Abstract
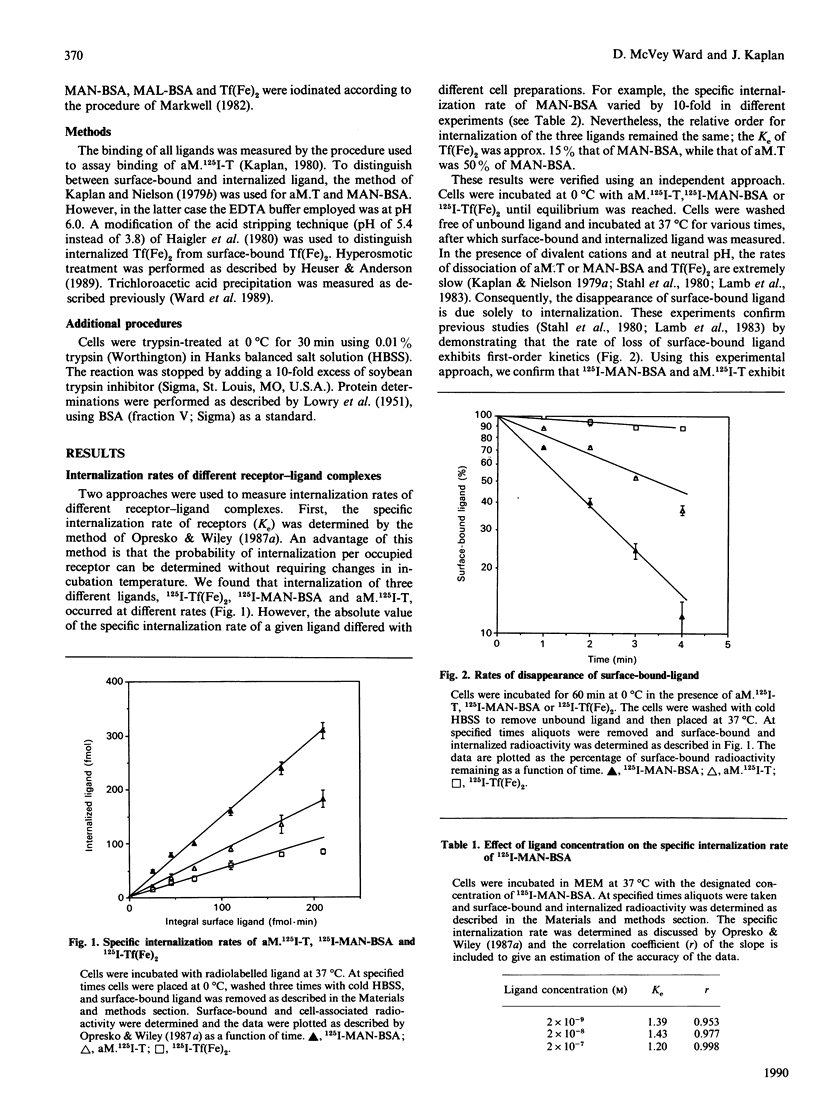
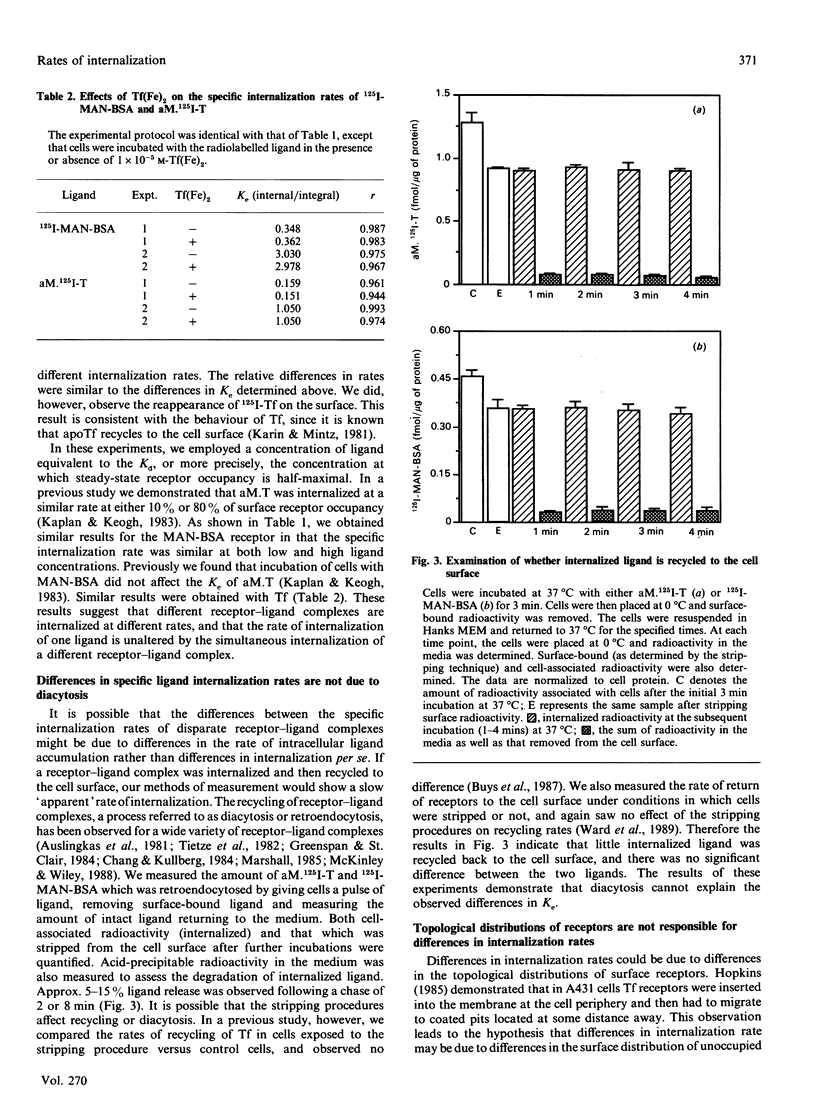
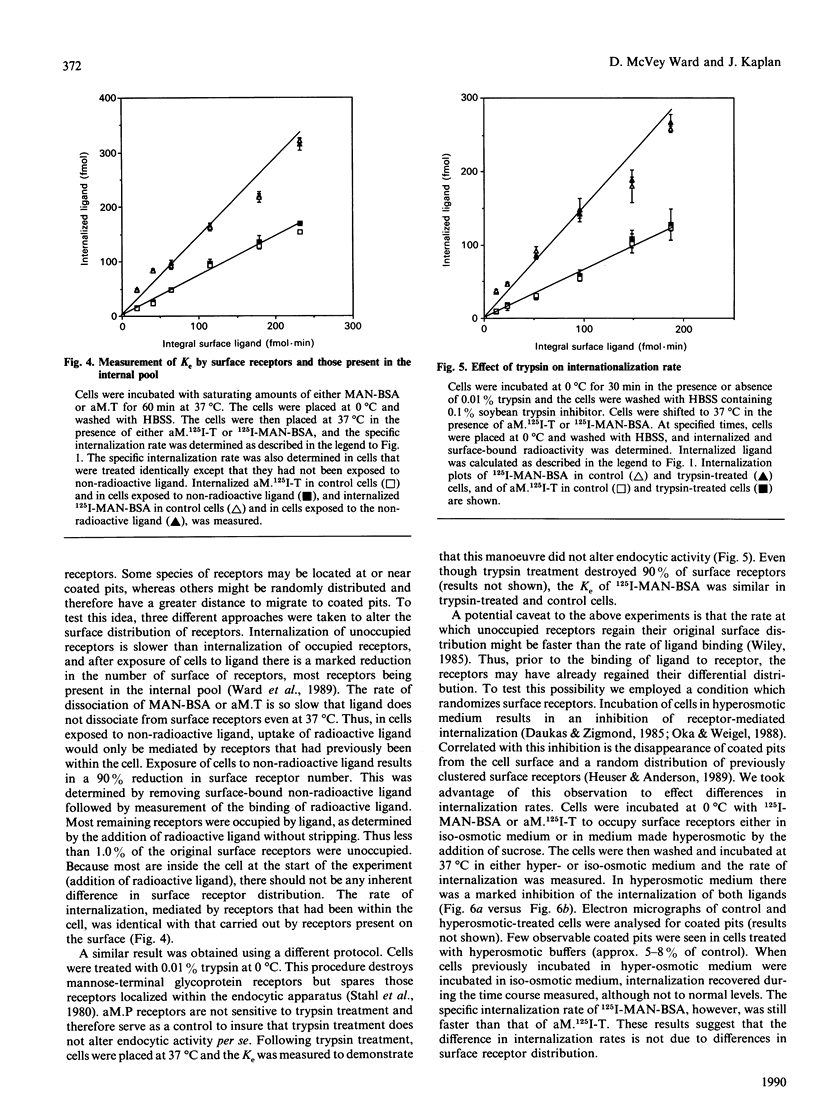
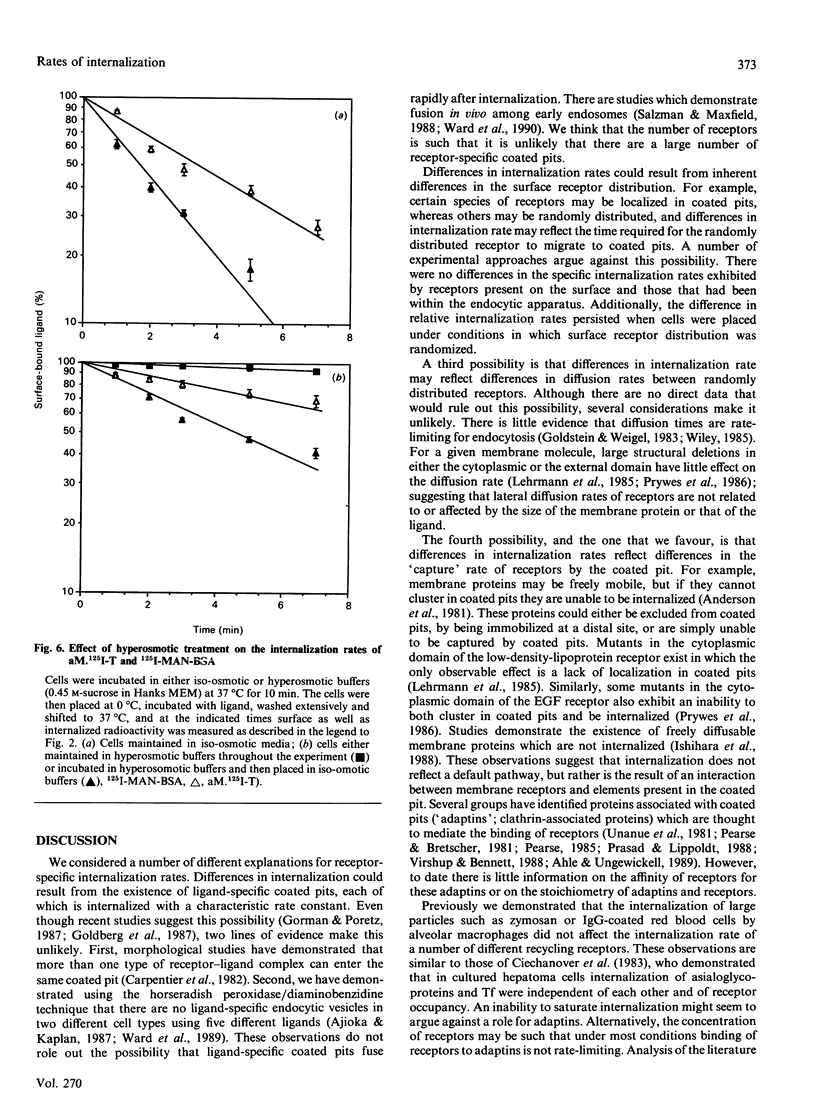
To probe the mechanisms of endocytosis in alveolar macrophages, we examined the internalization rates of three different receptors. Initial rates of internalization for mannosylated BSA, diferric transferrin and alpha-macroglobulin-proteinase complexes were all different. Although the absolute rates of internalization varied depending on the cell preparation, transferrin was internalized at 10-20% and alpha-macroglobulin-proteinase complex at 40-60% of the rate of manosylated-BSA. Incubation of cells with transferrin did not affect the rate of internalization of mannosylated BSA or alpha-macroglobulin-proteinase complexes, and the rates of internalization were independent of receptor occupancy. These different internalization rates could not be ascribed to different rates of diacytosis. Altering the distribution of unoccupied surface receptors by either trypsin treatment of cells at 0 degree C or exposure to hyperosmotic solutions resulted in the absolute internalization rates being affected by the experimental condition, but the hierarchy in receptor internalization rates was maintained. The fact that a variety of conditions affect receptor internalization rates to the same degree implies the existence of co-ordinate regulation at a single rate-limiting step. Based on these results, we suggest that differences in internalization rate reflect the ability of ligand-receptor complexes to be captured by coated pits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahle S., Ungewickell E. Identification of a clathrin binding subunit in the HA2 adaptor protein complex. J Biol Chem. 1989 Nov 25;264(33):20089–20093. [PubMed] [Google Scholar]
