Abstract
Herpes stromal keratitis (HSK) is a prevalent and frequently vision-threatening disease associated with herpes simplex virus type 1 (HSV-1) infection. In mice, HSK progression occurs after viral clearance and requires T cells and neutrophils. One model implicates Th1-like CD4 T cells with cross-reactivity between the HSV-1 protein UL6 and a corneal autoantigen. HSK can be prevented by establishing specific immunological tolerance. However, HSK can also occur in T-cell receptor-transgenic X SCID mice lacking HSV-specific T cells. To study the pathogenesis of HSK in the natural host species, we measured local HSV-specific T-cell responses in HSK corneas removed at transplant surgery (n = 5) or control corneas (n = 2). HSV-1 DNA was detected by PCR in two specimens. HSV-specific CD4 T cells were enriched in three of the five HSK specimens and were not detectable in the control specimens. Reactivity with peptide epitopes within the tegument proteins UL21 and UL49 was documented. Responses to HSV-1 UL6 were not detected. Diverse HLA DR and DP alleles restricted these local responses. Most clones secreted gamma interferon, but not interleukin-5, in response to antigen. HSV-specific CD8 cells were also recovered. Some clones had cytotoxic-T-lymphocyte activity. The diverse specificities and HLA-restricting alleles of local virus-specific T cells in HSK are consistent with their contribution to HSK by a proinflammatory effect.
Herpes simplex keratitis (HSK) is a common and frequently vision-threatening disease. In the natural human host, interstitial inflammation leading to opacification of the light path occurs in 28 to 40% of persons with herpes simplex virus (HSV) infection of the corneal epithelium. Recovery of live virus from the corneal stroma, in the absence of epithelial ulceration, is uncommon, and antiviral therapy does little to prevent the progression from epithelial to stromal disease. In contrast, corticosteroids and cyclosporin A, compounds with antileukocyte effects, are therapeutic. These clinical observations and murine models have recently been reviewed (21, 37). Despite the lack of spontaneous HSV reactivation in mice, these models partially replicate human HSK in that initial productive viral infection is followed by leukocyte infiltration, virus clearance, and an interval of normal histology. A second phase of leukocyte infiltration and chronic inflammation then occurs in the absence of live virus. Hypotheses developed in these models are addressed here using human corneal specimens.
Murine models show that both virus and host factors are involved in the pathogenesis of HSK. Live virus scarification into the cornea is required. Inoculation into the anterior chamber leads to antigen-specific tolerance rather than immunopathology (18, 45). The conclusions from various murine models are widely disparate, however. In the congenic mouse strains C.AL-20 and C.B-17, which differ in susceptibility to HSK, an elegant three-way molecular mimicry has been shown to explain both susceptibility and resistance to HSK. Adult susceptible mice contain CD4 T cells, which cross-react between the HSV type 1 (HSV-1) protein UL6, amino acids 299 to 314, and a corneal autoantigen. Resistant mice express a protein (immunoglobulin G2ab [IgG2ab] heavy chain) which, at a polymorphic locus, contains a cross-reactive peptide epitope (amino acids 292 to 308) that leads to deletion of these T cells from the repertoire. Single Th1-like T-cell clones with this cross-reactive specificity mediate HSK upon passive transfer, and deletion viruses missing the pathogenic UL6 epitope fail to induce HSK. Treatment with either the viral or IgG2ab peptide in a manner which induces immunological tolerance prevents disease after virus challenge (3, 71). In marked contrast, Gangappa et al. have recently shown that T-cell receptor (TCR) transgenic mice, crossed onto a SCID background and treated to prevent acute virus dissemination and death, can develop HSK in the absence of any detectable HSV-specific CD4 immune response (17). Infiltration of transgenic CD4 T cells does occur, leading to a bystander damage hypothesis. Regardless of the specificity of the infiltrating T cells, several models have shown that local Th1-like cytokines, such as interleukin-2 (IL-2) and gamma interferon (IFN-γ) are associated with severe disease while local IL-10 can ameliorate disease (11, 22, 47).
We have therefore investigated the localization of HSV-specific CD4 T cells in human HSK, using tissue obtained at corneal transplantation. HSV-specific CD4 T cells are present and appear to be locally enriched in HSK. Reactivity with two HSV tegument proteins, but not UL6 of HSV-1, was documented. The local CD4 response to HSV differed in fine specificity and HLA restriction between patients. The cytokine profile was generally one of Th1-like cytokine secretion in response to antigen and antigen-presenting cells (APC). Unexpectedly, CD8 cells with proliferative and cytotoxic responses to HSV-1 were also recovered. These data are consistent with a pathogenic role for HSV-specific CD4 Th1 profile T cells. (Presented in part at Immunology 2000, Seattle, Washington, 12 to 16 May 2000.)
MATERIALS AND METHODS
Subjects and specimens.
Adult subjects participated in an institutional review board-approved protocol. The subjects required transplantation of their native corneas for HSK (experimental subjects) or other diseases (control). One-half of each excised cornea was collected in T-cell medium (TCM; RPMI 1640 with 25 mM HEPES, 1% penicillin-streptomycin, 2 mM l-glutamine, and 10% heat-inactivated human serum) with 50 μM acyclovir.
Diagnostic tests.
Serum was evaluated for HSV-1 and HSV-2 antibodies by immunoblotting (2). The half cornea not studied in vitro was submitted for routine pathology. DNA (Qiagen tissue kit) from a portion of the cornea was tested for HSV DNA by real-time PCR. Positive samples were typed by PCR with type-specific primers (53). A portion was homogenized and cultured for HSV (48).
Cell lines and clones.
The bulk of the available cornea was minced into 15 to 20 pieces. Half were cultured in a 0.96-cm2 dish in TCM with 7.5 × 105 irradiated (3,300 rad) peripheral blood mononuclear cells (PBMC), 0.8 μg of purified phytohemagglutinin (PHA) (Murex)/ml, and 50 μM acyclovir. IL-2 (32 U/ml; Hemagen) was added at 48 h. The cells were fed with IL-2 every other day and expanded for 2 to 3 weeks to yield bulk cultures. To generate clones, half the fragments were cultured in TCM with IL-2 alone for 4 days. Emigrating cells were separated from tissue pieces and cloned at three 10-fold dilutions using PHA and IL-2 as described previously (30). The set of plates yielding less than 37% of cultures with visible growth at 2 weeks were considered to be clonal (60). The clones were expanded by a modification of published methods (4) using anti-CD3 monoclonal antibody (MAb), feeders, and IL-2. To test the antigenicity of home-made HSV antigen preparations (see below), the genital-lesion-derived HSV-2-reactive CD4 T-cell clones 4.2E1, 2.3, and ESL2.20 (31) were used for UL49, UL50, and UL21, respectively, while the genital-lesion-derived clone ESL2.2 (33a) was used for UL19. Long-term B-cell lines (B-LCL) were established with Epstein-Barr virus and maintained in fetal calf serum based LCL medium as described previously (30). HLA-typed B-LCL (http://www.ebi.ac.uk/imgt/hla/) were kindly provided by Gerald Nepom and John Hansen.
Viruses and viral antigens.
