Abstract
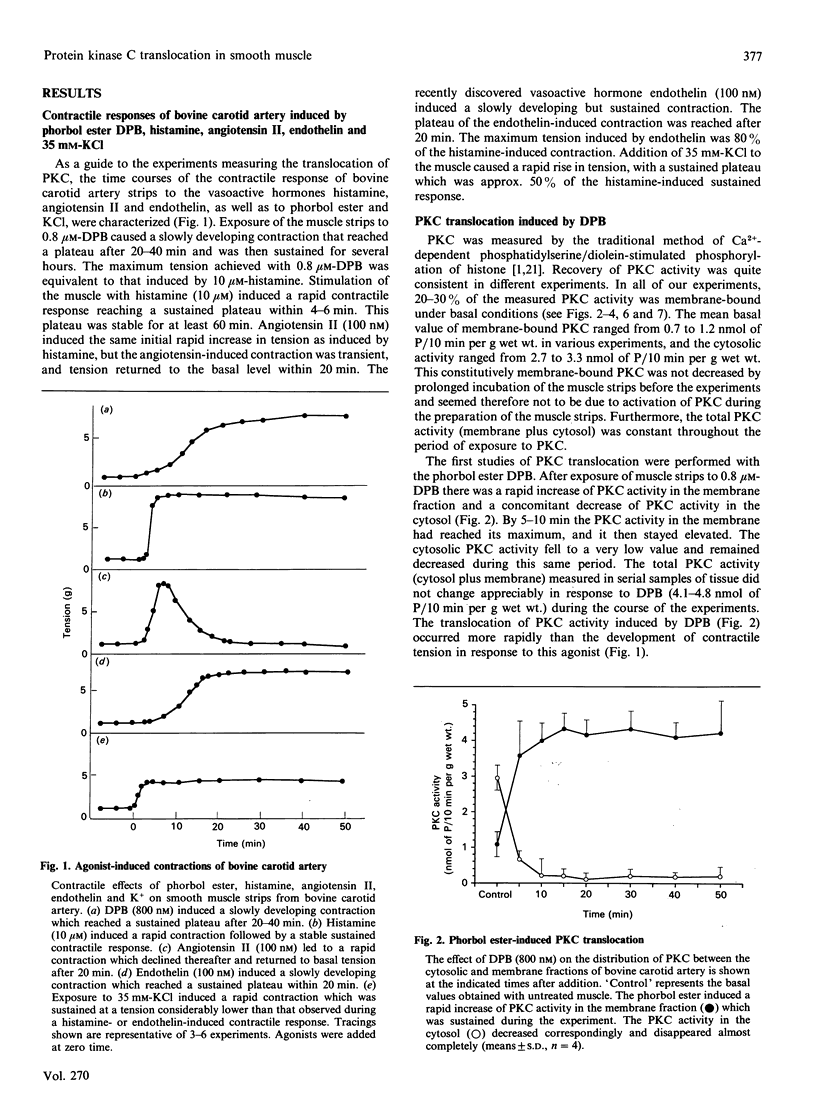
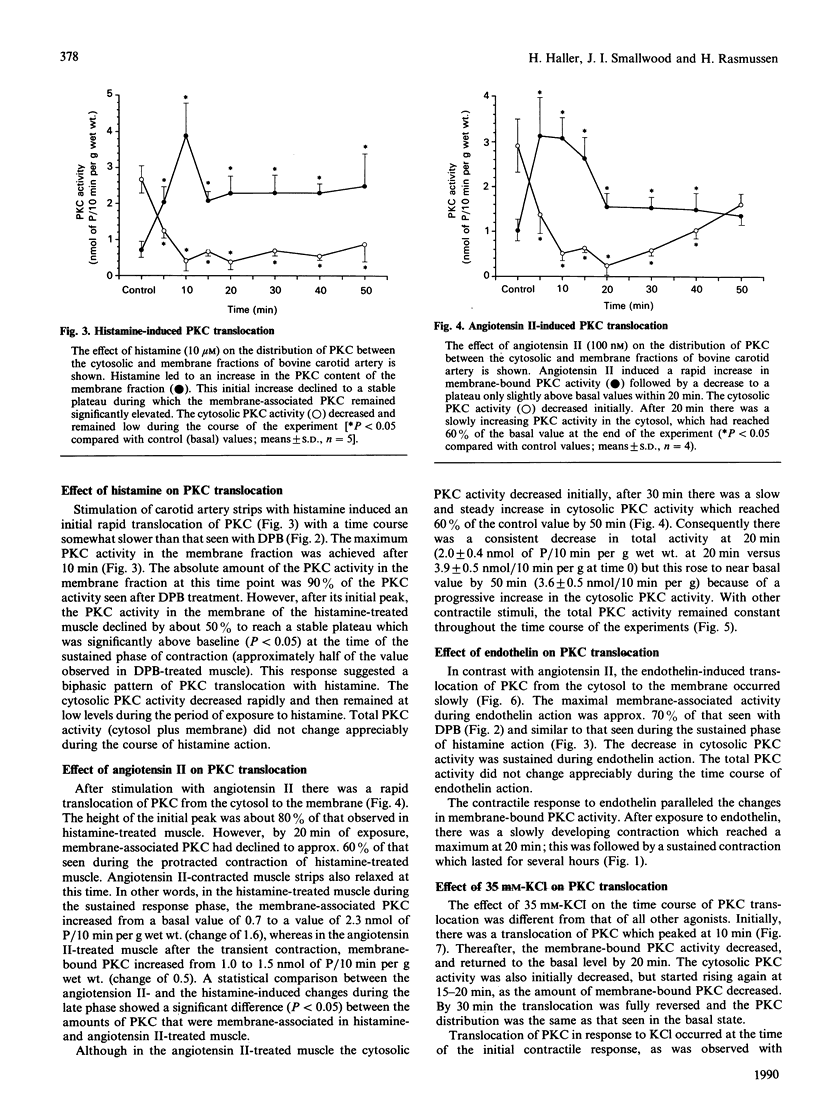
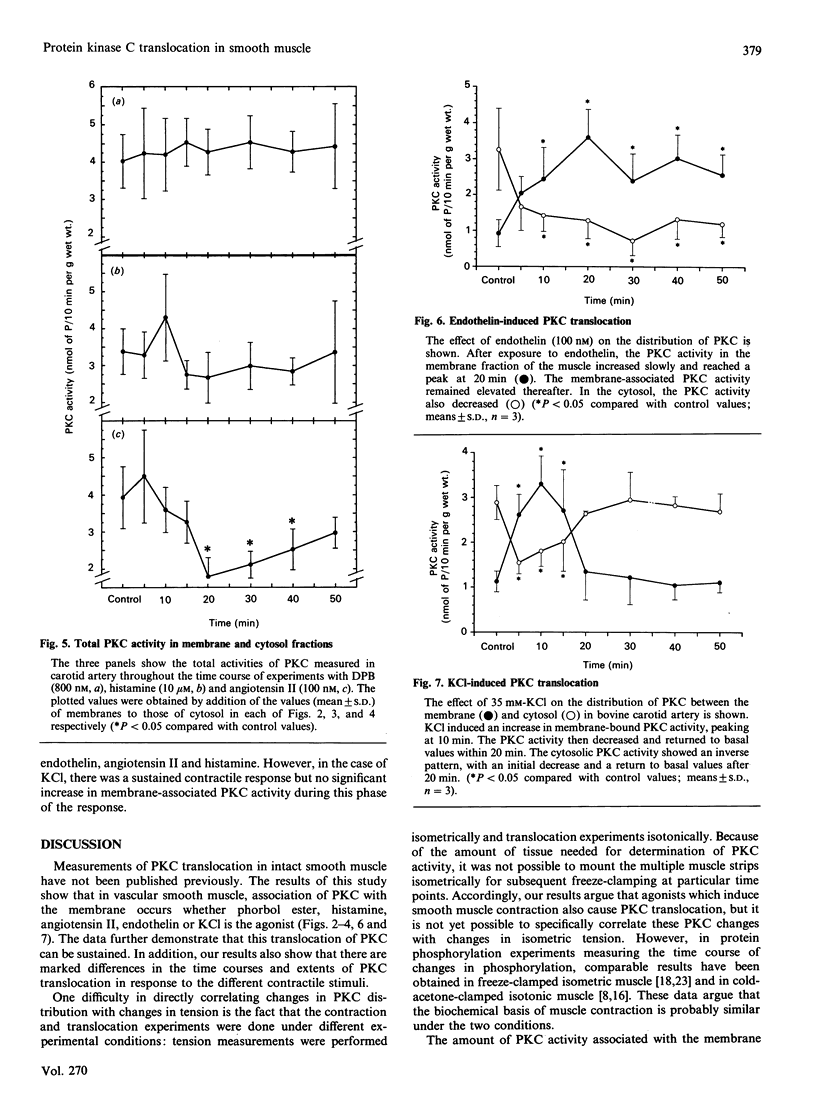
Using intact muscle strips from the bovine carotid artery, the time course of translocation of protein kinase C (PKC) from the cytosol to the membrane fraction was measured in response to various agonists that induce contractile responses. PKC activity was assessed by Ca2+/phospholipid-dependent phosphorylation of histone. Exposure of the muscle strips to phorbol ester (12-deoxyphorbol 13-isobutyrate) induced a rapid and sustained translocation of PKC from the cytosol to the membrane fraction, and a slowly developing but sustained contractile response. Histamine induced a comparable initial translocation of PKC to the membrane which then decreased somewhat to a stable plateau significantly above basal values. Histamine also led to a rapid and sustained increase in tension. Angiotensin I, which caused a rapid but transient contraction, induced a rapid initial translocation of PKC to the membrane. The membrane-associated PKC then declined to a stable plateau significantly lower than that seen after a histamine-induced response, and only slightly above the basal value. Endothelin, which induced a sustained contraction, caused a sustained translocation of PKC from the cytosol to the membrane. In contrast, although exposure to 35 mM-KCl induced a rapid and sustained contraction, it caused only a transient translocation of PKC; the membrane-associated PKC returned to its basal value within 20 min. These results demonstrate that PKC in intact smooth muscle can be rapidly translocated to the membrane and remains membrane-bound during sustained phorbol ester- or agonist-induced contractions, but that such a sustained translocation of PKC does not occur during prolonged stimulation with KCl.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Alexander R. W., Brock T. A., Gimbrone M. A., Jr, Rittenhouse S. E. Angiotensin increases inositol trisphosphate and calcium in vascular smooth muscle. Hypertension. 1985 May-Jun;7(3 Pt 1):447–451. [PubMed] [Google Scholar]