- Ajioka R. S., Kaplan J. Characterization of endocytic compartments using the horseradish peroxidase-diaminobenzidine density shift technique. J Cell Biol. 1987 Jan;104(1):77–85. doi: 10.1083/jcb.104.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Brown M. S., Goldstein J. L. Inefficient internalization of receptor-bound low density lipoprotein in human carcinoma A-431 cells. J Cell Biol. 1981 Feb;88(2):441–452. doi: 10.1083/jcb.88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulinskas T. H., van der Westhuyzen D. R., Bierman E. L., Gevers W., Coetzee G. A. Retro-endocytosis of low density lipoprotein by cultured bovine aortic smooth muscle cells. Biochim Biophys Acta. 1981 May 22;664(2):255–265. doi: 10.1016/0005-2760(81)90048-5. [DOI] [PubMed] [Google Scholar]
- Beisiegel U., Kita T., Anderson R. G., Schneider W. J., Brown M. S., Goldstein J. L. Immunologic cross-reactivity of the low density lipoprotein receptor from bovine adrenal cortex, human fibroblasts, canine liver and adrenal gland, and rat liver. J Biol Chem. 1981 Apr 25;256(8):4071–4078. [PubMed] [Google Scholar]
- Buys S. S., Gren L. H., Kaplan J. Phorbol esters and calcium ionophores inhibit internalization and accelerate recycling of receptors in macrophages. J Biol Chem. 1987 Sep 25;262(27):12970–12976. [PubMed] [Google Scholar]
- Buys S. S., Kaplan J. Effect of phagocytosis on receptor distribution and endocytic activity in macrophages. J Cell Physiol. 1987 Jun;131(3):442–449. doi: 10.1002/jcp.1041310317. [DOI] [PubMed] [Google Scholar]
- Buys S. S., Keogh E. A., Kaplan J. Fusion of intracellular membrane pools with cell surfaces of macrophages stimulated by phorbol esters and calcium ionophores. Cell. 1984 Sep;38(2):569–576. doi: 10.1016/0092-8674(84)90511-7. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. M., Kullberg D. W. Diacytosis of 125I-asialoorosomucoid by rat hepatocytes. A non-lysosomal pathway insensitive to inhibition by inhibitors of ligand degradation. Biochim Biophys Acta. 1984 Nov 13;805(3):268–276. doi: 10.1016/0167-4889(84)90082-x. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Daukas G., Zigmond S. H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Rosen O. M., Rubin C. S. Identification and characterization of a latent pool of insulin receptors in 3T3-L1 adipocytes. J Biol Chem. 1982 May 25;257(10):5350–5358. [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldberg R. I., Smith R. M., Jarett L. Insulin and alpha 2-macroglobulin-methylamine undergo endocytosis by different mechanisms in rat adipocytes: I. Comparison of cell surface events. J Cell Physiol. 