HSV-1 strain E115 and HSV-2 strain 333 were grown and titers were determined on Vero cells as described previously (30). Glycoproteins B and D of HSV-2 (gB2 and gD2) expressed in CHO cells and VP16 of HSV-2 (the product of the UL48 gene) expressed in baculovirus were provided by Rae Lyn Burke and Michael Tigges, Chiron Corporation. To express the HSV proteins we recently described as CD4 T-cell antigens (31), the full-length HSV-2 UL21, UL49, and UL50 genes were amplified from HSV-2 strain HG52 DNA by PCR with appropriate primers and a proofreading thermostable DNA polymerase. The HSV-2 proteins UL19 and US8 (gE) were also shown to be CD4 T-cell antigens by using expression cloning and lesion- or cervix-derived T-cell clones (unpublished data) and were also cloned. The full-length gene for UL6 from HSV-1 was amplified from strain 17 (39). The primers were as follows: UL19 5′, TATGGTACCGAACCCAATGGCCGCTCCTG (AccI); UL19 3′, TGCTCTAGATTACAGAGACAGGCCCTTTA (XbaI); UL21 5′, CTGGGATCCATGGAGCTCAGCTATGCCACC (BamHI); UL21 3′, CGCGAATTCTCACACAGACTGGCCGTGCTG (EcoRI); UL49 5′, CCGGAATTCTTGTCTGTCGTCTGAACGCG (EcoRI); UL49 3′, GGAAGATCTACCTCTCGCCGCTCCGTCA (BglII); UL50 5′, CCGGAATTCATGAGTCAGTGGGGGCCC (EcoRI); UL50 3′, AAACTGCAGCTATCTTCCAGTGGAGCCAAACCCC (PstI); US8 5′, CGGGGTACCTGCTCGCGGGGCCGGGTTGGTG (KpnI); US8 3′, TGCTCTAGAGCCTTACCAGAGGACGGACGG (XbaI); UL6 HSV-1 5′, CTAGGATCCATGACCGCACCACGCTCG (BamHI); and UL6 HSV-1 3′, TGCTCTAGATCATCGTCGGCCGTCGCG (XbaI). (Restriction sites are underlined.) Similarly, to prepare truncated versions of the protein encoded by gene UL21 of HSV-2, PCR primers were designed spanning the region of interest and a plasmid encoding the full-length gene was used as a template. The PCR products were gel purified and digested with restriction endonucleases corresponding to tail sequences inserted into the PCR primers.
The genes were cloned into the member of the pcDNA3.1-his ABC series (Invitrogen, Carlsbad, Calif.) suitable for in-frame expression with vector leader peptide, with the exception of UL49 of HSV-2, which was cloned into pEGFP-C1 (Clontech, Palo Alto, Calif.); in-frame fusion was confirmed for each HSV gene by sequencing. DNA prepared with Qiagen low-endotoxin kits was transfected with Fugene 6 (Boehringer Mannheim) into Cos-7 cells in 100-cm2 dishes according to the manufacturer's protocol. After 48 h, the cells were scraped and sonicated, and low-speed supernatants and pellets (reconstituted to 1 ml/dish) were used as antigens. Peptides were synthesized on a Symphony/Multiplex (Rainin Instruments) running 9-fluorenylmethoxy carbonyl chemistry and dissolved in dimethyl sulfoxide to 1 to 2 mg/ml prior to use.
Lymphocyte functional assays.
Screening proliferation assays were performed in duplicate in 96-well U-bottom plates. The APC were either 2.5 × 105 HSV-1- or mock-infected (multiplicity of infection, 5; overnight) autologous B-LCL/well that were gamma (8,000 rad) and UV (10 min at 10 cm from a new GT038 bulb) irradiated, or 105 autologous, gamma-irradiated (3,300 rad) PBMC/well plus either UV-treated whole HSV-1 antigen or control Vero cell preparation (30). A final volume of 200 μl of TCM was used. After 3 days, 1 μCi of [3H]thymidine was added, and the cells were counted in a TopCount (Packard) scintillation counter. Clones with stimulation indices (mean counts per minute of [3H]thymidine incorporation for HSV-1 divided by mean control counts per minute) greater than 10 in at least two assays were considered positive. Triplicate confirmatory and work-up assays, including cytokine secretion assays, used PBMC as APC and inactivated HSV-1 antigen. If the clone had a stimulation index of greater than 10 for whole UV-treated HSV-2 antigen, as well as HSV-1, it was considered to have type-common reactivity. Cytokine secretion assays used fetal calf serum-based LCL medium rather than human serum-based medium. From these wells, supernatants were saved for cytokine enzyme-linked immunosorbent assay (ELISA) prior to the addition of [3H]thymidine. To establish HLA-restricting loci, supernatants of hybridoma cells secreting anti-HLA-DR MAb L243, anti-DP B7.21, and anti-DQ SPVL3 were included in proliferation assays at 1:4 final dilutions as described previously (30). The controls in proliferation assays were mock-infected Vero cells for HSV-1 antigen, Cos-7 cells transfected with empty vector for recombinant HSV proteins, or dimethyl sulfoxide for peptides. Some results are listed as delta cpm [3H]thymidine incorporation, equal to mean thymidine incorporation for viral antigen minus mean thymidine incorporation for control antigen.
Proliferation assays to establish the antigenicity of HSV-2 protein preparations made by transient transfection were performed similarly, using autologous or HLA-matched irradiated PBMC as APC.
To estimate the frequencies of precursors of cells with proliferative responses to HSV-1 whole-virus antigen in the peripheral blood, a limiting-dilution assay was performed on PBMC from three subjects. The method reported in our previous publications for HSV-2 (29, 49) was used with UV-treated HSV-1 strain E115 antigen at a 1:1,000 final concentration. Results were analyzed by chi-square minimization (60) and are reported as the estimated frequency and 95% confidence intervals using a computer program (7) kindly provided by C. Orosz and written by L. Sirinek, Ohio State University, Columbus.
Triplicate cytotoxicity assays in 96-well U-bottom plates used autologous HSV-1-infected (multiplicity of infection, 10; overnight) or uninfected B-LCL as target cells. The targets were loaded with 1 μM peptide for 90 min at 37°C for some assays. The purified anti-HLA class I MAb W6/32 (5) was included in some assays at 10 μg/ml. Specific release was calculated as described previously (30). Spontaneous release was generally less than 20% of total release.
HLA typing.
HLA typing for alleles of the DRB1, DRB3, DRB4, DRB5, and DQB1 loci was performed by the Immunogenetics Laboratory at the Puget Sound Blood Center. High-resolution typing was performed by PCR amplification with sequence-specific oligonucleotide primers. Exon 2 of DPB1 was sequenced using a direct automated-sequencing method (52). Raw sequencing data was reviewed manually, and alleles were assigned using alignments with known DPB1 sequences.
Flow cytometry.
Lymphocytes were washed and stained with a fluorochrome-labeled MAb specific for CD4, CD8, CD3, a combination of CD16 and CD56, TCR αβ, or TCR γδ, or isotype controls (Sigma or Becton Dickenson) as described previously (33).
ELISA.
Supernatants from proliferation assays (see above) were saved at −80°C until they were assayed. Cytokine levels were measured by standard sandwich ELISA format using matched capture-detection antibody pairs from the following sources: Endogen (IFN-γ, IL-5, and IL-10) and R&D Systems (MIP-1α). Assay sensitivities were in the range of 0.2 to 0.6 pg/ml for IFN-γ, 5 to 10 pg/ml for IL-5, 10 to 76 pg/ml for IL-10, and 30 pg/ml for MIP-1α. For all assays, false-positive signals (due to human anti-mouse antibody interference) were inhibited by the addition of nonspecific mouse IgG to the assay buffer.
RESULTS
Native corneas from five subjects with HSK and two control subjects were studied. All seven persons were HSV-1 seropositive and HSV-2 seronegative. Four of the five subjects were receiving suppressive oral antiviral therapy at the time of surgery, and none was receiving immunosuppressive therapy or undergoing active flares of HSK. The control subjects had Fuch's dystrophy, an idiopathic dysfunction of the corneal endothelium, or disturbed vision from laser corneal surgery. HSK specimens from subjects 9599 and 9679, obtained while the patients were receiving suppressive antiviral therapy, were positive for HSV-1 DNA by PCR (Table 1). HSV cultures were negative for all specimens evaluated. Lymphocyte clones (3 to 96) were obtained from each subject by culturing cells emigrating from the minced cornea under limiting-dilution conditions.
TABLE 1.
