Abstract
Background:
Heart failure with preserved ejection fraction (HFpEF) is a multifactorial condition with a variety of pathophysiological causes and morphological manifestations. The inclusion criteria and patient classification have become overly simplistic due to the customary differentiation regarding the ejection fraction (EF) cutoff. EF is considered a measure of systolic function; nevertheless, it only represents a portion of the true contractile state and has been shown to have certain limits due to methodological and hemodynamic irregularities.
Methods:
As a result, broader randomized clinical trials have yet to incorporate the most recent criteria for HFpEF diagnosis, leading to a lack of data consistency and confusion in interpreting the results. The primary variations between the bigger clinical trials published in this context concerning patient selection and echocardiographic characteristics were analyzed. For all these reasons, we aim to clarify the main features and clinical impact of HFpEF in a study combining imaging, bio-humoral analysis, and clinical history to identify the specific subgroups that respond better to tailored treatment.
Results:
Disparate clinical characteristics and a lack of uniform diagnostic standards may cause suboptimal therapeutic feedback. To optimize treatment, we suggest shifting the paradigm from the straightforward EF measurement to a more comprehensive model that considers additional information, such as structural traits, related disorders, and biological and environmental data. Therefore, by evaluating certain echocardiographic and clinical factors, a stepwise diagnostic procedure may be useful in identifying patients at high risk, subjects with early HFpEF, and those with evident HFpEF.
Conclusions:
The present assessment underscores the significance of the precision medicine approach in guaranteeing optimal patient outcomes by providing the best care according to each distinct profile.
Keywords: HFpEF phenotype, treatment, SGLT2i, ARNIs, MRA, GLP-1
1. Introduction
Heart failure with preserved ejection fraction (HFpEF) affects around half of all heart failure (HF) cases, and throughout the past 20 years, this percentage has been rising [1, 2]. HFpEF prevalence in the adult population is around 1–3%, although it is increasing in the general global population [3]. Additionally, the 1-year mortality is about 6.3%, reaching 75.7% at 5 years, which is comparable to heart failure with reduced ejection fraction (HFrEF). While cardiovascular death remains the primary outcome for HF, non-cardiovascular causes are increasingly significant in HFpEF [4, 5, 6].
Compared to HF with impaired systolic function, HFpEF is regarded as a distinct entity with specific molecular vascular myocardial cell adaptations and a detailed pathophysiological profile (HFrEF) [7]. This evaluation is based on certain presumptions regarding the anatomy of the left ventricle (LV), the composition and structure of the heart, the geometry of the intrinsic fiber myocytes, the thickness of the vessels, and the effects of therapy [8]. Consequently, it is a diverse syndrome with a high comorbidity burden characterized by several related diseases and different clinical phenotypes. The considerable variety of enrollment criteria in the larger multicenter trials showed a lack of diagnostic consistency, and criteria uniformity is required for both the time course evaluation and the adopted treatment [9]. Understanding the underlying pathophysiological processes that generate hemodynamic changes and cardiovascular dysfunctions is crucial for addressing the optimized therapy according to different phenotypes. For example, patients with HFpEF and a prevalent coronary microvascular impairment as the main pathophysiological mechanism may benefit more from drugs acting on microcirculation that reduce coronary resistance, improving coronary blood flow. Alternatively, patients with HFpEF and prevalent extracellular fibrosis may benefit more from drugs with anti-fibrotic properties, which reduce collagen extracellular storage. Patients with early supraventricular tachyarrhythmias (SVTs) may benefit from early ablation procedures because recurrent supraventricular tachyarrhythmia (SVT) may trigger electrical disorder persistence and HFpEF progression. In conclusion, the emerging role of genetics and epigenetics may open new perspectives in HFpEF classification and treatment. The contrasting results of novel and conventional treatments emphasize the need to search for a more suitable mechanistic strategy based on more customized therapy that incorporates data on lifestyle, genetic, biochemical, environmental, and cardiovascular (CV) risk factors [10]. This review aims to revise the knowledge concerning this complex syndrome, particularly regarding the different phenotypes and the current and potential therapeutic targets in HFpEF.
2. Evolution of Classification and Diagnostic Innovations in HFpEF
2.1 Rising Awareness and Understanding of HFpEF
The understanding of HFpEF has transformed from a relatively overlooked condition to one now receiving growing recognition. HFpEF is a clinical syndrome distinguished by the manifestation of HF signs and symptoms due to augmented LV filling pressure, even in the presence of a normal or near-normal (50%) LV ejection fraction (LVEF) [11]. It is also characterized by a marked increase in natriuretic peptides (NPs), the presence of left ventricular hypertrophy (LVH), and/or left atrial (LA) enlargement, along with some level of diastolic dysfunction. For this reason, it was frequently considered a diagnosis of exclusion. However, the absence of well-defined criteria posed difficulties in both research and clinical contexts; thus, it became evident that the syndrome is heterogeneous, involving various comorbidities and contributing factors [12]. As a clinical syndrome, the delineation of HFpEF is complex, mainly due to the considerable variability in the clinical manifestations of patients along several dimensions: multiple comorbidities can alter clinical symptoms and signs; it also involves various organ systems, encompassing both cardiac and non-cardiac expressions [13, 14].
The increasing knowledge about HFpEF syndrome and the complexity of this condition are responsible for the evolutive classification of the HFpEF concept over the previous decade [15]. Until recently, HFpEF was a diagnosis of exclusion, while the term HFpEF implies a definition based solely on LVEF values, which is a rough and simplistic parameter not always associated with the pathophysiological background [16, 17]. However, over the recent years, novel modalities of classification have been introduced, which involve several aspects of the disease, including clinical, imaging, laboratory, metabolomic, and epigenetic parameters. To unravel this complex syndrome, it is necessary to increase the complexity of the classification to distinguish the diagnostic and therapeutic pathways [11, 15, 16].
In this regard, Fayol et al. [18] emphasized the role of etiology in HFpEF, demonstrating its usefulness in deciphering HFpEF heterogeneity better. They stratified 2180 patients with HFpEF according to etiology, identifying three main phylogroups. In particular, the best prognosis was observed in patients with idiopathic HFpEF, a group characterized by a high rate of non-cardiac comorbidities, compared to patients with secondary HFpEF due to myocardial hemodynamic loading abnormalities. On the other hand, Shah et al. [19] stratified patients according to prognosis to identify useful predictors. Patients with the best prognosis were relatively young, with mild diastolic dysfunction and low natriuretic peptide (NP) levels. Patients with “metabolic HF” were obese with LA dilatation, LVH, and diastolic dysfunction. This group was found to have a risk of death four times higher than the first. The third group included patients with a higher incidence of atrial fibrillation (AF), the highest NP levels, and the most severe diastolic dysfunction, and they were at the highest risk of death.
A different approach was used by Cohen et al. [20], who performed a secondary analysis on the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, dividing patients according to vascular and cardiac remodeling. The first subgroup included patients with mild LVH and chronic pulmonary hypertension with normal vascular stiffness and a rise in the expression of metalloproteinase; the second subgroup was characterized by older patients with multiple comorbidities, reduced vascular compliance, parietal hypertrophy, and tissue calcification; the third subgroup included obese patients with several metabolic alterations, an increase in renin-angiotensin-aldosterone system mediators levels, and an alteration in lipidic profile and tissue inflammation. The latter group showed a better response to the mineralocorticoid receptor antagonists, demonstrating that the response to therapy in the broad spectrum of HFpEF varies according to the subgroup considered. Moreover, Kyodo et al. [21] found that male patients older than 70 years with atherosclerotic vascular disease kidney and heart organ damage had a worse prognosis compared to other HFpEF patients. Beyond the clinical, etiological, and imaging parameters, a new idea of HFpEF classification has integrated several circulating biomarkers, markers of metabolic dysfunction, and microRNAs, which may be useful for diagnosis, prognosis, and cutting-edge personalized therapy [22]. The main circulating biomarkers identified have been related to myocardial injury (i.e., NPs, cardiac troponins), extracellular fibrosis (i.e., ST2, galectin-3, metalloproteinases), inflammation markers (i.e., interleukin-6 (IL-6), pentraxin, tumor necrosis factor-alfa (TNF-alfa)), and markers of endothelial dysfunction (i.e., endothelin-1, vascular cell adhesion molecule (VCAM)). From a metabolomic point of view, impairment of several pathways was identified. In particular, the role of lipids, energy, inflammation, endothelial impairment, and increased collagen synthesis has been shown [22].