1987 Nov;133(2):203–212. doi: 10.1002/jcp.1041330202. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Wiegel F. W. The effect of receptor clustering on diffusion-limited forward rate constants. Biophys J. 1983 Jul;43(1):121–125. doi: 10.1016/S0006-3495(83)84330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gorman R. M., Poretz R. D. Resolution of multiple endosomal compartments associated with the internalization of epidermal growth factor and transferrin. J Cell Physiol. 1987 May;131(2):158–164. doi: 10.1002/jcp.1041310204. [DOI] [PubMed] [Google Scholar]
- Greenspan P., St Clair R. W. Retroendocytosis of low density lipoprotein. Effect of lysosomal inhibitors on the release of undegraded 125I-low density lipoprotein of altered composition from skin fibroblasts in culture. J Biol Chem. 1984 Feb 10;259(3):1703–1713. [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Harford J., Bridges K., Ashwell G., Klausner R. D. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J Biol Chem. 1983 Mar 10;258(5):3191–3197. [PubMed] [Google Scholar]
- Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989 Feb;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R. The appearance and internalization of transferrin receptors at the margins of spreading human tumor cells. Cell. 1985 Jan;40(1):199–208. doi: 10.1016/0092-8674(85)90323-x. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Holifield B., Jacobson K. Analysis of lateral redistribution of a plasma membrane glycoprotein-monoclonal antibody complex [corrected]. J Cell Biol. 1988 Feb;106(2):329–343. doi: 10.1083/jcb.106.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Evidence for reutilization of surface receptors for alpha-macroglobulin.protease complexes in rabbit alveolar macrophages. Cell. 1980 Jan;19(1):197–205. doi: 10.1016/0092-8674(80)90401-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Keogh E. A. Studies on the physiology of macrophage receptors for alpha-macroglobulin X protease complexes. Ann N Y Acad Sci. 1983;421:442–456. doi: 10.1111/j.1749-6632.1983.tb18138.