Characteristics of subjects donating corneal specimensa
| Subject no. | Age, sexb | Serologyc | Duration of HSKd | Antiviral at surgerye | HSV PCR | HSV culture | DRB1 | DPB1 | DQB1 |
|---|---|---|---|---|---|---|---|---|---|
| 9447 | 47, F | HSV-1 | 5 | FAM | NDf | Negative | 1601 | 0502 | 0401 |
| 0701 | 0303 | ||||||||
| 9599 | 60, F | HSV-1 | 18 | ACV | Positive | Negative | 1101 | 0301 | |
| 0701 | 0303 | ||||||||
| 9679 | 18, M | HSV-1 | 5 | VAL | Positive | Negative | 0403 | 0302 | 0402 |
| 0407 | |||||||||
| 9752 | 32, M | HSV-1 | 4 | ACV | Negative | Negative | 03011 | 0201 | |
| 1301 | 0603 | ||||||||
| 9772 | 40, M | HSV-1 | 3 | VAL | Negative | Negative | 1501 | 0602 | |
| 1303 | 0301 | ||||||||
| Controls | |||||||||
| 9840 | 47, M | HSV-1 | S/p laser surgery | None | Negative | Negative | ND | ND | ND |
| 9841 | 82, M | HSV-1 | Fuch's dystrophy | None | Negative | Negative | ND | ND | ND |
Diagnostic tests were performed as described in Materials and Methods.
Age in years. F, female; M, male.
Sera were tested for antibodies to HSV-1 and HSV-2. All study sera contained only antibodies to HSV-1.
Years of clinical diagnosis of HSK prior to corneal transplant. For controls, the diagnosis requiring transplantation is given. S/p, status post.
Medications were given orally at standard doses for at least 1 month prior to surgery. FAM, famciclovir; ACV, acyclovir; VAL, valacyclovir.
ND, not done.
T-cell clones or lines with HSV-specific proliferative responses were obtained from four of five HSK specimens (Table 2). Between 3 and 27% of the clones were HSV specific, using a criterion of a stimulation index of 10 or greater in at least two assays. For HSK subject 9752, only three clones were available and no HSV-specific responses were detected. No HSV-specific T-cell clones were recovered from the control corneas among 101 clones screened. The precursor frequency of cells with proliferative responses to inactivated HSV-1 antigen in the peripheral blood was measured for three HSK subjects by limiting-dilution assays. Between 1 in 2,808 and 1 in 6,663 PBMC responded to HSV-1.
TABLE 2.
Frequencies and phenotypes of T-cell clones from cornea specimens with HSV-specific proliferative responses and precursor frequencies of PBMC with HSV-1-specific proliferative responses
| Subject no. | No. of clones screened | No. (percent) of clones positive | No. CD4+/CD8− | No. CD4−/CD8+ | No. HSV-1 type specific/type commonc | pProlif, PBMCd |
|---|---|---|---|---|---|---|
| 9447a | 72 | 2 (3) | 2b | 0/2 | 1/3,298 (1/1,213–1/5,983) | |
| 9599 | 15 | 4 (27) | 0 | 4 | NDe | ND |
| 9679 | 66 | 8 (12) | 8 | 8/0 | 1/6,663 (1/5,290–1/9,201) | |
| 9752 | 3 | 0 (0) | ND | |||
| 9772 | 72 | 16 (22) | 15 | 1 | 8/2 | 1/2,808 (1/2,339–1/4,401) |
| Controls | ||||||
| 9840 | 5 | 0 (0) | ND | |||
| 9841 | 96 | 0 (0) | ND |
Initial screening assay for this subject used autologous, HSV-1-infected B-LCL as APC. Confirmatory assays used PBMC.
One clone was initially a mixed population of CD4+/CD8− and CD4−/CD8+ cells, but CD4+/CD8− subclones were derived that maintained HSV-specific proliferative responses to UL21 (see the text).
Stimulation indices of >10 required for reactivity (see Materials and Methods). For subject 9772, not all clones were evaluated.
Estimated precursor frequency within peripheral blood mononuclear cells of cells with proliferative responses to whole UV-treated HSV-1 antigen (95% confidence intervals in parentheses) (see Materials and Methods).
ND, not done.
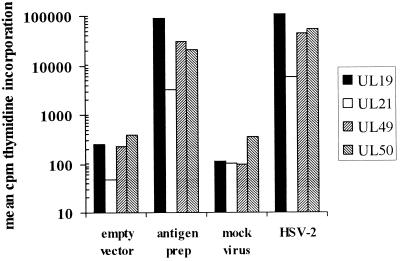
As expected from HSV sequence data (13, 41), some T-cell clones were type common, reacting with both HSV-1 and HSV-2, while others were type specific, reacting only with HSV-1. Among 20 clones evaluated, 16 (80%) were type specific for HSV-1. The type-common clones were evaluated with a panel of recombinant HSV-2 proteins. In addition to the published antigens gB2, gD2, VP16, UL21, UL49, and UL50 (30, 31), we also included the major capsid protein UL19 and glycoprotein E, encoded by gene US8. The last two proteins were shown by expression cloning to be recognized by genital HSV-2 lesion and cervix-derived CD4 T-cell clones, respectively (unpublished data). Antigens initially recovered as fragments of HSV-2 proteins during expression cloning (UL21, UL29, and UL50 [31] and UL19 and US8) were used as full-length recombinant proteins. Antigen preparations made by transient transfection of primate cells were shown to be highly antigenic in preliminary assays using [3H]thymidine proliferation of the index T-cell clones (Fig. 1). For UL19, UL21, UL49, and UL50 of HSV-2, these antigen preparations were used at a final dilution of 1:100, shown to be highly antigenic in preliminary [3H]thymidine proliferation assays using lesion-derived CD4 T-cell clones (reference 31 and unpublished data) as responders and autologous or HLA-matched PBMC as APC. Appropriate APC were not available to test the HSV-2 US8 protein preparation. For the HSV-1 UL6 antigen preparation, no reactive CD4 T-cell clones were available for testing. All antigen preparations were made on the same day using the same method, and in-frame fusion with the vector-derived fusion peptide was confirmed in each case by sequencing.
FIG. 1.
Validation of HSV-2 protein preparations as antigens capable of driving proliferative responses by CD4 T-cell clones. The responses measured are [3H]thymidine incorporation by HSV-specific CD4 T-cell clones derived from genital HSV-2 lesions as described in Materials and Methods and previously shown to be specific for the indicated proteins. Antigen preparations made by transient transfection of Cos-7 cells with HSV gene-containing or empty vector were used at a final concentration of 1:100. UV-treated mock virus or HSV-2 was used at a final concentration of 1:100.