2.2 Innovations in HFpEF Diagnosis and Classification
Diagnosing HFpEF is based on integrating clinical, imaging, and laboratory parameters [11]. Echocardiography is key in assessing HFpEF beyond estimating the LV diameters, volumes, and LVEF. Echocardiography allows the comprehensive evaluation of left ventricular diastolic function, a hallmark feature of HFpEF. Parameters such as early diastolic mitral inflow velocity (E), late diastolic mitral inflow velocity (A), and the E/A ratio provide information about the diastolic filling pattern. In addition, tissue Doppler imaging enables the assessment of myocardial velocities during diastole, offering insights into LV relaxation [23]. The spironolactone improved diastolic function (E/e’) ratio, which relates mitral inflow velocity to early diastolic tissue velocity, is a valuable parameter indicating elevated left ventricular filling pressures when the ratio exceeds 14. As the diastolic dysfunction progresses, there is a decrease in LV compliance during the atrial contraction phase, which is associated with impaired LV relaxation [24]. Moreover, it has been shown that reduced LV compliance is one of the strongest predictors of less favorable outcomes [25]. The diminished LV compliance leads to an increase in mean LA pressure and dimensions. Echocardiography is the gold standard in the evaluation of the LA volume, and it ought to be assessed after the systolic phase of the LV. Commonly, maximal LA volume is used with high prognostic power [24]; however, minimal LA volume has emerged as a viable alternative in identifying patients who are more at risk of CV events [26, 27]. Beyond the traditional evaluation of the LA volume, the resting LA strain has earned growing importance in the diastolic and HFpEF analysis [28]. Biomarkers have gained prominence in HFpEF diagnosis. NPs are cardiac hormones with cardioprotective properties, secreted by cardiomyocytes in response to pressure or volume overload and neuroendocrine–immune system stimulation. They have been extensively studied as diagnostic and prognostic factors for the disease, and the thresholds have been defined by the last European Society of Cardiology (ESC) guidelines to exclude those unlike HF [11, 29]. Integrating N-terminal pro-B-type natriuretic peptide (NT-proBNP) into the diagnostic algorithm, along with clinical evaluation and imaging studies, holds promise for improving the precision of HFpEF diagnoses and patient care optimization. A recent meta-analysis of 51 studies revealed that NPs demonstrate reliable diagnostic accuracy for identifying HFpEF in non-acute scenarios [30]. However, it is essential to underline different situations that interfere with NP levels, notably with NT-proBNP: age, gender, weight, genetic polymorphisms, renal insufficiency, and arrhythmia (AF in particular) [31].
The underlying myocardial changes in HFpEF still need to be defined. Myocardial histopathological features have been characterized by invasive methods such as endomyocardial biopsy, but cardiac magnetic resonance (CMR) or other imaging techniques can identify them more easily and non-invasively [32]. Over the years, several studies have investigated CMR-derived diastolic functional indices, including transmitral and pulmonary venous velocities, LV and LA strain using myocardial tagging, and, more recently, feature tracking [33]. Additionally, CMR provides early markers for detecting myocardial disease using tissue characterization imaging, which may improve the diagnosis and treatment. Myocardial fibrosis, hypertrophy of cardiomyocytes, coronary microvascular dysfunction (CMD), and inflammation have been recognized as key pathological processes impacting the myocardium in HFpEF [32]. Among CMR parameters, various studies have shown that global longitudinal strain (GLS) and extracellular volume (ECV) can completely discriminate the different phenotypes of HFpEF, with ECV appearing to be the strongest imaging marker that can distinguish hypertension from HFpEF independently [34]. The importance of CMR in HFpEF also lies in its ability to predict patients’ prognosis and outcome. Quantification of myocardial focal fibrosis by late gadolinium enhancement (LGE) may be useful for risk stratification in HFpEF patients, as a larger LGE was significantly associated with a high rate of future CV death and hospitalization for HF [35] (Table 1, Ref. [16, 17, 26, 27, 28, 29, 30, 31, 32]).
Table 1.
Summary evolution of the diagnostic framework of HFpEF.
| HFpEF diagnosis | Evolution summary | Reference |
| Biomarkers integration | The integration of circulating biomarkers, markers of metabolic dysfunction, and microRNAs has been incorporated into HFpEF classification. Identified biomarkers include those related to myocardial injury, extracellular fibrosis, inflammation, and endothelial dysfunction, providing valuable information for diagnosis, prognosis, and personalized therapy. | [16] |
| Advancements in imaging techniques | Echocardiography assesses diastolic function, while CMR-derived indices, including GLS and ECV, discriminate between different HFpEF phenotypes. LGE on CMR aids in risk stratification. | [17, 26, 27, 28, 29] |
| Scoring systems and challenges | H2FPEF score complements traditional diagnostic tools. Future research should focus on prospective validation and integrating these scores into routine clinical practice. | [30, 31, 32] |
| Diagnostic algorithm development | Development of stepwise diagnostic algorithms such as HFA-PEFF, incorporating pre-assessment, echocardiography, functional testing, and final etiology phases. The H2FPEF score provides a practical tool for initial risk stratification. | [30, 31, 32] |
HFpEF, heart failure with preserved ejection fraction; CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; GLS, global longitudinal strain; ECV, extracellular volume; H2FPEF, heart 2 preserved ejection fraction; HFA-PEFF, Heart Failure Association-preserved ejection fraction.
Thus, the diagnostic approach can be complex, requiring a global assessment beyond the single echocardiographic and serum parameters. A stepwise algorithm is recommended for the diagnosis of HFpEF and its severity. Several scores have been developed, incorporating common parameters in this pathology [36]. The consensus recommendation of the Heart Failure Association (HFA) of the ESC has developed the Heart Failure Association-preserved ejection fraction (HFA-PEFF) diagnostic algorithm, which consists of four different phases: Pre-assessment (P), echocardiography (E), functional testing (F1), and final etiology (F2). Pre-assessment is typically performed in the ambulatory setting and includes the clinical evaluation of HF symptoms and signs, typical clinical morbidities, and diagnostic laboratory tests aiming to identify the risk of HFpEF (low, intermediate, or high) based on the patient’s risk factors. Echocardiography, as mentioned, provides strong evidence of diastolic dysfunction and high LV filling pressure, which the NT-proBNP measurement must accompany. When ambiguous results are reached in the first steps, an additional stress test or an invasive hemodynamic study is needed to unmask the most challenging presentations [37]. Furthermore, the heart 2 preserved ejection fraction (H2FPEF) score represents a clinical tool that incorporates five easily assessable parameters: Hypertension, AF, age 60 years, obesity, and E/e’ ratio 9. The simplicity of this score makes it practical for initial risk stratification in patients suspected of HFpEF, enabling clinicians to identify individuals who may benefit from further diagnostic evaluation [38]. These scores complement traditional diagnostic tools, offering an additional layer of assessment that is particularly valuable in cases with unclear clinical displays. While scoring systems provide valuable contributions, challenges include potential variations in patient populations and the need for continuous refinement. Future research should focus on prospective validation of these scores and their integration into routine clinical practice. Additionally, ongoing efforts to identify and incorporate novel biomarkers and advanced imaging modalities may further enhance the accuracy of diagnostic scoring systems.
The field of HFpEF classification and diagnosis continues to evolve, with ongoing research exploring genetic and molecular factors and the potential role of artificial intelligence in refining diagnostic accuracy [21]. Multimodal approaches integrating clinical, imaging, and biomarker data hold promise for a comprehensive understanding of HFpEF, paving the way for personalized and targeted therapeutic strategies.
3. Different HFpEF Phenotypes
Many questions are currently being raised regarding the influence of common cardiovascular risk factors in the general population that can induce HFpEF, the role that comorbidities play in the development of this syndrome, and the precise correlation between this syndrome and underlying cardiac dysfunction. These questions result from the inconsistent findings of clinical trials and the generation of different hypotheses. More recently, some epidemiological studies have proposed that HFpEF represents a terminal phase of various diseases with diverse clinical courses, outcomes, and phenotypes. Distinct patterns and HFpEF subtypes have been revealed by certain cluster analyses of big randomized clinical trials (RCTs). For example, in the TOPCAT trial, latent class analysis identified three primary categories based on left ventricular geometry, clinical profile, risk factor burden, and vascular characteristics [20]. The authors identified three main phenogroups: Group 1 with low NP levels, normal LV geometry, low arterial stiffness, and cardiac events; Group 2 was characterized by high NP levels, increased vascular stiffness associated with LV concentric hypertrophy, LA dilatation and an elevated incidence of cardiovascular events; Group 3 is the typical metabolic pattern with an elevated level of rennin, an abnormal glycemic and lipid profile, an increased inflammatory pattern and a better response to spironolactone treatment [20, 21].
