rule AM Metal Concentrations in E-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect 2018, 126 (02). 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wei B; Connor RJO; Goniewicz ML; Hyland A Emerging Chemicals of Health Concern in Electronic Nicotine Delivery Systems. Chem. Res. Toxicol 2020. 10.1021/acs.chemrestox.0c00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Salamanca JC; Meehan-Atrash J; Vreeke S; Escobedo JO; Peyton DH; Strongin RM E-Cigarettes Can Emit Formaldehyde at High Levels under Conditions That Have Been Reported to Be Non-Averse to Users. Sci. Rep 2018, 8 (1), 6–11. 10.1038/s41598-018-25907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Uchiyama S; Noguchi M; Sato A; Ishitsuka M; Inaba Y; Kunugita N Determination of Thermal Decomposition Products Generated from E-Cigarettes. Chem. Res. Toxicol 2020. 10.1021/acs.chemrestox.9b00410. [DOI] [PubMed] [Google Scholar]
- (17).Tierney PA; Karpinski CD; Brown JE; Luo W; Pankow JF Flavour Chemicals in Electronic Cigarette Fluids. Tob. Control 2016, 25 (E1), e10–e15. 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Omaiye E; McWhirter K; Luo W; Pankow J; Talbot P Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. Chem. Res. Toxicol 2018, 1–19. 10.1101/490607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Erythropel HC; Anastas PT; Krishnan-Sarin S; O’Malley SS; Jordt SE; Zimmerman JB Differences in Flavourant Levels and Synthetic Coolant Use between USA, EU and Canadian Juul Products. Tob. Control 2020, 1–4. 10.1136/tobaccocontrol-2019-055500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rawlinson C; Martin S; Frosina J; Wright C Chemical Characterisation of Aerosols Emitted by Electronic Cigarettes Using Thermal Desorption–Gas Chromatography–Time of Flight Mass Spectrometry. J. Chromatogr. A 2017, 1497, 144–154. 10.1016/j.chroma.2017.02.050. [DOI] [PubMed] [Google Scholar]
- (21).Herrington JS; Myers C Electronic Cigarette Solutions and Resultant Aerosol Profiles. J. Chromatogr. A 2015, 1418, 192–199. 10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- (22).Wang J; Chow W; Chang J; Wong JW Ultrahigh-Performance Liquid Chromatography Electrospray Ionization Q-Orbitrap Mass Spectrometry for the Analysis of 451 Pesticide Residues in Fruits and Vegetables: Method Development and Validation. J. Agric. Food Chem 2014, 62 (42), 10375–10391. 10.1021/jf503778c. [DOI] [PubMed] [Google Scholar]
- (23).Hollender J; Schymanski EL; Singer HP; Ferguson PL Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ. Sci. Technol 2017, 51 (20), 11505–11512. 10.1021/acs.est.7b02184. [DOI] [PubMed] [Google Scholar]
- (24).Zubarev RA; Makarov A Orbitrap Mass Spectrometry. Anal. Chem 2013, 85 (11), 5288–5296. 10.1021/ac4001223. [DOI] [PubMed] [Google Scholar]
- (25).Pan Q; Zhuo X; He C; Zhang Y; Shi Q Validation and Evaluation of High-Resolution Orbitrap Mass Spectrometry on Molecular Characterization of Dissolved Organic Matter. ACS Omega 2020, 5 (10), 5372–5379. 10.1021/acsomega.9b04411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sanchís J; Jaén-Gil A; Gago-Ferrero P; Munthali E; Farré MJ Characterization of Organic Matter by HRMS in Surface Waters: Effects of Chlorination on Molecular Fingerprints and Correlation with DBP Formation Potential. Water Res. 2020, 176. 10.1016/j.watres.2020.115743. [DOI] [PubMed] [Google Scholar]
- (27).Knolhoff AM; Kneapler CN; Croley TR Optimized Chemical Coverage and Data Quality for Non-Targeted Screening Applications Using Liquid Chromatography/High-Resolution Mass Spectrometry. Anal. Chim. Acta 2019, 1066, 93–101. 10.1016/j.aca.2019.03.032. [DOI] [PubMed] [Google Scholar]
- (28).Waldner BJ; Machalett R; Schönbichler S; Dittmer M; Rubner MM; Intelmann D Fast Evaluation of Herbal Substance Class Composition by Relative Mass Defect Plots. Anal. Chem 2020. 10.1021/acs.analchem.0c01447. [DOI] [PubMed] [Google Scholar]