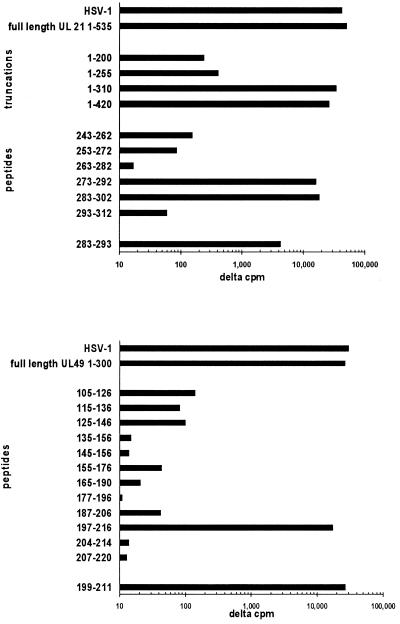
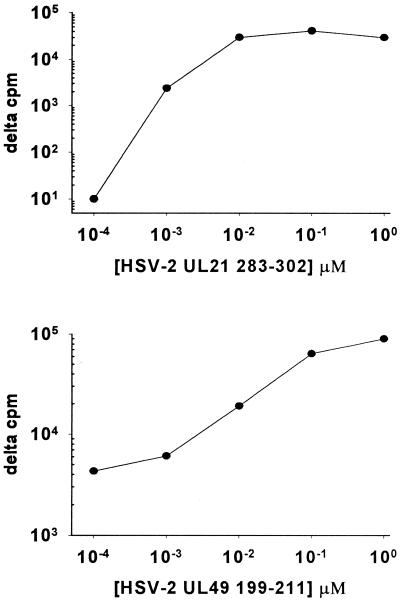
Reactivity with tegument proteins UL21 and UL49 was detected for T-cell clones recovered from subject 9447 (Fig. 2). Clone 9447.28 was reactive with full-length UL49 of HSV-2. Synthetic HSV-2 peptides from the midregion of the protein (31) were used to locate the epitope to HSV-2 197 to 216, and directed truncations identified HSV-2 199 to 211 (KRVFCAAVGRLAA) as an antigenic fragment. The corresponding HSV-1 epitope 198 to 210 has an identical sequence (13, 41). This epitope is distinct from the three CD4 T-cell epitopes in the midregion of UL49 recognized by genital HSV-2 lesion-derived clones (31). The 50% effective concentration in proliferation assays for the HSV-2 199 to 211 epitope was in the 10- to 100-nM range (Fig. 3).
FIG. 2.
Proliferative responses of cornea-derived CD4 T-cell clones from subject 9447 to defined HSV antigens. The measures are means of triplicate net [3H]thymidine incorporation compared to control antigen. (Top) Clone 9447.72 responds to full-length UL21 of HSV-2. Truncated proteins allow localization of the epitope to approximately amino acids 250 to 300. Synthetic peptides localize the epitope to amino acids 283 to 293. (Bottom) Clone 9447.28 responds to full-length UL49 of HSV-2. The epitope is localized to amino acids 199 to 211, corresponding to amino acids 198 to 210 of UL49 of HSV-1.
FIG. 3.
Dose-response curves for proliferative responses of cornea-derived CD4 T-cell clone 9447.28 to peptide UL49 (HSV-2) 199 to 211 and clone 9447.72 to UL21 (HSV-2) 283 to 302.
Clone 9447.72 was reactive with full-length UL21 of HSV-2. This clone originally was a mixture of CD4−/CD8+ and CD4+/CD8− cells by flow cytometry. Therefore, subclones were made which were CD4+/CD8− and retained proliferative responses to full-length UL21 of HSV-2. A series of truncations were made to localize the epitope to approximately amino acids 250 to 300, followed by synthesis of internal peptides. Peptides 273 to 292 and 283 to 302 and overlap HSV-2 peptide 283 to 293 (RELWWVFYAGD) stimulated proliferation. The corresponding overlap HSV-1 peptide, UL21 285 to 295, has an almost identical sequence (RELWWVFYAAD). The 50% effective concentration for peptide 283 to 302 was in the 1- to 10-nM range (Fig. 3). The type-common clones from the other HSK subjects did not react with our panel of HSV-2 proteins, indicating that additional specificities are present.
Each HSV-reactive T-cell clone was evaluated for reactivity with recombinant UL6 of HSV-1 using autologous PBMC as APC. The gene-cloning and antigen preparation conditions were identical to those used for UL21 and UL49 of HSV-2 and were also validated for UL19 and UL50 of HSV-2 (Fig. 1). No clones with stimulation indices of greater than 2.0 were detected. We also evaluated bulk cultures of cornea-derived T cells for reactivity with UL6, and again no reactivity was detected. No reactivity for any of the clones was detected for gB2, gD2, VP16, UL19, UL50, or US8 of HSV-2 (all stimulation indices were less than 2.0). Bulk cultures from subject 9679 were reactive with gB2, consistent with the presence of gB-specific type-common T cells in this population.
At least five HLA class II alleles restrict the HSV-specific CD4 T-cell clones from subjects 9447, 9679, and 9772. The HLA-restricting allele for clone 9447.28 is HLA DRB1*1601 (Table 3), as proliferation to autologous APC plus antigen was inhibited by anti-DR MAb and partially matched KAS011 APC bearing HLA DRB1*1601-presented peptide antigen. While the inhibition by anti-DR was only 31%, it was greater than that seen with anti-DP or anti-DQ, and KAS011 cells express DQB1*0502 and DPB1*0401 and *1401 and are thus not matched with subject 9447 at either of these loci. The peptide antigen contains the DRB1*1601 binding motif (http://134.2.96.221/), making it most likely that this is the presenting allele. The HLA-restricting allele for clone 9447.72 is DPB1*0401, as proliferation to autologous APC plus antigen was inhibited by anti-DP MAb and the subject is homozygous for this allele. Clone 9679.66 was inhibited by anti-DR MAb, while proliferation of the other clones from 9679 was inhibited by anti-DP MAb. This subject is heterozygous for the closely related (40) DRB1*0403 and -0407 alleles and homozygous for DPB1*0402. The HLA-restricting allele for clone 9772.45 and other clones from this subject (not shown) is DRB1*1303, again defined using anti-class II MAb and partially matched APC.
TABLE 3.
HLA-restricting loci and alleles of selected HSV-reactive CD4 T-cell clonesa
| Clone | Expt 1 (determination of restricting HLA locus)
|
Expt 2 (determination of restricting HLA allele)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No MAb | α-DR | α-DP | α-DQ | Autologous | Partial match 1 | Delta cpm | Partial match 2 | Delta cpm | |
| 9447.28 | 25,289 | 17,588 | 29,552 | 31,534 | 72,745 | KAS011 DRB1*1601 | 67,851 | PITOUT DRB1*0701 | 0 |
| 9447.72 | 28,851 | 30,138 | 7,955 | 28,089 | 9,652 | HERFLUFF DPB1*0401 | 4,040 | ARENT DPB1*2001 | 932 |
| 9679.20 | 55,922 | 41,043 | 1,540 | 47,485 | |||||
| 9772.45 | 22,578 | 1,963 | 10,317 | 13,332 | 3,061 | HAG DRB1*1303 | 6,996 | EB5491 DRB1*1501 | 0 |
Data for experiment 1 are mean delta counts per minute [3H]thymidine incorporation in comparison to mock viral antigen, autologous PBMC as APC, and no MAb. For experiment 2, several APC and antigen were used. For clone 9447.28, the APC are autologous or HLA-typed LCL, the antigen is 1 μg of peptide UL49 of HSV-2 199-211/ml, and the data are thymidine incorporation as described above in comparison to LCL plus 1% DMSO. As an additional control, proliferation for T-cell clone, peptide, and no APC was 44 cpm. For clone 9447.72, the APC are similar, the antigen is 1:20-diluted Cos-7 cell sonicate after transfection with UL21 (HSV-2) DNA as described in Materials and Methods, and the data are thymidine incorporation as described above in comparison to LCL plus sonicate of cells transfected with empty vector. For clone 9772.45, the APC-antigen combination reagent was autologous or HLA-typed LCL infected with HSV-1 and irradiated as described in Materials and Methods, and the data are thymidine incorporation in comparison to uninfected APC. Restricting alleles are in boldface. Partially matched APC are from http://www.ebi.ac.uk/imgt/hla/ except EB5491, which is in house. Subject 9679 is homozygous for DPB1*0402.
The cytotoxic activity of CD4 HSV-specific T-cell clones was examined in detail (Table 4). Knowledge of the peptide epitopes within UL21 and UL49 for CD4 T-cell clones from subject 9447 allowed us to check targets pulsed with a high concentration (1 μM) of peptide, as well as HSV-1 infected targets. Cytotoxicity was only detectable when a very high effector-to-target ratio (200:1) and peptide loading was used for the UL49-specific clone. For both clones, strong antigen-specific proliferative responses were present in assays performed on the same day with the same effector cells (data not shown). Larger panels of CD4 clones from subjects 9679 and 9772 were also tested. Using an arbitrary cutoff of greater than 10% specific release at an effector-to-target ratio of 20:1, neither of the clones from subject 9447, three of four clones (75%) from subject 9772, and one of two clones from subject 9679 demonstrated cytotoxic-T-lymphocyte (CTL) activity.