Five distinct groups were chosen by another analysis based on shared laboratory characteristics: Age, gender, and related disorders, which came from the SwedHF registry. Similarly to the previous analysis, Cluster 1 included younger patients with low comorbidities and NP values. Cluster 2 encompasses patients with a high prevalence of AF and diabetes associated with impaired renal function; Cluster 3 demonstrated a higher incidence of AF, older age, the prevalence of females, and those associated with a high NP level; Cluster 4 could be identified by the elevated prevalence of hypertension and diabetes with high body mass index (BMI); Cluster 5 identified older patients with cardio–renal disorders and more advanced New York Heart Association (NYHA) classes. The current classifications show a poorer outcome among groups with cardio–renal and hypertension–diabetic patterns [39]. Similarly, an analysis of the Alberta cohort recognized four phenotypes ranging from healthy subjects at risk of HFpEF without signs of HF up to congested, older patients with elevated comorbidity burdens [40]. The more recent Prospective Comparison of angiotensin receptor–neprilysin inhibitor (ARNI) with angiotensin receptor blocker (ARB) Global Outcomes in HFpEF (PARAGON-HF) included older individuals with a mean age of 72 years, a mean EF of 57%, a significant prevalence of diabetes (43%), and chronic kidney disease (47%), reflecting the real HFpEF patients [41]. Looking at more detailed morphological investigations, these patients had similar LA sizes but lower evidence of increased LV mass values and tricuspid regurgitation velocity compared to previous trials. Conversely, the E/e’ ratio in the PARAGON trial was more noticeable, emphasizing the variability in the cardiac phenotype associated with this disease [42]. The comparison of this research highlights the differences in patient characteristics, especially the observation that only half of the people enrolled in the most well-known trials met the HFpEF diagnostic criteria. Interestingly, 50% of patients exhibited increased LV mass, yet while advanced diastolic dysfunction was common, treatment with sacubitril/valsartan was the most beneficial for those with an EF below normal [43] (Fig. 1).
Fig. 1.
Different HFpEF phenotypes and clinical scenarios may respond differently to treatment. HFpEF, heart failure with preserved ejection fraction.
Current discrepancies demonstrated that different mechanisms may primarily be responsible for disease onset and progression depending on different baseline conditions, burden of risk factors, and cardiovascular adaptation to the underlying triggers [44]. A certain amount of overlap and negligence between phenomapping analyses may reflect different HFpEF phases and selection criteria. Comprehensive morphological analysis, combined with environmental lifestyle and CV risk factors, may result in a more homogeneous classification and improved risk stratification of HFpEF [45, 46].
Screening and treating underlying causes and comorbidities, both cardiovascular and non-cardiovascular, are advised for HFpEF patients.
When AF causes HF, the clinical course tends to be more favorable compared to other causes of HF (known as tachycardiomyopathy) [47]. Nevertheless, the presence of AF may attenuate the prognostic benefits associated with beta-blockers and impede the efficacy of ivabradine [48]. Certain HF treatments, such as angiotensin-converting enzyme inhibitors (ACE-I), may reduce the risk of developing AF.
To manage HFpEF, beta-blockers, long-acting nitrates, calcium channel blockers (CCBs), ivabradine, ranolazine, trimetazidine, nicorandil, and their associations should be contemplated for angina relief, notwithstanding the absence of demonstrable benefits on HF progression or coronary endpoints [49, 50, 51, 52, 53].
Hypertension has emerged as a key driver of HFpEF, posing a significant treatment challenge with uncertain optimal approaches.
ARBs, ACE-I, and CCBs cause more effective LVH regression than beta-blockers or diuretics. However, caution is warranted to prevent hypotension in HFpEF patients with LVH and limited preload reserves.
Further, obesity strongly correlates with HFpEF, revealing distinct pathophysiological pathways in obese patients [8]. Weight reduction and increased physical activity may enhance symptom management and exercise capacity, especially in suitable candidates [54].
Chronic kidney disease (CKD) and declining renal function are more prevalent in HFpEF patients compared to heart failure with mildly reduced ejection fraction (HFmrEF) and HFrEF; however, their impact on outcomes appears less severe in HFpEF relative to the other classifications [55].
Management of HF in chronic obstructive pulmonary disease (COPD) patients generally proceeds without major concerns. While beta-blockers may exacerbate pulmonary function in select cases, they are not contraindicated in COPD or asthma [56, 57].
4. ACE-I, ARBs, Beta-Blockers and Diuretics in HFpEF
Before the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure (EMPEROR-Preserved) and Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) studies, American and European guidelines lacked recommendations for the use of disease-modifying HFrEF therapies as clinical trials with ACE-I, ARB, mineralocorticoid receptor antagonists (MRA), while ARNIs failed to achieve their primary endpoints in HFpEF patients.
Notable trials include PEP-CHF (perindopril) [58], CHARM-Preserved (candesartan) [59], I-PRESERVE (irbesartan) [60], TOPCAT (spironolactone) [61], DIG-Preserved (digoxin) [62], and PARAGON-HF (sacubitril/valsartan) [63]. Candesartan and spironolactone showed reductions in hospitalizations for heart failure, with a trend toward reductions observed while using sacubitril/valsartan. However, since these trials were neutral for their primary endpoints, these findings are considered hypothesis-generating only.
In the SENIORS trial, although nebivolol significantly decreased the combined primary endpoints of all-cause mortality and CV hospital admission, it only included 15% of participants with an LVEF greater than 50% [64].
Despite no treatment showing conclusive evidence of reducing mortality and morbidity in HFpEF patients, most of them have underlying hypertension and/or coronary artery disease (CAD), and many have already been treated with ACE-I/ARB, beta-blockers, or MRAs.
In congested patients with HFpEF, diuretics are recommended to mitigate symptomatic distress and clinical manifestations. Loop diuretics are preferred, while thiazide diuretics may also prove efficacious in managing hypertension.
5. ARNIs Action Mechanisms in HFpEF
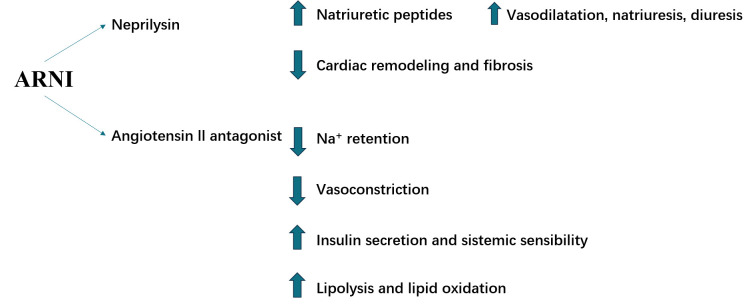
The action mechanisms of ARNIs in HFpEF involve a multifaceted approach targeting various pathological processes, including neurohormonal activation, inflammation, and fibrosis. ARNIs represent a relatively novel class of drugs that combine the actions of ARBs and neprilysin inhibitors. The primary components of ARNIs are sacubitril and valsartan, combined in a unique crystalline salt complex with high stability and good pharmacodynamics. Sacubitril inhibits neprilysin, an enzyme responsible for degrading NPs, which has beneficial effects on blood pressure regulation and sodium balance. Conversely, valsartan is an ARB that blocks the effects of angiotensin II, a vasoconstrictor and pro-fibrotic peptide. Their combination is crucial because increasing the concentration of circulating neprilysin and sacubitril causes a reflex renin-angiotensin-aldosterone (RAAS) activation that limits its benefit, while valsartan inhibits this reflex [65].
The potential beneficial mechanisms of ARNIs in HFpEF are multifaceted:
• NP enhancement: By inhibiting neprilysin, ARNIs increase the levels of NPs, such as atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). These peptides promote vasodilation, natriuresis, and diuresis, which help reduce fluid overload and improve renal function.
• Angiotensin II blockade: Valsartan blocks the effects of angiotensin II, contributing to vasoconstriction, sodium retention, and myocardial remodeling.
• Improvement in cardiac remodeling: In HF, cardiac remodeling is often caused by microvascular inflammation and is often mediated by a reduction of intracellular cyclic guanosine monophosphate (cGMP). Increasing cGMP neprilysin counteracts this action, reducing left ventricular remodeling, myocardial hypertrophy, and fibrosis, thus reducing cardiomyocyte stiffness and ultimately improving cardiac function and structure.
• Beneficial metabolic effects: ARNIs act in both lipid and carbohydrate metabolism, increasing lipolysis and lipid oxidation, insulin secretion, and insulin sensitivity through many pathways. This is crucial because metabolic dysregulation is an important risk factor for HFpEF, especially affecting cardiac diastolic function [66] (Fig. 2).
Fig. 2.
ARNIs have potential beneficial molecular effects in HFpEF acting through different molecular mechanisms. ARNI, angiotensin receptor–neprilysin inhibitor; HFpEF, heart failure with preserved ejection fraction.
6. Clinical Trials on ARNIs in HFpEF
HFpEF management is still a topic of debate because of the lack of specific therapies, even if many drugs designed for HFrEF have also been investigated for HFpEF. According to both the 2021 ESC guidelines and the 2022 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on HF, ARNIs may be considered in HFpEF (Class IIB) [11, 67]. Unfortunately, the class of recommendation is still low because, to date, clinical trials have failed to show the benefit of these drugs in hard endpoints such as cardiovascular deaths or hospitalizations.