- Bank B., DeWeer A., Kuzirian A. M., Rasmussen H., Alkon D. L. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry N., Ase K., Kishimoto A., Nishizuka Y. Activation of resting human T cells requires prolonged stimulation of protein kinase C. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2294–2298. doi: 10.1073/pnas.87.6.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block L. H., Emmons L. R., Vogt E., Sachinidis A., Vetter W., Hoppe J. Ca2+-channel blockers inhibit the action of recombinant platelet-derived growth factor in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2388–2392. doi: 10.1073/pnas.86.7.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chatterjee M., Tejada M. Phorbol ester-induced contraction in chemically skinned vascular smooth muscle. Am J Physiol. 1986 Sep;251(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1986.251.3.C356. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Acute desensitization to angiotensin II: evidence for a requirement of agonist-induced diacylglycerol production during tonic contraction of rat aorta. Eur J Pharmacol. 1986 Jul 15;126(1-2):135–139. doi: 10.1016/0014-2999(86)90749-1. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Phorbol ester-induced contraction of arterial smooth muscle and inhibition of alpha-adrenergic response. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1103–1109. doi: 10.1016/0006-291x(84)91397-4. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Thomas T. P., Anderson W. B. Altered cytosol/membrane enzyme redistribution on interleukin-3 activation of protein kinase C. Nature. 1985 May 16;315(6016):235–237. doi: 10.1038/315235a0. [DOI] [PubMed] [Google Scholar]