TABLE 4.
Cytotoxic activity of cornea-derived CD4+ HSV-reactive T-cell clones against autologous B-LCL
| Clone | % Specific release for Target B-LCLa
|
||
|---|---|---|---|
| Mock infected | HSV-1 infected | Peptide pulsed (1 μM) | |
| 9447.28 | 0 | 0.1 | 1.3 |
| 9447.72 | 1.6 | 0 | 2.1 |
| 9447.72b | 1.4 | 0 | 16.6 |
| 9772.22c | 0 | 0 | NA |
| 9772.33 | 0 | 35.1 | NA |
| 9772.34 | 0 | 18.1 | NA |
| 9772.48 | 0 | 19.6 | NA |
| 9679.20c | 0 | 1.3 | NA |
| 9679.66 | 0 | 13.6 | NA |
Data are percent specific release at an effector-to-target ratio of 20:1. Results of less than zero are reported as zero. NA, not applicable.
Effector-to-target ratio was 200:1 for this assay only.
Representative CD4+ clones from subjects 9679 and 9772 with no CTL activity (<10% specific release for HSV-1 infected targets).
The cytokine secretion profiles of CD4+ clones from subjects 9447, 9679, and 9772 were studied using autologous APC and mock or HSV-1 antigen (Table 5). None of the T-cell clones made detectable amounts of IL-5 or IL-10 in response to APC and mock virus antigen. Clones 9679.34 and 9679.66 made less than 10 pg of IFN-γ/ml under control conditions. All clones made significant amounts of IFN-γ after specific stimulation with HSV-1 antigen. Clone 9679.66 made IL-5, but the remaining clones from this subject and from the other two subjects did not. Secretion of IL-10 was noted for most clones from subjects 9447 and 9679 but was low or absent in clones from subject 9772. Clones from subject 9772 were analyzed for secretion of MIP-1α. An increment of specific secretion by clone, APC, and antigen in comparison to clone, APC, and mock antigen was noted for each clone. For MIP-1α, but not the other cytokines, substantial secretion was also detected with mock antigen (Table 5).
TABLE 5.
Cytokine secretion by HSV-specific CD4+-T-cell clones from HSK corneal specimens
| Clone | Amt secreted
|
||||
|---|---|---|---|---|---|
| IFN-γ | IL-5 | IL-10 | MIP-1α (net HSV-1-induced)a | MIP-1α (control)a | |
| 9447.28 | 705 | 0 | 1,415 | ND | |
| 9447.72 | 2,213 | 0 | ND | ND | |
| 9679.1 | 2,316 | 0 | 316 | ND | |
| 9679.20 | >3,000 | 0 | >2,000 | ND | |
| 9679.22 | >3,000 | 0 | 236 | ND | |
| 9679.29 | 1,725 | 0 | 329 | ND | |
| 9679.34 | >3,000 | 0 | 536 | ND | |
| 9679.66 | >3,000 | 161 | 694 | ND | |
| 9772.1 | 2,605 | 0 | 22.3 | 15,126 | 6,145 |
| 9772.2 | 1,209 | 0 | 7.3 | 11,293 | 5,256 |
| 9772.9 | 973 | 0 | 0 | 12,671 | 5,132 |
| 9772.20 | 3,023 | 0 | 27.4 | 44,811 | 4,352 |
| 9772.33 | 505 | 0 | 0 | 6,683 | 7,472 |
| 9772.34 | 184 | 0 | 0 | 3,060 | 6,601 |
| 9772.45 | 1,985 | 0 | 28.3 | 24,217 | 7,170 |
| 9772.48 | 74.9 | 0 | 10.5 | 2,166 | 5,863 |
| 9772.59 | 567 | 0 | 0 | 11,823 | 5,177 |
| 9772.68 | 79 | 0 | 0 | 3,444 | 4,454 |
| 9772.71 | 767 | 0 | 0 | 22,429 | 6,493 |
Most clones secreted substantial amounts of MIP-1α upon incubation with autologous PBMC as APC and mock antigen (right column). The net increment observed above this value upon incubation with UV HSV-1 antigen is reported. ND, not done.
Most clones with HSV-specific proliferative responses were CD3+, CD4+, and CD8−. However, some clones obtained from subjects 9599 and 9772 were CD3+, CD4−, CD8+, CD16−, CD56−, and TCR αβ+ (Table 6). Stimulation indices and delta CPM values were typically lower than for CD4+ clones. Cytotoxic activity (not shown) and secretion of IFN-γ in response to antigen were generally low for these clones.
TABLE 6.
Functional responses of CD8 T-cell clones recovered from HSK corneas
| Clone | Antigen (cpm)a
|
IFN-γ (pg/ml)b | |
|---|---|---|---|
| Mock | HSV-1 | ||
| 9599.9 | 4,063 ± 3,017 | 19,847 ± 4,092 | 120 |
| 9599.10 | 1,357 ± 363 | 27,657 ± 6,159 | 115 |
| 9599.11 | 108 ± 36 | 10,859 ± 3,066 | 105 |
| 9599.14 | 504 ± 152 | 7,726 ± 884 | 50 |
| 9772.7 | 263 ± 58 | 3,956 ± 591 | <2 |
Mean and standard deviation of triplicate [3H]thymidine incorporation.
Net IFN-γ release in picograms per milliliter at 72 h in response to UV HSV-1 antigen and PBMC as APC; mean of triplicates.
DISCUSSION
More than 50% of adults are infected with HSV-1. The virus is latent in the trigeminal ganglia, and most infections are asymptomatic (8, 9). Among symptomatic persons, most have orolabial infection, and only a minority suffer from corneal infection. The virus or host factors contributing to corneal infection are unknown. There are an estimated 50,000 new cases of HSV corneal infection per year in the United States, with peak incidence in the third and fourth decades, well after the peak age of acquisition of HSV-1 infection (36, 37).
The complex nomenclature of HSV corneal syndromes (25, 37) emphasizes division by the anatomical layer(s) involved and the extent of active viral replication. Most cases begin as superficial epithelial infections. For unknown reasons, many patients progress to interstitial keratitis (36, 37). The risk factors for disease progression are unknown. Progression from epithelial to interstitial keratitis is not prevented by suppressive antiviral therapy (23, 24). Once the infection is established, local or systemic corticosteroids and cyclosporin A (19, 34) are therapeutic, consistent with a pathogenic role for infiltrating leukocytes.
The pathogenesis of HSK has been intensively studied in inbred mice, generating several hypotheses which are difficult to evaluate in the natural host. At polar extremes are models in which strict molecular mimicry (driven by the HSV-1 protein UL6) causes the disease (71) and models in which HSK occurs without HSV-specific T-cell responses (17). Several intermediary models implicate local HSV-specific CD4 Th1-like T-cell responses (14, 21, 46, 47, 57). These cells could directly damage corneal cells through cytotoxicity, mediate autoimmunity by release of normally sequestered autoantigens, or recruit damaging leukocytes via secretion of cytokines. The local inflammatory response overcomes local immune-suppressive effects mediated by corneal Fas ligand expression and secreted factors, such as IL-10 and transforming growth factor β (61).