In 2012, the Prospective Comparison of ARNI with ARB on the Management of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) was the first trial that clearly demonstrated the potential role of ARNIs in HFpEF. PARAMOUNT is a phase 2 randomized double-blind trial comparing sacubitril/valsartan to valsartan alone in 301 patients with HFpEF (EF 45%) for a 36-week follow-up. It demonstrated a reduction in NT-proBNP levels in the sacubitril/valsartan group after 12 weeks, with an improvement in NYHA class and a reverse LA remodeling at 36 weeks [68]. Despite these results, subsequent trials have been controversial. In 2019, three important trials were published: the PARAGON-HF, the Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER-HF), and the Prospective Comparison of ARNI vs. Comorbidity-Associated Conventional Therapy on Quality of Life and Exercise Capacity (PARALLAX) trials [63, 69, 70].
Using the same study groups as the PARAMOUNT trial but with 4822 patients, who were observed for a median follow-up of 35 months, the PARAGON-HF trial did not show a benefit in deaths or hospitalizations among sacubitril/valsartan patients, despite an improvement in the NYHA class and the quality of life, measured using the Kansas City Cardiomyopathy Questionnaire (KCCQ). Nevertheless, a subgroup analysis suggested potential ARNI survival benefits in women and patients with lower EF (45–57%). Furthermore, the benefit of ARNIs was larger in patients recently hospitalized for HF [63].
The PARALLAX trial with 2572 patients, EF 40%, and a median follow-up of 24 weeks showed a reduction in NT-proBNP in patients treated with sacubitril/valsartan compared to patients treated with an ACE-I or ARB. However, there was no significant improvement in the NYHA class, KCCQ, or the 6-minute walk distance [69]. Compared to the PARAMOUNT trial, these findings suggest that NT-proBNP reduction is probably an early effect of ARNI treatment, while other benefits are shown later. Furthermore, the ARNI effects on NT-proBNP are not limited to a chronic setting but are also important in acute HF, as shown by the PIONEER-HF trial in HFrEF [70] and by the Prospective comparison of ARNI with ARB Given following the stabiLization In DEcompensated HFpEF (PARAGLIDE) trial. However, also in the PARAGLIDE trial, sacubitril/valsartan did not reduce mortality, even if the hierarchical outcome showed a positive but not significant trend (unmatched win ratio: 1.19; 95% CI: 0.93–1.52; p = 0.16) [71] (Table 2, Ref. [63, 68, 69, 71]).
Table 2.
Main randomized clinical trials on ARNIs in patients with HFpEF with improved soft endpoints but no clear effect on CV deaths.
| Study | Year | Drugs | Patients | EF | Primary endpoint | p-value | Follow-up |
| PARAMOUNT | 2012 | Sacubitril/valsartan vs. valsartan | 149 | 45% | Change from baseline in NT-proBNP level at week 12 [68] | p = 0.01 | 36 weeks |
| PARAGON-HF | 2019 | Sacubitril/valsartan vs. valsartan | 4822 | 45% | Composite of total hospitalizations for HF and death from CV causes [63] | p = 0.06 | 35 months |
| PARALLAX | 2021 | Sacubitril/valsartan vs. enalapril, valsartan, or placebo | 4632 | 40% | Change from baseline in plasma NT-proBNP level at week 12. | p 0.001 | 24 weeks |
| Change from baseline in the 6-minute walk distance at week 24 [69] | p = 0.42 | ||||||
| PARAGLIDE | 2023 | Sacubitril/valsartan vs. valsartan | 466 | 40% | Time averaged reduction in NT-proBNP after an acute HF episode [71] | p = 0049 | 8 weeks |
ARNI, angiotensin receptor–neprilysin inhibitor; CV, cardiovascular; EF, ejection fraction; HF, heart failure; PARAMOUNT, Prospective Comparison of ARNI with ARB on Management of Heart Failure with Preserved Ejection Fraction; PARAGON-HF, Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF; PARALLAX, Prospective Comparison of ARNI vs. Comorbidity-Associated Conventional Therapy on Quality of Life and Exercise Capacity; PARAGLIDE, prospective comparison of ARNI with ARB Given following stabiLization In DEcompensated HFpEF; HFpEF, heart failure with preserved ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ARB, angiotensin receptor blocker.
In conclusion, the role of ARNIs in HFpEF is still debated; however, it seems to lower HF hospitalizations in HFpEF patients [72]. Furthermore, a recent pooled analysis of the PARAGON and PARAGLIDE trials showed a significant reduction in deaths or hospitalizations in patients treated with sacubitril/valsartan compared to valsartan alone, with a larger benefit in patients with LVEF 60% [73].
7. MRAs Action Mechanisms in HFpEF
MRAs, such as spironolactone and eplerenone, antagonize mineralocorticoid receptors, reducing aldosterone effects that can harm HFpEF, promoting sodium and water retention, and exacerbating cardiac fibrosis and inflammation. The role of MRAs in HFpEF should probably be discussed alongside HF pathophysiology. Indeed, MRAs are probably more effective in patients with hypertension as an etiology of HF [74]. Their potential beneficial mechanisms in HFpEF include:
• Sodium and water balance: MRAs counteract sodium and water retention caused by aldosterone, reducing edema and congestion.
• Reduction in myocardial fibrosis: Aldosterone is implicated in cardiac fibrosis; thus, MRAs may have a role in reducing myocardial remodeling.
• Anti-inflammatory effects: MRAs may have anti-inflammatory properties, which could help ameliorate the systemic inflammation often seen in HFpEF patients [75, 76] (Fig. 3).
Fig. 3.
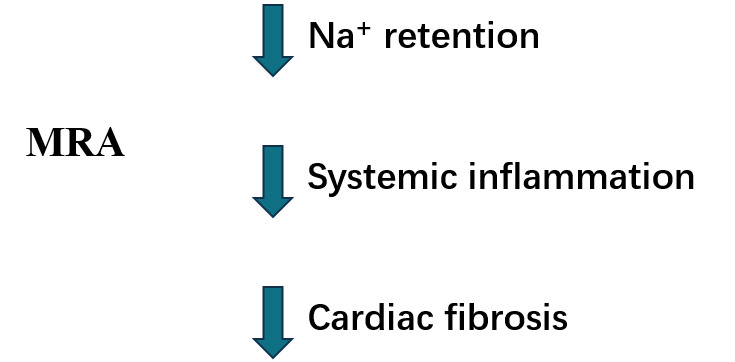
MRA potential beneficial mechanisms in HFpEF. MRA, mineralocorticoid antagonists; HFpEF, heart failure with preserved ejection fraction.
8. Clinical Trials on MRAs in HFpEF
As for ARNIs, MRAs could also be used in HFpEF according to the latest ESC guidelines and ACC/AHA guidelines on HF (Class IIB) [11, 67]. However, their effects on CV deaths and hospitalizations remain uncertain and under investigation.
In 2013, the Aldosterone Receptor Blockade in Diastolic Heart Failure (ALDO-HF) trial showed that E/e’ and reduced left ventricular mass in the treatment group (n = 213) compared to the placebo (n = 209), even if it did not benefit the maximal exercise capacity or the patient’s symptoms and quality of life [77]. In 2014, the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial showed a decrease in hospitalizations but not in mortality in patients with HFpEF [61, 78], even though a subsequent sub-analysis seems to illustrate a survival benefit in the American cohort of the trial compared to the Russian cohort; differences in drug compliance have been hypothesized to clarify these results [79]. Furthermore, in 2016, the Spironolactone in Myocardial Dysfunction with Reduced Exercise Capacity (STRUCTURE) trial showed an improvement in exercise capacity using the cardiopulmonary test in patients with HFpEF and exertional dyspnea [80].
Currently, the only large study on MRAs with positive results on the survival rate is the Canrenone Effects on Cardiovascular Mortality in Patients with Congestive Heart Failure (COFFEE-IT) trial, a retrospective study that showed the survival benefit of canrenone on older people (68–83 years old) after 10 years of treatment [81]. Despite this, canrenone remains poorly used among MRAs.