- Forder J., Scriabine A., Rasmussen H. Plasma membrane calcium flux, protein kinase C activation and smooth muscle contraction. J Pharmacol Exp Ther. 1985 Nov;235(2):267–273. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Ho A. K., Thomas T. P., Chik C. L., Anderson W. B., Klein D. C. Protein kinase C: subcellular redistribution by increased Ca2+ influx. Evidence that Ca2+-dependent subcellular redistribution of protein kinase C is involved in potentiation of beta-adrenergic stimulation of pineal cAMP and cGMP by K+ and A23187. J Biol Chem. 1988 Jul 5;263(19):9292–9297. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Machado-de Domenech E., Söling H. D. Effects of stimulation of muscarinic and of beta-catecholamine receptors on the intracellular distribution of protein kinase C in guinea pig exocrine glands. Biochem J. 1987 Mar 15;242(3):749–754. doi: 10.1042/bj2420749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S., Pritchard K., Redwood C., Taggart M. Ca2+ regulation of the thin filaments: biochemical mechanism and physiological role. Biochem Soc Trans. 1988 Aug;16(4):494–497. doi: 10.1042/bst0160494. [DOI] [PubMed] [Google Scholar]
- McArdle C. A., Conn P. M. Hormone-stimulated redistribution of gonadotrope protein kinase C in vivo: dependence on Ca2+ influx. Mol Pharmacol. 1986 Jun;29(6):570–576. [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Activation of tracheal smooth muscle contraction: synergism between Ca2+ and activators of protein kinase C. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8835–8839. doi: 10.1073/pnas.82.24.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Phillips W. A., Fujiki T., Rossi M. W., Korchak H. M., Johnston R. B., Jr Influence of calcium on the subcellular distribution of protein kinase C in human neutrophils. Extraction conditions determine partitioning of histone-phosphorylating activity and immunoreactivity between cytosol and particulate fractions. J Biol Chem. 1989 May 15;264(14):8361–8365. [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Silver P. J., Stull J. T. Phosphorylation of myosin light chain and phosphorylase in tracheal smooth muscle in response to KCl and carbachol. Mol Pharmacol. 1984 Mar;25(2):267–274. [PubMed] [Google Scholar]