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. II. Internalization of alpha-macroglobulin . trypsin complexes by rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7329–7335. [PubMed] [Google Scholar]
- Kaplan J., Ray F. A., Keogh E. A. Recognition of nucleophile-treated alpha 2-macroglobulin by the alveolar macrophage alpha-macroglobulin . protease complex receptor. J Biol Chem. 1981 Aug 10;256(15):7705–7707. [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., FARISS B. Lysozyme content of alveolar and peritoneal macrophages from the rabbit. J Immunol. 1961 Feb;86:133–136. [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Marshall S. Dual pathways for the intracellular processing of insulin. Relationship between retroendocytosis of intact hormone and the recycling of insulin receptors. J Biol Chem. 1985 Nov 5;260(25):13524–13531. [PubMed] [Google Scholar]
- McKinley D. N., Wiley H. S. Reassessment of fluid-phase endocytosis and diacytosis in monolayer cultures of human fibroblasts. J Cell Physiol. 1988 Sep;136(3):389–397. doi: 10.1002/jcp.1041360302. [DOI] [PubMed] [Google Scholar]
- Oka J. A., Weigel P. H. Effects of hyperosmolarity on ligand processing and receptor recycling in the hepatic galactosyl receptor system. J Cell Biochem. 1988 Feb;36(2):169–183. doi: 10.1002/jcb.240360208. [DOI] [PubMed] [Google Scholar]
- Opresko L. K., Wiley H. S. Receptor-mediated endocytosis in Xenopus oocytes. I. Characterization of the vitellogenin receptor system. J Biol Chem. 1987 Mar 25;262(9):4109–4115. [PubMed] [Google Scholar]
- Opresko L. K., Wiley H. S. Receptor-mediated endocytosis in Xenopus oocytes. II. Evidence for two novel mechanisms of hormonal regulation. J Biol Chem. 1987 Mar 25;262(9):4116–4123. [PubMed] [Google Scholar]
- Pearse B. M. Assembly of the mannose-6-phosphate receptor into reconstituted clathrin coats. EMBO J. 1985 Oct;4(10):2457–2460. doi: 10.1002/j.1460-2075.1985.tb03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Prasad K., Lippoldt R. E. Molecular characterization of the AP180 coated vesicle assembly protein. Biochemistry. 1988 Aug 9;27(16):6098–6104. doi: 10.1021/bi00416a040. [DOI] [PubMed] [Google Scholar]