Results from two large trials investigating the impact of spironolactone on cardiovascular deaths and hospitalizations should be published in the near future from the Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction (SPIRRIT-HF) [82] and the Spironolactone In The Treatment of Heart Failure (SPIRIT-HF) trials [83]. Furthermore, compared to traditional MRAs, non-steroidal MRAs are emerging as drugs with higher selectivity for mineralocorticoid receptors and without sex–steroid-related side effects [84]. Among them, finerenone is the most investigated drug, and subgroup analyses of the FIDELIO-DKD [85] and FIGARO-DKD on patients with HF (symptomatic HFrEF was an exclusion criterion in both trials) suggest a potential role of this drug in HFpEF [86]. Indeed, a reduction in the composite outcome of cardiovascular death, non-fatal myocardial infarction, stroke, or hospitalization for heart failure was observed in a mean follow-up of 2.6 years. As a result, the Finerenone in Heart Failure Patients (FINEARTS-HF) trial is currently ongoing, investigating finerenone effects on morbidity in more than 6000 patients with HF and EF 40% [87] (Table 3, Ref. [77, 79, 80, 82, 83, 87]).
Table 3.
Main randomized clinical trials on MRAs in patients with HFpEF with improved secondary endpoints but no clear effect on CV deaths.
| Study | Year | Drugs | Patients | EF | Primary endpoint | p-value | Follow-up |
| ALDO-HF | 2013 | Spironolactone vs. placebo | 422 | 50% | Change in diastolic function (E/e’). | p 0.001 | 12 months |
| Change in peak oxygen uptake on cardiopulmonary exercise testing [77] | p = 0.81 | ||||||
| TOPCAT | 2014 | Spironolactone vs. placebo | 3445 | 45% | Composite of death from CV causes, aborted cardiac arrest, or hospitalization for HF [79] | p = 0.014 | 3.3 years |
| STRUCTURE | 2016 | Spironolactone vs. placebo | 150 | 50% | Improvement in peak oxygen uptake. | p 0.001 | 6 months |
| Improvement exertional E/e’ ratio [80] | p 0.001 | ||||||
| SPIRRIT-HF | On going | Spironolactone vs. no spironolactone | 2000 | 40% | Incidence rate for total HF hospitalizations or CV death [82] | 5 years | |
| SPIRIT-HF | On going | Spironolactone vs. placebo | 1300 | 40% | Cumulative number of primary composite events of CV death and total HF hospitalizations [83] | 48 months | |
| FINEARTS-HF | On going | Fineronone vs. placebo | 6016 | 40% | Number of CV deaths and HF events [87] | 42 months |
CV, cardiovascular; EF, ejection fraction; HF, heart failure; MRAs, mineralocorticoids antagonists; HFpEF, heart failure with preserved ejection fraction; ALDO-HF, Aldosterone Receptor Blockade in Diastolic Heart Failure; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist; STRUCTURE, Spironolactone in Myocardial Dysfunction with Reduced Exercise Capacity; SPIRRIT-HF, Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction; SPIRIT-HF, Spironolactone In The Treatment of Heart Failure; FINEARTS-HF, Finerenone in Heart Failure Patients; E/e’, spironolactone improved diastolic function.
9. Sodium–Glucose Cotransporter-2 Inhibitor (SGLT2i) in HFpEF: Potential Mechanism of Action and Results from Trials
Recently, the ESC guidelines recommended SGLT2i therapy for treating HFpEF [11], despite the exact mechanisms yet being fully understood. However, several potential mechanisms of action have been proposed based on clinical and pre-clinical evidence [85]. SGLT2i plays the potential role of anti-HFpEF through the direct or indirect synergy of multiple targets and pathways [88].
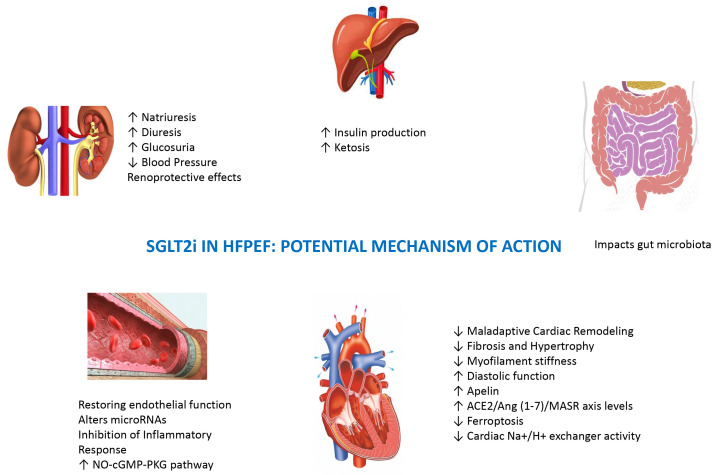
SGLT2i inhibits the absorption of sodium and glucose in the proximal renal tubule, leading to natriuresis, glucosuria, and elevated urine output. Thus, it has been associated with a reduction in blood pressure. Lowering blood pressure can alleviate cardiac workload and improve overall cardiovascular function.
The vascular and metabolic effects of SGLT2i have always been held responsible for cardiovascular benefits [89]. In HFpEF studies, researchers propose potent reno-protective effects of SGLT2i, with their impact on intraglomerular pressure prevailing over other mechanisms. SGLT2i, by restoring afferent arteriole tone, synergizes with renin-angiotensin system inhibitors, reducing intraglomerular pressure and preventing renal complications. Notably, despite a decline in the estimated glomerular filtration rate (eGFR), SGLT2 inhibition influences primary endpoints [90].
In a post-hoc analysis of the EMPEROR-Preserved Trial, empagliflozin was linked to a slight increase in the risk of volume depletion for patients concurrently using diuretics [91]. However, it was also associated with a decreased probability of initiating or escalating diuretic doses and an increased likelihood of reducing or permanently discontinuing diuretics [91]. Natriuresis derived from empagliflozin is not associated with neurohormonal activation, potassium loss, or impaired renal function (favorable diuretic profile) [92].
SGLT2i provides metabolic advantages, promotes weight loss, and enhances insulin sensitivity for improved cardiovascular health. It also exhibits intriguing effects, which include inducing a transcriptional paradigm, nutrient deprivation, hypoxia, increasing ketosis, erythropoietin, and autophagic flux [93]. Consequently, inflammasome activation is reduced, mitigating cardiomyocyte dysfunction and coronary microvascular injury. Moreover, alterations in iron homeostasis contribute to enhanced cardiac energetics and function. Additionally, SGLT2i reduces epicardial adipose tissue and modifies adipokine signaling, potentially contributing to the observed reductions in inflammation and oxidative stress associated with their use [93].
Dapagliflozin has a significant impact on energy metabolism related to fatty acid intake and mitochondrial dysfunction in HFpEF; it is achieved by elevating -hydroxybutyric acid (-OHB) levels, activating citrate synthase, reducing acetyl coenzyme A (acetyl-CoA) pools, regulating adenosine 5’-triphosphate production, and enhancing the expression of mitochondrial oxidative phosphorylation system complexes I–V. SGLT2i is beneficial in preventing and treating cardiac remodeling and dysfunction in HFpEF models by mitigating cardiometabolic dysregulation [94].
Endothelial dysfunction is a pivotal mechanism in HFpEF, diabetes mellitus (DM), and frailty. Treatment with the SGLT2i empagliflozin altered certain microRNAs, counteracting the changes observed in HFpEF patients. This suggests a potential restoration of endothelial function through empagliflozin treatment [95].
Chronic activation of the SGLT2i pathway may contribute to maladaptive cardiac remodeling. Inhibiting SGLT2 receptors could potentially mitigate adverse remodeling processes, improving cardiac structure and function [96].
SGLT2i can affect the extracellular matrix, contributing to collagen turnover and mitigating fibrosis—a significant feature in HFpEF. Notably, these inhibitors have demonstrated a capacity to reduce myofilament stiffness and remodel the extracellular matrix in the heart. This action improves diastolic function, offering potential benefits in the context of HFpEF [93].
Canagliflozin (CANA) treatment reduces myocardial hypertrophy and fibrosis and improves left ventricular diastolic function and remodeling. These positive effects are attributed to CANA’s ability to upregulate apelin, activate angiotensin-converting enzyme 2 (ACE2), and increase ACE2/Ang (1–7)/mast cell receptor (MASR) axis levels [97]. Canagliflozin treatment was found to counteract ferroptosis, a recently identified mechanism of iron-dependent non-apoptotic cell death in HF. In a rat model of HF induced by a high-salt diet, the study observed iron overloading and lipid peroxidation, both of which had been alleviated by administering canagliflozin [98]. SGLT2i mitigates the risk of hospitalization for HF in individuals with HFpEF. However, the specific hemodynamic mechanisms responsible for these benefits are poorly understood. In the CAMEO-DAPA trial, dapagliflozin treatment in HFpEF patients was associated with reduced resting and exercise pulmonary capillary wedge pressure (PCWP) and positive effects on plasma volume and body weight [99].
Emerging evidence also indicates that these drugs impact cardiomyocyte ionic homeostasis. Empagliflozin was observed to diminish the activity of the cardiac / exchanger, potentially enhancing cardiac function. Subsequently, it was discovered that dapagliflozin and canagliflozin also inhibited / exchanger activity, leading to a decrease in cytosolic . Moreover, empagliflozin reduced the activity of /calmodulin-dependent kinase II (CaMKII) and CaMKII-dependent sarcoplasmic reticulum leakage [100].