HSV-specific CD4 T-cell clones were recovered at relatively high frequencies from HSK tissue. Overall, 25 of 230 (10.9%) clones from HSK corneas were HSV-specific CD4 T cells. In our studies to date, none of the 101 clones from control corneas from HSV-1-infected persons was HSV specific. There are scattered reports of recovery of HSV-1 DNA (10, 28) from apparently normal corneas. It is possible that local HSV antigen could elicit local HSV-specific responses, which might not cause clinically overt keratitis. Neither of the control corneas in our small series were PCR positive for HSV-1. Possibly, autoreactive T cells that might be present in other inflammatory corneal diseases could cross-react with UL6 or other HSV-1 proteins, leading to the detection of HSV-specific responses even in the absence of HSV-1 infection in the subject. We plan to test this possibility by studying additional control tissues from HSV-1-infected and uninfected persons, including subjects with corneal inflammatory diseases, to confirm the disease association between HSK and local HSV-specific CD4 T cells.
The frequency of T cells with proliferative responses to HSV-1 in the peripheral blood of HSV-1-infected persons is on the order of 0.2 to 0.01% (1/500 to 1/104). Measurements using precursor frequency analysis in limiting-dilution proliferation assays, by ELISPOT, or by intracellular flow cytometry after stimulation with whole viral antigen and staining for IFN-γ have generally been in this range, and these responders are mostly CD4 cells (1, 7, 29, 50, 54, 55). We were able to study three of our HSK subjects, and their values were in this range. We were unable to estimate the precursor frequency of HSV-specific cells in the cornea by the same method used for the blood due to the very small number of lymphocytes in our specimens and the difficulty of extracting them for immediate ex vivo analysis. It is possible that some cell division occurred among the HSV-specific CD4 T cells emigrating from corneal fragments during the 4 days prior to initiation of clonal cultures. However, the magnitude of the difference between our results for the cornea and peripheral blood (Table 2) argues for spatial enrichment in HSK tissues.
We were unable to document proliferative responses to the HSV-1 protein UL6 among bulk or cloned T cells recovered from five subjects with HSK. Our results are similar to those recently reported by Verjans et al. (64), who evaluated bulk cornea-derived lymphocyte lines from 12 subjects with HSK (necrotizing and nonnecrotizing variants) and found no reactivity with HSV-1 UL6 expressed in vaccinia virus. The antigenicity of our UL6 protein is unproven, as we do not have a positive control CD4 T-cell clone reactive with UL6. However, each of the four evaluable, similar preparations containing HSV-2 proteins, and the series of truncations of UL21 of HSV-2, were highly antigenic in our system when tested with CD4 T-cell clones and PBMC as APC. UL6 was cloned using a DNA polymerase with proofreading function, reducing the likelihood of a protein-coding error, and both ends of the vector–viral-gene junction were checked by sequencing. Possibly, UL6-reactive CD4 T cells initiate HSK, sensitizing cross-reactive T cells capable of recognizing a corneal autoantigen. Since our subjects had HSK for many years, the UL6-reactive cells might no longer be present or might persist locally at a level below our threshold of detection. Additional work will be required to rule out reactivity with UL6 in human HSK. The corneal autoantigen in the murine system (3, 71) has not been defined, so it cannot be determined if the homologous human antigen shares molecular cross-recognition with UL6.
It is possible that corneal autoantigens homologous to proteins other than UL6 are involved in human HSK. A search of the database for human-expressed proteins homologous to the UL21 and UL49 epitopes defined here has not led to any obvious homologues. However, HSV-reactive (32) and other CD4 T cells tolerate extensive amino acid substitutions, and sophisticated computation and library methods have been required to uncover microbial/self mimicries (20). Reactivity with identical or overlapping peptides in different subjects, if found in future studies, will focus the search for cross-reactive human epitopes. It is also possible that CD8 T-cell cross-reactivity between HSV and self antigens could be present. Extensive CD8 reactivity with insulin in a murine diabetes model has rekindled interest in these effector cells in autoimmune diseases (66). Our screening methodology for corneal T-cell clones used proliferation but not CTL assays, and thus would have missed typical CD8 CTL clones. The infiltrate in HSK may include both CD4 and CD8 T cells (59, 69, 70). We hope to incorporate immunohistology and CTL screening in future studies.
The UL21 epitope found in this study (HSV-2 283 to 293, corresponding to HSV-1 285 to 295) is distinct from the region recognized by a DR-restricted clone from a genital lesion (HSV-2 148 to 81) (31). We previously identified three different CD4 T-cell epitopes in the midregion of UL49 of HSV-2 (31), and we identify a fourth epitope here. UL49 of HSV-1 (and HSV-2) exhibits facilitated intercellular spread in vitro after virus infection or transfection (15) and is of interest as a delivery molecule in gene therapy (12). In common with other gene delivery agents, host immune responses occur which may limit the use of this viral protein. The abundance of UL49 in virions (35) and its facilitated intercellular spread could contribute to its antigenicity. As noted by others (56), caution is required with vaccines for HSV-2 and HSV-1 to minimize elicitation of potentially harmful immunologic reactions in tissues such as the cornea.
Functional studies of corneal lymphocytes in human HSK are limited. Verjans et al. derived HSV-specific CD4 T-cell clones from the corneas of two subjects with ulcerative necrotizing HSK. Both had HSV DNA detected by PCR, although cultures were negative. The disease-specific enrichment of HSV-specific CD4 T cells was difficult to determine, since the cells were expanded in bulk prior to cloning and no control corneas were included. HLA-restricting alleles and antigenic specificity were not determined, but TCR sequencing and limited HSV type specificity studies showed that a fairly limited number (two to four) of discrete clones were recovered per subject. These clones had a Th0-like pattern of cytokine secretion, producing IL-2, IFN-γ, and IL-4 and IL-5 (65). Our subjects, in contrast, had nonnecrotizing HSK. The available data are consistent with a Th1-like secretion pattern. Secretion of IL-5 by clone 9679.66 in response to antigen indicates that this cytokine is readily measurable in our assay. The diversity of the local response in our subjects awaits performance of detailed TCR-sequencing experiments. The diversity of HSV corneal syndromes and the probable contribution of virus replication to the necrotizing variants (34, 37) emphasizes the importance of correlating the precise clinical history and pathologic findings with in vitro T-cell studies.
Neutrophils, and chemokines associated with neutrophil chemotaxis, are required for HSK in mice (62). One chemoattractant for neutrophils is the C-C chemokine MIP-1α. MIP-1α-deficient mice have normal clearance of HSK after corneal inoculation, followed by HSK which is clinically milder than that seen in controls (63). Both tissue cells and infiltrating leukocytes contribute to ocular chemokine synthesis in mice (6). Herpetic vesicle fluid in humans is enriched in MIP-1α and MIP-1β (44). IFN-γ and IL-2 may also participate in attracting and maintaining neutrophils by upregulation of adhesion molecules, such as PECAM-1 (58), and prolonging neutrophil survival (27). Possibly, HSV-reactive T cells, such as those isolated in the present work, contribute to this inflammatory reaction.
HSV-specific CD4 T-cell clones derived from PBMC (68) and genital lesions (30) are frequently cytotoxic towards HSV-infected B-LCL and can also have cytotoxic activity towards interferon-treated, HSV-infected keratinocytes (42, 43). Endogenously synthesized HSV antigen appears to be able to access the HLA class II antigen-processing pathway, as reported for other viruses (38). Cytotoxic activity towards HSV-infected cells is heterogeneous between specific HSV-reactive CD4 clones. It is not known if differential access of endogenously synthesized viral proteins to the HLA class II antigen-processing pathway controls the presentation of antigen and thus the measured CTL activity by CD4 clones. However, when B-LCL APC were optimally loaded with antigen by exogenous addition of high concentrations (1 μM) of peptide, every HSV-specific CD4 T-cell clone in our previous studies exerted clear cytotoxicity at moderate effector-to-target ratios (31). It was therefore somewhat surprising that the CD4 tegument-specific clones from subject 9447 had very weak to absent CTL activity against peptide-pulsed targets. HSV-specific CD4 CTL have recently been shown to use granule-based killing mechanisms (67). Cytotoxicity could be quite harmful in the eye, and cytotoxic CD4 clones were recovered from two of our five subjects. Further study will be required to determine the molecular basis for the heterogeneity in CTL activity between T-cell clones.