- Presky D. H., Schonbrunn A. Receptor-bound somatostatin and epidermal growth factor are processed differently in GH4C1 rat pituitary cells. J Cell Biol. 1986 Mar;102(3):878–888. doi: 10.1083/jcb.102.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Livneh E., Ullrich A., Schlessinger J. Mutations in the cytoplasmic domain of EGF receptor affect EGF binding and receptor internalization. EMBO J. 1986 Sep;5(9):2179–2190. doi: 10.1002/j.1460-2075.1986.tb04482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Intracellular fusion of sequentially formed endocytic compartments. J Cell Biol. 1988 Apr;106(4):1083–1091. doi: 10.1083/jcb.106.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaloff D. L., Ascoli M. Removal of the surface-bound human choriogonadotropin results in the cessation of hormonal responses in cultured Leydig tumor cells. J Biol Chem. 1981 Nov 25;256(22):11420–11423. [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Ungewickell E., Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981 Nov;26(3 Pt 1):439–446. doi: 10.1016/0092-8674(81)90213-0. [DOI] [PubMed] [Google Scholar]
- Virshup D. M., Bennett V. Clathrin-coated vesicle assembly polypeptides: physical properties and reconstitution studies with brain membranes. J Cell Biol. 1988 Jan;106(1):39–50. doi: 10.1083/jcb.106.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M., Ajioka R., Kaplan J. Cohort movement of different ligands and receptors in the intracellular endocytic pathway of alveolar macrophages. J Biol Chem. 1989 May 15;264(14):8164–8170. [PubMed] [Google Scholar]
- Ward D. M., Hackenyos D. P., Kaplan J. Fusion of sequentially internalized vesicles in alveolar macrophages. J Cell Biol. 1990 Apr;110(4):1013–1022. doi: 10.1083/jcb.110.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Wiley H. S. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988 Aug;107(2):801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]