The effects of empagliflozin on HFpEF are primarily mediated by inhibiting / exchanger 1 (NHE1), influencing cardiomyocyte oxidative stress modulation, cardiomyocyte stiffness, myocardial extracellular matrix remodeling, heart concentric hypertrophy, and systemic inflammation [101]. Empagliflozin enhances the nitric oxide (NO)—soluble guanylate cyclase (sGC)—cGMP cascade and protein kinases GI (PKGI) activity by reducing PKGI oxidation in HFpEF [102]. Additionally, dapagliflozin inhibits the inflammatory response and activates the NO–cGMP–protein Kinases G (PKG) pathway in animal models [103].
Prior evidence showed that metabolites produced by gut microbiota play a crucial role in heart failure development. SGLT2i have been discovered to impact the gut microbiota in rodent studies. The EMPAGUM trial seeks to validate these human changes and explores the role of gut microbiota and their metabolites in the HFpEF process [104]. It is important to note that ongoing research further elucidates the mechanisms of SGLT2i in HFpEF, and the field continues to evolve (Fig. 4).
Fig. 4.
Potential SGLT2i effects beyond the cardiovascular system on renal, liver, and intestinal districts. SGLT2i, sodium–glucose cotransporter-2 inhibitors; HFpEF, heart failure with preserved ejection fraction; ACE2, angiotensin-converting enzyme 2; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; MASR, mast cell receptor; PKG, protein Kinases G.
The EMPEROR-Preserved study study was carried out on 5988 patients (median age: 72, 45% women, median LVEF: 54%); half of the patients had diabetes, and half had an eGFR below 60 mL/min/1.73 . The primary endpoint (cardiovascular events and HF hospitalization) was met (hazard ratio (HR) 0.73; 95% confidence interval (CI), 0.6–0.88; p 0.001), marking the first instance where drug therapy achieved this goal in an HFpEF study.
The trial validated a 21% risk reduction in cardiovascular death or HF hospitalization from using empagliflozin on HF patients with an LVEF 40% [105]. Subgroup analysis has shown the highest benefit in LVEF 50% (HR 0.71; 95% CI, 0.57–0.88), a lower benefit in LVEF 50 to 60% (HR 0.80; 95% CI, 0.64–0.99), and no apparent benefit in LVEF 60% (HR 0.87; 95% CI, 0.69–1.10). Pooled analysis across EMPEROR-Reduced and EMPEROR-Preserved trials have indicated consistent HF outcomes with empagliflozin in patients with LVEF 25% to 65%, with diminished effects in those with LVEF 65% [106]. A potential drawback in the EMPEROR-Preserved trial was raised from the under-representation of females and a predominantly low NYHA class within the studied population. Additionally, there was no observed benefit of empagliflozin on overall mortality, as indicated by a hazard ratio of 1 (95% CI, 0.87–1.15).
In the Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure trial of 6263 heart failure patients (mean age: 71, 44% female, mean LVEF: 54%), dapagliflozin led to an 18% risk reduction in the primary combined endpoint (HR 0.82; 95% CI, 0.73–0.92) and a non-significant 12% reduction in cardiovascular mortality (HR 0.88; 95% CI, 0.74–1.05) [107] over 2.3 years. Dapagliflozin lowered the risk of HF worsening or cardiovascular death by 18%, regardless of LVEF. Unlike prior trials, DELIVER included all LVEF ranges, but it is unclear if the benefit in patients with LVEF 60% specifically relates to a reduction in total CV events, HF events, or both.
DELIVER permitted randomization during or soon after hospitalization for HF in clinically stable patients without intravenous HF therapies. Starting dapagliflozin during, or soon after, HF hospitalization in patients with mildly reduced or preserved LVEF has appeared safe and effective; moreover, dapagliflozin has reduced the risk of worsening HF or cardiovascular death similarly in patients with and without a history of recent HF hospitalization [108]. In patients recently hospitalized with HF, initiating dapagliflozin has had trivial effects on blood pressure and has not worsened renal status [109].
In a meta-analysis [110] performed to investigate SGLT2i effects on HFpEF or HFmrEF, by pooling data from all clinical RCTs available and thus increasing power to testify, the authors have demonstrated that in patients with LVEF 40%, SGLT2i significantly reduces the composite risk of cardiovascular death and hospitalization for heart failure, although the risk of cardiovascular death and all-cause death did not reduce.
10. Rationale for the Use of Glucagon-Like Peptide 1 Receptor Agonists
Type 2 diabetes mellitus (T2DM) and obesity could be considered metabolic disorders characterized by high CV risk [111].
Recently, a multinational, cross-sectional study of cardiovascular disease prevalence and etiology in adults with T2DM across 13 countries (CAPTURE study) showed that, among 9823 T2DM patients, one-third have a CV disease [112]. Moreover, the overall mean BMI was 29.0 , highlighting a pathophysiological link between T2DM and obesity [112]. Importantly, according to U.S. National Health Interview Survey data, overall mortality in T2DM patients has reduced from 11.3% during 1988–1994 to 5.9% during 2010–2015. However, despite a paradigm shift in T2DM treatment beyond the hypoglycemic effect, the overall prevalence of CV complication is 32.2%, consisting mainly of CAD [113, 114, 115].
Obesity and T2DM are very common comorbidities in HFpEF patients and are closely involved in their pathophysiology and prognosis [116, 117]. T2DM-related metabolic derangements such as hyperglycemia, lipotoxicity, and hyperinsulinemia, associated with coronary microvascular rarefaction and advanced glycation end-products deposition, favor the development of DM-related cardiomyopathy (DMC) with HFpEF phenotype and concentric LV remodeling with diastolic LV dysfunction. Importantly, this phenotype is more prevalent in obese patients with T2DM [118]. Recently, a new paradigm of HFpEF syndrome has been developed: Particularly, HFpEF comorbidities are believed to induce a chronic systemic inflammatory state. In obese subjects, macrophages infiltrate adipose tissue, releasing proinflammatory cytokines. This condition promotes coronary microvascular endothelial inflammation, leading to a reduction in NO bioavailability. Reductions in both cGMP and protein kinase G activity increase wall tension, myocardial stiffness, and interstitial fibrosis [119]. These pathophysiological aspects suggest why innovative drugs targeting endothelial dysfunction are crucial in treating T2DM and obese patients and could be useful in diabetic and obese HFpEF phenotypes.
11. Mechanisms of Action of Glucagon-Like Peptide 1 Receptor Agonists and Their Potential Role in HFpEF
Incretin hormones—glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP1)—are released from gut endocrine cells and potentiate meal-stimulated insulin secretion [120]. Biologically active GLP1 refers to both GLP1 (7–36) amide and GLP1 (7–37), which act on a single identified GLP1 receptor (GLP1R) on pancreatic islet -cells, -cells, and -cells, respectively, to increase insulin and somatostatin secretion and decrease glucagon secretion.
Synthetic GLP1R agonists (GLP1RAs) mediate the same biological effects of endogenous GLP1, binding to the GLP1R and stimulating glucose-dependent insulin release from the pancreatic islets.
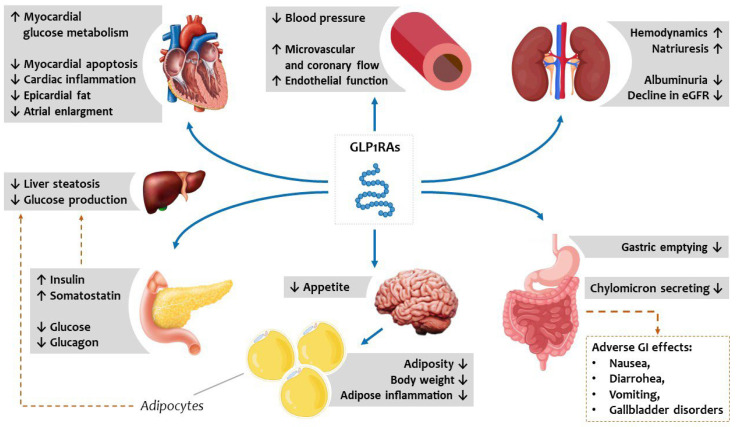
A single, canonical GLP1R, expressed at low levels in the human atria and ventricles (including in cardiomyocytes) and in blood vessels, mediates the major CV actions of GLP1RAs, reducing blood pressure and improving microvascular and coronary flow, counteracting atherosclerosis, and promoting plaque stability [121]. Specifically, GLP1RAs might reduce myocardial apoptosis and inflammation in the heart, inducing glucose metabolism [120]. In addition, GLP1 and GLP1RAs have multiple extra-pancreatic effects that might indirectly reduce CV morbidity, inducing weight loss and acting on postprandial lipemia and inflammation [122]. Enterocytes and hepatocytes do not express GLP1R, although studies on mouse liver showed that a subset of GLP1R+ endothelial and intrahepatic T cells mediates a component of the anti-inflammatory effect of GLP1RAs [123]. As proof of this, patients with either T2DM, obesity, or non-alcoholic steatohepatitis (NASH) treated with semaglutide had shown a decrease in circulating triacylglycerol, low density lipoprotein cholesterol (LDLc), and non-high density lipoprotein (HDL) cholesterol [124]. Similarly, several GLP1RAs were shown to reduce postprandial plasma levels of triacylglycerol with little effect on fasting plasma LDLc, even in patients treated with statins [125]. The effect of a reduction in the fasting plasma levels of cholesterol and triacylglycerol could indirectly reflect the extent of weight loss achieved in these patients [119]. Weight loss is determined by reducing hunger by stimulating the GLP1Rs expressed in the central nervous system and slowing down gastric emptying [120].