The detection of proliferative responses among CD8+ clones to UV-treated whole HSV-1 virus as antigen, and irradiated PBMC as APC, indicates that exogenous antigen is capable of accessing the antigen-processing pathway used to present peptide to these cells. Similar CD8 proliferative responses have been reported previously among PBMC-derived T-cell clones (26). Cytotoxicity in these clones was weak. Antigen-specific CD8 T cells with weak-to-absent cytotoxicity have been described in other systems and hypothesized to have regulatory functions (16, 51). Full assessment of these CD8 T cells will require the determination of their restricting HLA alleles, peptide specificities, and cytokine secretion profiles.
In summary, our data are consistent with local enrichment of HSV-1-reactive CD4 T cells in end-stage HSK in humans, the natural host for this viral agent. A variety of HSV proteins are recognized by these local T cells, including tegument proteins previously shown to be antigenic in genital HSV-2 lesions. Several different HLA-restricting loci and alleles are used by these clones, consistent with a diverse ocular immune response to HSV within the population. Similar to genital HSV-2 infection, local CD4 responses to viral tegument proteins were detected. Reactivity with the HSV-1 protein UL6 was not detected in our limited panel of subjects. Further studies are required to elucidate the contributions of virus and host factors to the pathogenesis of HSK in humans.
ACKNOWLEDGMENTS
This work was supported by NIH grants CA70017 and AI30731 and by a Howard Hughes Medical Institute Pilot Research Initiative and a grant from the University of Washington Royalty Research Fund.
Gerald Nepom and John Hansen supplied HLA-typed B-LCL, and Rae Lyn Burke and Michael Tigges supplied HSV-2 proteins. Meei-Li Huang performed HSV PCR, and the Virology Laboratory at Children's Hospital and Medical Center, Seattle, Wash., under the direction of Rhoda L. Ashley, performed HSV serology and culture. Matthew Wavra provided valuable technical assistance.
REFERENCES
- 1.Asanuma H, Sharp M, Maecker H T, Maino V C, Arvin A M. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus and cytomegalovirus determined by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–866. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 2.Ashley R A, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunoblot for detecting antibodies to herpes simplex types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery A C, Zhao Z-S, Rodriguez A, Bikoff E K, Soheilian M, Foster C S, Cantor H. Resistance to herpes stromal keratitis conferred by an IgG2a-derived peptide. Nature. 1995;376:431–434. doi: 10.1038/376431a0. [DOI] [PubMed] [Google Scholar]
- 4.Brodie S J, Lewinsohn D A, Patterson B K, Jiyamapa D, Krieger J, Corey L, Greenberg P D, Riddell S R. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky F M, Parham P. New aspects of HLA serology and biochemistry defined using monoclonal antibodies. In: Ferrone S, Solheim B G, editors. HLA typing: methodology and clinical aspects. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1982. pp. 23–38. [Google Scholar]
- 6.Chen W, Tang Q, Hendricks R L. Ex vivo model of leukocyte migration into herpes simplex virus-infected mouse corneas. J Leukoc Biol. 1996;60:167–173. doi: 10.1002/jlb.60.2.167. [DOI] [PubMed] [Google Scholar]
- 7.Clouse K A, Adams P W, Orosz C G. Enumeration of viral antigen-reactive helper T lymphocytes in human peripheral blood by limiting dilution for analysis of viral antigen-reactive T-cell pools in virus-positive and virus-negative individuals. J Clin Microbiol. 1989;27:2316–2323. doi: 10.1128/jcm.27.10.2316-2323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey L, Spear P G. Infections with herpes simplex viruses. N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. , 749–757. [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Wald A. Genital Herpes. In: Holmes K K, Sparling P F, Mardh P A, Lemon S M, Stamm W E, Piot P, Wasserheit J M, editors. Sexually transmitted diseases. 3rd ed. New York, N.Y: McGraw-Hill, Inc.; 1999. pp. 285–312. [Google Scholar]
- 10.Crouse C, Pflugfelder S C, Perieria I E, Rabinowitz S S, Levine J A, Atherton S S. Detection of herpes viral genomes in normal and disease corneal epithelium. Curr Eye Res. 1990;9:569–574. doi: 10.3109/02713689008999597. [DOI] [PubMed] [Google Scholar]
- 11.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse B T. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid encoding IL-10. J Immunol. 1997;159:1945–1952. [PubMed] [Google Scholar]
- 12.Dilber M S, Phelan A, Aints A, Mohamed A J, Elliott G, Smith C I E, O'Hare P. Intercellular delivery of the thymidine kinase prodrug activating enzyme by the herpes simplex virus protein VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- 13.Dolan A, Jamieson F E, Cunningham C, Barnett B C, McGeoch D J. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doymaz M Z, Rouse B T. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Investig Ophthalmol Vis Sci. 1992;33:2165–2167. [PubMed] [Google Scholar]
- 15.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–232. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 16.Erard F, Garcia-Sanz J A, Moriggl R, Wild M T. Presence or absence of TGF-beta determines IL-4-induced generation of type 1 or type 2 CD8 T cell subsets. J Immunol. 1999;162:209–214. [PubMed] [Google Scholar]
- 17.Gangappa S, Deshpande S P, Rouse B T. Bystander activation of CD4 T cells can represent an exclusive means of immunopathology in a virus infection. Eur J Immunol. 1999;29:3674–3682. doi: 10.1002/(SICI)1521-4141(199911)29:11<3674::AID-IMMU3674>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Griffith T S, Yu X, Herndon J M, Green D R, Ferguson T A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1995;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 19.Heiligenhaus A, Steuhl K P. Treatment of HSV-1 stromal keratitis with topical cyclosporin A: a pilot study. Graefes Arch Clin Exp Ophthalmol. 1999;237:435–438. doi: 10.1007/s004170050257. [DOI] [PubMed] [Google Scholar]
- 20.Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus S E, McFarland H F, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks R L. Immunopathogenesis of viral ocular infections. Chem Immunol. 1999;73:120–136. doi: 10.1159/000058743. [DOI] [PubMed] [Google Scholar]
- 22.Hendricks R L, Tumpey T M, Finnegan A. IFN-γ and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 23.Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. Arch Ophthalmol. 1997;115:703–712. doi: 10.1001/archopht.1997.01100150705001. [DOI] [PubMed] [Google Scholar]
- 24.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 25.Holland E J, Schwartz G S. Classification of herpes simplex keratitis. Cornea. 1999;18:144–154. doi: 10.1097/00003226-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Inatsuki A, Yasukawa M, Kobayashi Y. The effect of human T cell leukemia type I infection on a herpes simplex virus-specific CD8+ cytotoxic T cell clone. Br J Hematol. 1991;77:311–314. doi: 10.1111/j.1365-2141.1991.tb08576.x. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman S, Heiligenhaus A, Rodriguez A, Soukiasian S, Dorf M E, Foster C S. Exacerbation of murine herpes simplex virus-mediated keratitis by Th2 type T cells. J Immunol. 1993;151:5777–5789. [PubMed] [Google Scholar]