- (29).Talih S; Salman R; El-Hage R; Karam E; Karaoghlanian N; El-Hellani A; Saliba N; Shihadeh A Characteristics and Toxicant Emissions of JUUL Electronic Cigarettes. Tob. Control 2019, 28 (6), 678–680. 10.1136/tobaccocontrol-2018-054616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).El-Hellani A; Salman R; El-Hage R; Talih S; Malek N; Baalbaki R; Karaoghlanian N; Nakkash R; Shihadeh A; Saliba NA Nicotine and Carbonyl Emissions from Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob. Res 2018, 20 (2), 215–223. 10.1093/ntr/ntw280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wieczorek R; Phillips G; Czekala L; Trelles Sticken E; O’Connell G; Simms L; Rudd K; Stevenson M; Walele T A Comparative in Vitro Toxicity Assessment of Electronic Vaping Product E-Liquids and Aerosols with Tobacco Cigarette Smoke. Toxicol. Vitr 2020, 66 (April), 104866. 10.1016/j.tiv.2020.104866. [DOI] [PubMed] [Google Scholar]
- (32).Shao XM; Friedman TC Pod-Mod vs. Conventional e-Cigarettes: Nicotine Chemistry, PH, and Health Effects. J. Appl. Physiol 2020, 128 (4), 1056–1058. 10.1152/japplphysiol.00717.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Tayyarah R; Long GA Comparison of Select Analytes in Aerosol from E-Cigarettes with Smoke from Conventional Cigarettes and with Ambient Air. Regul. Toxicol. Pharmacol 2014, 70 (3), 704–710. 10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- (34).St. Helen G; Havel C; Dempsey DA; Jacob P; Benowitz NL Nicotine Delivery, Retention and Pharmacokinetics from Various Electronic Cigarettes. Addiction 2016, 111 (3), 535–544. 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Pappas RS; Gray N; Halstead M; Valentin-Blasini L; Watson C Toxic Metal-Containing Particles in Aerosols from Pod-Type Electronic Cigarettes. J. Anal. Toxicol 2020. 10.1093/jat/bkaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Harvanko AM; Havel CM; Jacob P; Benowitz NL Characterization of Nicotine Salts in 23 Electronic Cigarette Refill Liquids. Nicotine Tob. Res 2020, 22 (7), 1239–1243. 10.1093/ntr/ntz232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).BLANKZ! Refillable JUUL Pods. https://blankzpods.com/products/blankz-refillable-pods-juul-compatible.
- (38).Olmedo P; Navas-Acien A; Hess C; Jarmul S; Rule AM A Direct Method for E-Cigarette Aerosol Sample Collection. Environ. Res 2016, 149, 151–156. 10.1016/j.envres.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hilpert M; Ilievski V; Hsu S; Rule AM; Olmedo P E-Cigarette Aerosol Collection Using Converging and Straight Tubing Sections: Physical Mechanisms. J. Colloid Interface Sci 2021, 584, 804–815. 10.1016/j.jcis.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhao D; Navas-Acien A; Ilievski V; Slavkovich V; Olmedo P; Adria-Mora B; Domingo-Relloso A; Aherrera A; Kleiman NJ; Rule AM; Hilpert M Metal Concentrations in Electronic Cigarette Aerosol: Effect of Open-System and Closed-System Devices and Power Settings. Environ. Res 2019, 174 (April), 125–134. 10.1016/j.envres.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).ISO. ISO 20768: Vapour Products — Routine Analytical Vaping Machine — Definitions and Standard Conditions; 2018; Vol. 2018. [Google Scholar]
- (42).CORESTA. CORESTA Technical Guide No.22 for the Selection of Appropriate Intense Vaping Regimes for E-Vapour Devices. 2018, No. February, 1–12. [Google Scholar]
- (43).Rivas-Ubach A; Liu Y; Bianchi TS; Tolić N; Jansson C; Paša-Tolić L Moving beyond the van Krevelen Diagram: A New Stoichiometric Approach for Compound Classification in Organisms. Anal. Chem 2018, 90 (10), 6152–6160. 10.1021/acs.analchem.8b00529. [DOI] [PubMed] [Google Scholar]
- (44).Fouquet TNJ The Kendrick Analysis for Polymer Mass Spectrometry. J. Mass Spectrom 2019, 54 (12), 933–947. 10.1002/jms.4480. [DOI] [PubMed] [Google Scholar]
- (45).Sleiman M; Logue JM; Montesinos VN; Russell ML; Litter MI; Gundel LA; Destaillats H Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol 2016, 50 (17), 9644–9651. 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- (46).Talih S; Salman R; Karaoghlanian N; El-Hellani A; Saliba N; Eissenberg T; Shihadeh A Juice Monsters: Sub-Ohm Vaping and Toxic Volatile Aldehyde Emissions. Chem. Res. Toxicol 2017, 30 (10), 1791–1793. 10.1021/acs.chemrestox.7b00212. [DOI] [PubMed] [Google Scholar]
- (47).Baassiri M; Talih S; Salman R; Karaoghlanian N; Saleh R; El Hage R; Saliba N; Shihadeh A Clouds and “Throat Hit”: Effects of Liquid Composition on Nicotine Emissions and Physical Characteristics of Electronic Cigarette Aerosols. Aerosol Sci. Technol 2017, 51 (11), 1231–1239. 10.1080/02786826.2017.1341040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Podgorski DC; Hamdan R; McKenna AM; Nyadong L; Rodgers RP; Marshall AG; Cooper WT Characterization of Pyrogenic Black Carbon by Desorption Atmospheric Pressure Photoionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem 2012, 84 (3), 1281–1287. 10.1021/ac202166x. [DOI] [PubMed] [Google Scholar]