The activity of GLP-1 and GIP is limited by the dipeptidyl peptidase-4 (DPP-4) enzyme, which rapidly inactivates incretin hormones. This provides the rationale for developing DPP4 inhibitors, which limit the breakdown of endogenous GLP-1 by the DPP-4 enzyme. Concentrations of the active intact GLP-1 and GIP are therefore increased, leading to an increased and prolonged action of these hormones. The biological effects of GLP1RAs are summarized in Fig. 5.
Fig. 5.
Metabolic vascular and systemic effects of GLP1RAs potentially reduce the burden of cardiovascular risk and HFpEF deterioration. GLP1RAs act directly on pancreatic beta and alpha cells, the gastrointestinal tract, and the central nervous system to improve glucometabolic homeostasis and indirectly improve circulating lipid profiles by reducing hepatic steatosis. GLP1RAs, synthetic glucagon-like peptide receptor agonists; HFpEF, heart failure with preserved ejection fraction; eGFR, estimated glomerular filtration rate; GI, gastrointestinal.
GLP1RAs and DPP4 inhibitors were developed for T2DM treatment based on this evidence. However, these effects may be particularly relevant in the context of metabolic alterations and inflammation of HFpEF syndrome. As proof, GLP1RAs were shown to have protective effects in experimental HF models in several species, including mice, rats, pigs, and dogs, further suggesting a role in specific HFpEF phenotypes [119, 126]. The same molecule used in experimental animal models of T2DM cardiomyopathy improved diastolic dysfunction, as reflected by a decreased E/e’ ratio [127].
Recently, a meta-analysis of eight RCTs about CV events, mortality, and kidney outcomes with GLP1RAs in 60,080 T2DM patients showed a significant reduction (–14%) in major cardiovascular events (MACEs), all-cause mortality (–12%), and composite kidney outcome (–21%) with no increase in the risk of severe hypoglycemia, retinopathy, or pancreatic adverse events. Moreover, GLP1RAs significantly reduced hospitalizations for HF by 11% [128]. Effects on HF-related events were different depending on the patients treated. For example, a post-hoc analysis from Harmony Outcomes aiming to explore the effects of the GLP1RA albiglutide on HF outcomes in T2DM patients and cardiovascular disease, with and without HF history, showed that albiglutide had no effect in reducing HF-related events among those with a history of HF [129]. Similarly, in a post-hoc analysis from the REWIND trial, which included 5.4 years of follow-up, dulaglutide was not associated with a reduction in HF events in patients with T2DM regardless of baseline HF status [130].
Since the effects of GLP1RAs are mainly related to anti-inflammatory and lipolytic effects, their administration is associated with weight loss regardless of diabetic status [131]. Obesity and, more specifically, increased epicardial fat in HFpEF are associated with more symptoms and worse prognoses [132, 133, 134]. This has led to weight loss as a specific target for HFpEF treatment and the realted role of GLP1RAs in this context. However, the effect of weight loss in HF patients is still partially unsettled. Weight loss is a poor prognostic factor in patients with HFrEF [135, 136]. Conversely, evidence suggested that weight loss has beneficial effects in obese patients with HFpEF: Kitzman et al. [137] showed that among clinically stable obese older HFpEF patients, aerobic exercise training or caloric restriction increased peak oxygen consumption, and drug effects were additive [138]. Bariatric surgery was also shown to improve symptoms, as well as reduce HF rehospitalizations and reverse left ventricular remodeling in obese HFpEF patients [137, 139].
However, the same beneficial effects of weight loss have not been replicated in non-obese patients with HFpEF: the FLAGSHIP cohort study aimed to examine the association between weight loss and HFpEF prognosis in 573 hospitalized obese and non-obese patients [140]. In particular, in non-obese patients, weight loss was associated with higher all-cause mortality and rehospitalization rates than ones without weight loss. Moreover, 6 months after hospital discharge, a high proportion of non-obese patients with weight loss showed functional limitations and anorexia, suggesting an impairment of their physical function and poor nutritional status. Conversely, weight loss was not associated with adverse events in obese patients with HFpEF [141].
Based on these results, much evidence suggests that GLP1RAs, promoting weight loss by reducing the generation of reactive oxygen species and systemic inflammation, may be highly effective in the obese phenotype of HFpEF. Among GLP1RAs, semaglutide was shown to be more effective in weight loss compared with other agents: In the SUSTAIN trials, a slightly greater weight loss was observed with subcutaneous once-weekly semaglutide administration, compared with exenatide, dulaglutide, or liraglutide [142]. Similarly, a secondary analysis of PIONEER 4 showed a greater weight loss with once-daily oral semaglutide administration than with subcutaneous liraglutide [142, 143, 144, 145].
The STEP HFpEF trial has recently shown a significant improvement in symptoms, quality of life, and exercise tolerance, assessed by the 6-minute walking test (6MWT), along with weight loss, in 529 non-diabetic obese patients with HFpEF randomized to subcutaneous semaglutide (2.4 mg weekly) or placebo [140]. This trial also showed a significant reduction in C-reactive protein (CRP) in the semaglutide arm compared with the placebo. Importantly, a prespecified trial sub-analysis showed that semaglutide improved symptoms, physical limitations, and exercise function and reduced inflammation and body weight across obesity categories. Furthermore, the magnitude of benefit was directly related to the extent of weight loss (adjusting for age, sex, body weight at baseline, and other confounding variables) [146]. Semaglutide also greatly improved HF-related symptoms, physical limitations, exercise function, and NT-proBNP regardless of baseline health status [147]. In the STEP-HFpEF, semaglutide was also shown to reduce NPs. This evidence suggests a direct hemodynamic mechanism for semaglutide, regardless of caloric and metabolic improvement [148].
The STEP HFpEF DM trial (NCT04916470), which recently completed enrolment, will test the safety and benefit of semaglutide in obesity-related HFpEF patients with T2DM and explore the interaction with SGLT2i, as their use was rare in STEP-HFpEF but not in STEP HFpEF DM (32%) [140] (Table 4, Ref. [146, 147, 148, 149, 150]).
Table 4.
Characteristics of most recent clinical trials on semaglutide.