- 28.Kay S B, Lynas C, Patterson A, Risk J M, McCarthy K, Hort C A. Evidence for herpes simplex virus latency in the human cornea. Br J Ophthalmol. 1991;75:195–198. doi: 10.1136/bjo.75.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koelle D M, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of HSV-specific T lymphocyte clones from human recurrent HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 30.Koelle D M, Corey L, Burke R L, Eisenberg R J, Cohen G H, Pichyangkura R, Triezenberg S J. Antigenic specificity of human CD4+ T cell clones recovered from recurrent genital HSV-2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koelle D M, Frank J M, Johnson M L, Kwok W W. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol. 1998;72:7476–7483. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koelle D M, Johnson M L, Ekstrom A N, Byers P, Kwok W W. Preferential presentation of herpes simplex virus T-cell antigen by HLA DQA1*0501/DQB1*0201 in comparison to HLA DQA1*0201/DQB1*0201. Hum Immunol. 1997;53:195–205. doi: 10.1016/S0198-8859(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 33.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Koelle, D. M., M. Schomogyi, C. McClurkan, S. N. Reymond, and H. B. Chen. 2000. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glyco-proteins. J. Virol. 74:xx–xx. [DOI] [PMC free article] [PubMed]
- 34.Leibowitz H M, Waring G O. Herpes simplex keratitis. In: Leibowitz H M, Waring G O, editors. Corneal disorders. 2nd ed. Philadelphia, Pa: W. B. Saunders Company; 1998. pp. 662–688. [Google Scholar]
- 35.Leslie J, Rixon F J, McLauchlan J. Overexpression of the herpes simplex virus type 1 tegument protein VP22 increases its incorporation into virus particles. Virology. 1996;220:60–68. doi: 10.1006/viro.1996.0286. [DOI] [PubMed] [Google Scholar]
- 36.Liesegang T J. Epidemiology of ocular herpes simplex. Natural history in Rochester, MN 1950 through 1982. Arch Ophthalmol. 1989;107:1160–1165. doi: 10.1001/archopht.1989.01070020226030. [DOI] [PubMed] [Google Scholar]
- 37.Liesegang T J. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18:127–143. doi: 10.1097/00003226-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Malnati M S, Marti M, LaVaute T, Jaraquemada D, Biddison W, DeMars R, Long E O. Processing pathways for presentation of cytosolic antigen to MHC class II-restricted T cells. Nature. 1992;357:702–704. doi: 10.1038/357702a0. [DOI] [PubMed] [Google Scholar]
- 39.Marsden H S, Crombie I K, Subak-Sharpe J H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature sensitive mutants of HSV strain 17. J Gen Virol. 1976;31:347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- 40.Marsh S G E, Parham P, Barber L D. The HLA FactsBook. San Diego, Calif: Academic Press; 2000. p. 346. [Google Scholar]
- 41.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 42.Mikloska A, Cunningham A L. Herpes simplex virus type 1 glycoproteins gB, gC, and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol. 1998;79:353–361. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 43.Mikloska A, Kesson A M, Penfold M E T, Cunningham A L. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-γ. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 44.Mikloska Z, Danis V A, Adams S, Lloyd A R, Adrian D L, Cunningham A L. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions—do herpes simplex virus-infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 45.Niederkorn J Y. Anterior chamber-associated immune deviation. Chem Immunol. 1999;73:59–71. doi: 10.1159/000058740. [DOI] [PubMed] [Google Scholar]
- 46.Niemialtowski M G, Rouse B T. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 47.Niemialtowski M G, Rouse B T. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 48.Peterson E P, Schmidt O W, Goldstein L C, Nowinski R C, Corey L. Typing of clinical HSV isolates using mouse monoclonal antibodies to HSV-1 and HSV-2: comparison with type-specific rabbit antisera and restriction endonuclease analysis of viral DNA. J Clin Microbiol. 1983;17:92–96. doi: 10.1128/jcm.17.1.92-96.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posavad C M, Koelle D M, Corey L C. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol. 1996;70:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posavad C M, Koelle D M, Shaughnessy M F, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired HSV-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohrer J W, Coggin J H. CD8 T cell clones inhibit antitumor T cell function by secreting IL-10. J Immunol. 1995;155:5719–5727. [PubMed] [Google Scholar]
- 52.Rozemuller E H, Chadwick B, Charron D, Baxter-Lowe A, Eliaou J F, Johnston-Dow L, Tilanus M G J. Sequenase sequence profiles used for HLA-DPB1 sequence-based typing. Tissue Antigens. 1996;47:72–79. doi: 10.1111/j.1399-0039.1996.tb02516.x. [DOI] [PubMed] [Google Scholar]
- 53.Ryncarz A J, Goddard J, Wald A, Huang M-L, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting HSV DNA in clinical samples. J Clin Microbiol. 1999;37:1941–1947. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmid D S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4, CD8 T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988;140:3610–3616. [PubMed] [Google Scholar]
- 55.Schmid D S, Thieme M L, Gary H E, Reeves W C. Characterization of T cell responses to herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) using a TNF-beta ELISpot cytokine assay. Arch Virol. 1997;142:1659–1671. doi: 10.1007/s007050050187. [DOI] [PubMed] [Google Scholar]
- 56.Stanberry L R, Cunningham A L, Mindel A, Scott L L, Spruance S L, Aoki F Y, Lacey C J. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis. 2000;30:549–566. doi: 10.1086/313687. [DOI] [PubMed] [Google Scholar]
- 57.Streilein J W, Dana M R, Ksander B R. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 58.Tang Q, Hendricks R L. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J Exp Med. 1996;184:1435–1447. doi: 10.1084/jem.184.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang S, Scheiffarth O F, Stefani F H. Clinical and immunohistochemical correlation of herpetic keratitis with the expression of HLA-DR antigen. Graefes Arch Clin Exp Ophthalmol. 1993;231:162–165. doi: 10.1007/BF00920940. [DOI] [PubMed] [Google Scholar]
- 60.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 61.Taylor A W. Ocular immunosuppressive microenvironment. Chem Immunol. 1999;73:72–89. doi: 10.1159/000058738. [DOI] [PubMed] [Google Scholar]
- 62.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 63.Tumpey T M, Cheng H, Cook D N, Smithies O, Oakes J E, Lausch R N. Absence of macrophage inflammatory protein-1 alpha prevents the development of blinding herpes stromal keratitis. J Virol. 1998;72:3705–3710. doi: 10.1128/jvi.72.5.3705-3710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verjans G M G M, Remeijer L, Mooy C M, Osterhaus A D M E. Herpes simplex virus specific T-cells infiltrate the cornea of patients with herpes stromal keratitis: no evidence for intra-corneal autoreactive T-cells in human HSK. Investig Ophthalmol Vis Sci. 2000;41:2607–2612. [PubMed] [Google Scholar]
- 65.Verjans G M G M, Remeijer L, van Binnendijk R S, Cornelissen J G C, Volker-Dieben H J, Baarsma S G, Osterhaus A D M E. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis. 1998;177:484–488. doi: 10.1086/517382. [DOI] [PubMed] [Google Scholar]
- 66.Wong F S, Karttunen J, Dumont C, Wen L, Visintin I, Pilip I M, Shastri N, Pamer E G, Janeway C A. Identification of an MHC class I-restricted autoantigen in type I diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 67.Yasukawa M, Ohminami H, Yakushijin Y, Arai J, Hasegawa A, Ishida Y, Fujita S. Fas-independent cytotoxicity mediated by CD4+ CTL directed against herpes simplex virus-infected cells. J Immunol. 1999;162:6100–6106. [PubMed] [Google Scholar]
- 68.Yasukawa M, Zarling J M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA Class II MB and DR antigens. J Immunol. 1984;133:422–427. [PubMed] [Google Scholar]
- 69.Youinou P, Colin J, Ferec C. Monoclonal antibody analysis of blood and cornea T lymphocyte subpopulations in herpes simplex keratitis. Graefes Arch Clin Exp Ophthalmol. 1986;224:131–133. doi: 10.1007/BF02141485. [DOI] [PubMed] [Google Scholar]
- 70.Youinou P, Colin J, Mottier D. Immunologic analysis of the cornea in herpetic stromal keratitis. J Clin Lab Immunol. 1985;17:105–106. [PubMed] [Google Scholar]
- 71.Zhao Z S, Granucci F, Yeh L, Schaffer P A, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]