- (49).Schramm S; Carré V; Scheffler JL; Aubriet F Analysis of Mainstream and Sidestream Cigarette Smoke Particulate Matter by Laser Desorption Mass Spectrometry. Anal. Chem 2011, 83 (1), 133–142. 10.1021/ac1019842. [DOI] [PubMed] [Google Scholar]
- (50).Zhao J; Nelson J; Dada O; Pyrgiotakis G; Kavouras IG; Demokritou P Assessing Electronic Cigarette Emissions: Linking Physico-Chemical Properties to Product Brand, e-Liquid Flavoring Additives, Operational Voltage and User Puffing Patterns. Inhal. Toxicol 2018, 30 (2), 78–88. 10.1080/08958378.2018.1450462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).McCauley L; Markin C; Hosmer D An Unexpected Consequence of Electronic Cigarette Use. Chest 2012, 141 (4), 1110–1113. 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- (52).Eissenberg T; Maziak W Are Electronic Cigarette Users at Risk for Lipid-Mediated Lung Injury? Am. J. Respir. Crit. Care Med 2020, 201 (8), 1012–1013. 10.1164/RCCM.201910-2082LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Blount BC; Karwowski MP; Shields PG; Morel-Espinosa M; Valentin-Blasini L; Gardner M; Braselton M; Brosius CR; Caron KT; Chambers D; Corstvet J; Cowan E; de Jesús VR; Espinosa P; Fernandez C; Holder C; Kuklenyik Z; Kusovschi JD; Newman C; Reis GB; Rees J; Reese C; Silva L; Seyler T; Song MA; Sosnoff C; Spitzer CR; Tevis D; Wang L; Watson C; Wewers MD; Xia B; Heitkemper DT; Ghinai I; Layden J; Briss P; King BA; Delaney LJ; Jones CM; Baldwin GT; Patel A; Meaney-Delman D; Rose D; Krishnasamy V; Barr JR; Thomas J; Pirkle JL Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med 2020, 382 (8), 697–705. 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Fouquet TNJ; Cody RB; Ozeki Y; Kitagawa S; Ohtani H; Sato H On the Kendrick Mass Defect Plots of Multiply Charged Polymer Ions: Splits, Misalignments, and How to Correct Them. J. Am. Soc. Mass Spectrom 2018, 29 (8), 1611–1626. 10.1007/s13361-018-1972-4. [DOI] [PubMed] [Google Scholar]
- (55).Erythropel HC; Jabba SV; DeWinter TM; Mendizabal M; Anastas PT; Jordt SE; Zimmerman JB Formation of Flavorant-Propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res 2019, 21 (9), 1248–1258. 10.1093/ntr/nty192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Escobar YNH; Nipp G; Cui T; Petters SS; Surratt JD; Jaspers I In Vitro Toxicity and Chemical Characterization of Aerosol Derived from Electronic Cigarette Humectants Using a Newly Developed Exposure System. Chem. Res. Toxicol 2020, 33 (7), 1677–1688. 10.1021/acs.chemrestox.9b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lisko JG; Lee GE; Kimbrell JB; Rybak ME; Valentin-Blasini L; Watson CH Caffeine Concentrations in Coffee, Tea, Chocolate, and Energy Drink Flavored E-Liquids. Nicotine Tob. Res 2017, 19 (4), 484–492. 10.1093/ntr/ntw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Salin AV; Il’in AV; Faskhutdinov RI; Galkin VI; Islamov DR; Kataeva ON Tributylphosphine Catalyzed Addition of Diphenylphosphine Oxide to Unsubstituted and Substituted Electron-Deficient Alkenes. Tetrahedron Lett. 2018, 59 (17), 1630–1634. 10.1016/j.tetlet.2018.03.040. [DOI] [Google Scholar]
- (59).Takeshita A; Igarashi-migitaka J; Nishiyama K; Takahashi H; Takeuchi Y; Koibuchi N Acetyl Tributyl Citrate, the Most Widely Used Phthalate Substitute Plasticizer, Induces Cytochrome P450 3A through Steroid and Xenobiotic Receptor. Toxicol. Sci 2011, 123 (2), 460–470. 10.1093/toxsci/kfr178. [DOI] [PubMed] [Google Scholar]
- (60).Jing Y; Gong C; Xian K; Wang C; Lu P The Effects of Filter Ventilation on Flavor Constituents in Cigarette Smoke. Beiträge zur Tab. Int. to Tob. Res 2016, 21 (5), 280–285. 10.2478/cttr-2013-0794. [DOI] [Google Scholar]
- (61).National Library of Medicine (US). PubChem Compound Summary for CID 3564, Harmaline/pubchem.ncbi.nlm.nih.gov/compound/Harmaline (accessed Jan 19, 2021). [Google Scholar]
- (62).Kivell BM; Danielson K Neurological Effects of Nicotine, Tobacco, and Particulate Matter; Elsevier Inc., 2016; Vol. 1. 10.1016/B978-0-12-800213-1.00011-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