- Small J. V., Fürst D. O., De Mey J. Localization of filamin in smooth muscle. J Cell Biol. 1986 Jan;102(1):210–220. doi: 10.1083/jcb.102.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J. I., Gügi B., Rasmussen H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J Biol Chem. 1988 Feb 15;263(5):2195–2202. [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y., Kelley G., Takuwa N., Rasmussen H. Protein phosphorylation changes in bovine carotid artery smooth muscle during contraction and relaxation. Mol Cell Endocrinol. 1988 Nov;60(1):71–86. doi: 10.1016/0303-7207(88)90121-9. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Rasmussen H. Measurement of cytoplasmic free Ca2+ concentration in rabbit aorta using the photoprotein, aequorin. Effect of atrial natriuretic peptide on agonist-induced Ca2+ signal generation. J Clin Invest. 1987 Jul;80(1):248–257. doi: 10.1172/JCI113055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J Biol Chem. 1986 Nov 5;261(31):14670–14675. [PubMed] [Google Scholar]
- TerBush D. R., Bittner M. A., Holz R. W. Ca2+ influx causes rapid translocation of protein kinase C to membranes. Studies of the effects of secretagogues in adrenal chromaffin cells. J Biol Chem. 1988 Dec 15;263(35):18873–18879. [PubMed] [Google Scholar]
- Thomas T. P., Gopalakrishna R., Anderson W. B. Hormone- and tumor promoter-induced activation or membrane association of protein kinase C in intact cells. Methods Enzymol. 1987;141:399–411. doi: 10.1016/0076-6879(87)41086-0. [DOI] [PubMed] [Google Scholar]
- Trilivas I., Brown J. H. Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J Biol Chem. 1989 Feb 25;264(6):3102–3107. [PubMed] [Google Scholar]


