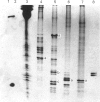
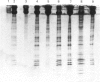
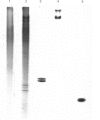
Abstract
Human saliva contains a large number of phosphopeptides derived by cleavage of acidic proline-rich proteins (APRPs). These peptides were purified by column chromatography and they constituted 0.5% of APRPs in parotid saliva, but 11% of APRPs in saliva expectorated from the mouth (whole saliva), indicating that there is considerable cleavage of APRPs after secretion from the gland. Similarly to APRP, the phosphopeptides bind Ca2+, but they accounted for only 4% of protein-bound Ca2+ in whole saliva. APRPs as well as the phosphopeptides inhibited formation of hydroxyapatite, but, whereas 19-20 micrograms of APRP was needed for 50% inhibition, only 0.7-3.3 micrograms of purified peptides was needed for the same degree of activity, and the phosphopeptides accounted for 18% of total inhibitory activity in whole saliva. All phosphopeptides adsorbed on hydroxyapatite in vitro, and adsorption of phosphopeptides on tooth surfaces in vivo could also be demonstrated, indicating that they would be able to inhibit unwanted mineral formation on the tooth surface in vivo. Degradation of APRPs after secretion therefore does not lead to a loss of their biological activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azen E. A., Hurley C. K., Denniston C. Genetic polymorphism of the major parotid salivary glycoprotein (Gl) with linkage to the genes for Pr, Db, and Pa. Biochem Genet. 1979 Apr;17(3-4):257–279. doi: 10.1007/BF00498967. [DOI] [PubMed] [Google Scholar]
- Bennick A., Cannon M. Quantitative study of the interaction of salivary acidic proline-rich proteins with hydroxyapatite. Caries Res. 1978;12(3):159–169. doi: 10.1159/000260326. [DOI] [PubMed] [Google Scholar]
- Bennick A., Chau G., Goodlin R., Abrams S., Tustian D., Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Arch Oral Biol. 1983;28(1):19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., McLaughlin A. C., Grey A. A., Madapallimattam G. The location and nature of calcium-binding sites in salivary acidic proline-rich phosphoproteins. J Biol Chem. 1981 May 25;256(10):4741–4746. [PubMed] [Google Scholar]
- Bennick A. The binding of calcium to a salivary phosphoprotein, protein A, common to human parotid and submandibular secretions. Biochem J. 1976 Apr 1;155(1):163–169. doi: 10.1042/bj1550163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. The binding of calcium to a salivary phosphoprotein, protein C, and comparison with calcium binding to protein A, a related salivary phosphoprotein. Biochem J. 1977 May 1;163(2):241–245. doi: 10.1042/bj1630241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. M., Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J Dent Res. 1987 Aug;66(8):1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Cisar J. O., Clark W. B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988 Nov;56(11):2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V., Peacock A. C. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973 Nov;56(1):43–51. doi: 10.1016/0003-2697(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Gron P. The state of calcium and inorganic orthophosphate in human saliva. Arch Oral Biol. 1973 Nov;18(11):1365–1378. doi: 10.1016/0003-9969(73)90110-6. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Bennick A., Schlesinger D. H., Minaguchi K., Madapallimattam G., Schluckebier S. K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f). Biochem J. 1988 Oct 1;255(1):15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Kauffman D. L., Keller P. J. The basic proline-rich proteins in human parotid saliva from a single subject. Arch Oral Biol. 1979;24(4):249–256. doi: 10.1016/0003-9969(79)90085-2. [DOI] [PubMed] [Google Scholar]
- Kousvelari E. E., Baratz R. S., Burke B., Oppenheim F. G. Immunochemical identification and determination of proline-rich proteins in salivary secretions, enamel pellicle, and glandular tissue specimens. J Dent Res. 1980 Aug;59(8):1430–1438. doi: 10.1177/00220345800590081201. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kim H. S., Azen E. A., Smithies O. Differential RNA splicing and post-translational cleavages in the human salivary proline-rich protein gene system. J Biol Chem. 1985 Sep 15;260(20):11123–11130. [PubMed] [Google Scholar]
- Mehansho H., Butler L. G., Carlson D. M. Dietary tannins and salivary proline-rich proteins: interactions, induction, and defense mechanisms. Annu Rev Nutr. 1987;7:423–440. doi: 10.1146/annurev.nu.07.070187.002231. [DOI] [PubMed] [Google Scholar]
- Minaguchi K., Bennick A. Genetics of human salivary proteins. J Dent Res. 1989 Jan;68(1):2–15. doi: 10.1177/00220345890680010201. [DOI] [PubMed] [Google Scholar]
- Moreno E. C., Varughese K., Hay D. I. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int. 1979 Aug 24;28(1):7–16. doi: 10.1007/BF02441212. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Yang Y. C., Diamond R. D., Hyslop D., Offner G. D., Troxler R. F. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem. 1986 Jan 25;261(3):1177–1182. [PubMed] [Google Scholar]
- Robinson R., Kauffman D. L., Waye M. M., Blum M., Bennick A., Keller P. J. Primary structure and possible origin of the non-glycosylated basic proline-rich protein of human submandibular/sublingual saliva. Biochem J. 1989 Oct 15;263(2):497–503. doi: 10.1042/bj2630497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger D. H., Hay D. I. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977 Mar 10;252(5):1689–1695. [PubMed] [Google Scholar]
- Shomers J. P., Tabak L. A., Levine M. J., Mandel I. D., Hay D. I. Properties of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982 Feb;61(2):397–399. doi: 10.1177/00220345820610020601. [DOI] [PubMed] [Google Scholar]
- Smith Q. T., Shapiro B. L., Hamilton M. J. Polyacrylamide gel patterns of parotid saliva proteins in Caucasoids and Amerindians. Arch Oral Biol. 1975 May-Jun;20(5-6):369–373. doi: 10.1016/0003-9969(75)90029-1. [DOI] [PubMed] [Google Scholar]