| Trial name, Author, Year | PIONEER 6, Husain et al. [149], 2019 | STEP-HFpEF, Kosiborod et al. [146, 147, 148], 2023 | SELECT, Lincoff et al. [150], 2023 |
| Trial design | Event-driven, randomized, double-blind, placebo-controlled trial | Multinational (96 centers among 13 countries), double-blind, randomized, placebo-controlled clinical trial | Randomized, international multicenter, double-blind, placebo-controlled clinical trial |
| Study population | Patients with T2DM and: | Symptomatic patients with HFpEF (EF 45%), obesity (BMI 30 ) and without T2DM | Patients with overweight or obesity (BMI 27 ), established CVD (previous MI or stroke, or PAD) and without T2DM |
| 50 years old and CV disease or CKD, or | |||
| 60 years old and CV risk factors only | |||
| Intervention and control | Oral semaglutide (target dose, 14 mg) or placebo once-daily | Subcutaneous semaglutide (2.4 mg) or placebo once weekly | Subcutaneous semaglutide (2.4 mg) or placebo once weekly |
| Primary endpoint | Time to the first occurrence of a MACE, a composite of death from CV causes, nonfatal myocardial infarction, or nonfatal stroke | Change in KCCQ -CSS from baseline (week 0) to end of treatment (week 52) | Composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, assessed in a time-to-first-event analysis |
| Change in body weight (%) from baseline (week 0) to end of treatment (week 52) | |||
| Main secondary endpoints | Time to the first occurrence of the following: an expanded composite outcome consisting of the primary outcome plus unstable angina resulting in hospitalization or HF resulting in hospitalization a composite of death from any cause, nonfatal myocardial infarction, or nonfatal stroke the individual components of these composite outcomes |
Change in 6-MWD (meters) from baseline (week 0) to end of treatment (week 52) Hierarchical composite of time to all-cause death from baseline (week 0) to end of study (week 57) Hierarchical composite of number of HF events requiring hospitalization or urgent HF visit from baseline (week 0) to end of study (week 57) Hierarchical composite of time to first HF event requiring hospitalization or urgent HF visit from baseline (week 0) to end of study (week 57) Change in CRP (%) from baseline (week -2) to end of treatment (week 52) |
Time-to-first-event analyses and tested in hierarchical order: Death from cardiovascular causes; A composite HF end point (death from cardiovascular causes or hospitalization or an urgent medical visit for HF); Death from any cause. Additional endpoints: Change in systolic blood pressure Change in body weight |
| Number of patients enrolled | 3183; semaglutide (n = 1591) or placebo (n = 1592) | 529; semaglutide (n = 263) or placebo (n = 266) | 17,604; semaglutide (n = 8803) or placebo (n = 8801) |
| Median duration of follow up | 64 weeks | 52 weeks | 160 weeks |
| Main results |
Primary endpoint (semaglutide vs. placebo): MACEs: 3.8% vs. 4.8% (HR, 0.79; 95% CI, 0.57 to 1.11 p 0.001 for noninferiority) Secondary endpoints (semaglutide vs. placebo): Death from CV causes: 0.9% vs. 1.9% (HR, 0.49; 95% CI, 0.27 to 0.92) Nonfatal myocardial infarction: 2.3% vs. 1.9% (HR, 1.18; 95% CI, 0.73 to 1.90) Nonfatal stroke: 0.8% vs. 1.0% (HR, 0.74; 95% CI, 0.35 to 1.57) First events of HF resulting in hospitalization: 1.3% vs. 1.5% (HR, 0.86; 95% CI, 0.48 to 1.55) Death from any cause: 1.4% vs. 2.8% (HR, 0.51; 95% CI, 0.31 to 0.84) |
Co-primary endpoint (semaglutide vs. placebo): Change in KCCQ-CSS: 16.6 vs. 8.7 (p 0.001) Percentage change in body weight: –13.3 vs. –2.6 (p 0.001) Secondary endpoints (semaglutide vs. placebo): Change in 6-MWD from baseline to week 52: 21.5 vs. 1.2 m (p 0.001) Percentage reduction from baseline to week 52 in NT-proBNP: –20.9 vs. –5.3 (p 0.05) Hospitalization or urgent visit for HF: 1 vs. 12 events (p 0.05) Reduction in CRP levels at week 52: 43.5 vs. 7.3 (p 0.001) Percentage reduction in NT-proBNP level at week 52: –20.9 vs. –5.3 Adverse events were similar |
Primary endpoint (semaglutide vs. placebo): |
| Composite of CV death, nonfatal MI, and nonfatal stroke, for semaglutide vs. placebo: 6.5% vs. 8.0% (HR 0.80, 95% CI 0.72–0.90, p 0.001) | |||
| Secondary endpoints (semaglutide vs. placebo): | |||
| CV death: 2.5% vs. 3.0% (HR 0.85, 95% CI 0.71–1.01, p = 0.07) | |||
| HF composite end point: 3.4% vs. 4.1% (HR 0.82, 95% CI 0.71–0.96) | |||
| All-cause death: 4.3% vs. 5.2% (HR 0.81, 95% CI 0.71–0.93) | |||
| Nonfatal MI: 2.7% vs. 3.7% (HR 0.72, 95% CI 0.61–0.85) | |||
| Hospitalization or urgent medical visit for HF: 1.1% vs. 1.4% (HR 0.79, 95% CI 0.60–1.03) | |||
| Additional endpoints: | |||
| Change in systolic blood pressure: –3.8 vs. –0.5 mm Hg | |||
| Mean change in body weight at 104 weeks: –9.4% vs. –0.9% |
BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; CVD, cardiovascular disease; CKD, chronic kidney disease; HR, hazard ratio; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score; MACE, major cardiovascular event; MI, myocardial; HFpEF, heart failure with preserved ejection fraction; T2DM, type 2 diabetes mellitus; CV, cardiovascular; HF, heart failure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAD, peripheral artery disease; EF, ejection fraction; 6-MWD, six minute walking test.
Given the results derived from RCTs, international scientific societies currently recommend using GLP1RAs as part of a comprehensive strategy to reduce the risk of CV events in patients with T2DM [21], although they have yet to be recommended to prevent HF [151, 152].
More recently, results of a SELECT study demonstrated the superiority of semaglutide when added to a typical standard of care, compared with placebo, in overweight or obese patients without T2DM, in reducing the incidence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke at a mean follow-up of 39.8 months [153]. Semaglutide also showed a non-significant reduction in the heart failure composite endpoint (HR 0.82 [0.71–0.96]).
New molecules are currently being developed and tested. For example, tirzepatide is a dual agonist of glucose-dependent insulinotropic polypeptide and GLP1R, thereby constituting a novel treatment option for T2DM. This agent exerts additional effects in addition to improvement in glycemic control, which can benefit individuals with T2DM, especially those at risk for or with established CV disease or HF. However, current evidence is limited, although it is suggestive of the cardiovascular safety of tirzepatide [150]. SUMMIT is an ongoing RCT that will assess the efficacy and safety of tirzepatide (LY3298176), compared with the placebo, in participants with the obesity phenotype of HFpEF [154, 155, 156, 157, 158, 159, 160, 161, 162].
12. Potential Effects of Vericiguat in HFpEF
Vericiguat is a drug that stimulates the cGMP pathway through direct and indirect stimulation of soluble guanylate cyclase. The current mechanism increases nitric oxide synthase (eNOS) at the endothelial level, directly affecting cardiac workload by reducing systemic resistance and improving vascular compliance [156, 157]. The NO availability enhancement leads to smooth muscle cell relaxation and reduces hypertrophy, inflammation, and fibrosis [158]. Two recent trials demonstrated a potential drug effect in HFpEF: The SOCRATES-PRESERVED study showed no significant effect on mortality and hospitalization in patients with worsening heart failure. However, using vericiguat was associated with better tolerance and quality of life after a 3-month follow-up period [159]. More recently, the VITALITY study did not confirm preliminary findings, showing no differences in quality of life and 6-minute walking distance score [160]. These contrasting data could depend on the high frailty burden of enrolled patients and the short observational period [161].
13. Future Perspectives
Even though recent post-hoc analyses revealed that all drugs endorsed for patients with reduced ejection fraction have positive effects in subjects with HFpEF up to the cutoff 60%, there are still doubts regarding the effective benefits of all agents [162]. Presently, only SGLT2i have demonstrated a comparable effect in HFrEF and HFpEF, but the remaining data are based on putative analyses, surrogate endpoints, and retrospective data. Since HFpEF encompasses several subtypes with different risk profiles, patients’ frailty, pathophysiological mechanisms, and hemodynamic cardiovascular disorders, a tailored management algorithm needs to be precise according to these features. In particular, there are two settings in which therapeutic advances demonstrated significant improvement: inflammatory and metabolic pathways can be regulated by treatment, resulting in improved functional status, reduced HF hospitalization, and lower CV mortality.
14. Conclusions
In the last 5 years, additional putative analyses and post-hoc investigations in certain HFpEF phenotypes have indicated that traditional treatment used for HFrEF can be extended to individuals affected by HFpEF. The efficacy of various treatments helped to understand the pathophysiological mechanisms behind the development and maintenance of the HFpEF condition, resulting in the identification of new therapeutic targets. However, more research is needed to understand how these agents influence the natural history of specific HFpEF phenotypes. This is extremely important given the high frequency and poor prognosis of patients with HFpEF.
Acknowledgment
Not applicable.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
AP: conception and design; PS: interpretation of data; AD: acquisition of data; VM: analysis and interpretation of data; LT: substantial contribution to the study design; MC: conception and design of the work; GD: acquisition and analysis; FF: investigation and analysis; SN: study conception and reviewing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. Alberto Palazzuoli is serving as one of the Editorial Board members of this journal. We declare that Alberto Palazzuoli had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Wengen Zhu.
References
- [1].Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England Journal of Medicine . 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- [2].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation . 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- [3].Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovascular Research . 2023;118:3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- [4].Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. European Journal of Heart Failure . 2017;19:1574–1585. doi: 10.1002/ejhf.813. [DOI] [PubMed] [Google Scholar]
- [5].Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. Journal of the American College of Cardiology . 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- [6].Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology . 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- [7].Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology . 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- [8].Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. European Heart Journal . 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Patel HC, Hayward C, Dungu JN, Papadopoulou S, Saidmeerasah A, Ray R, et al. Assessing the Eligibility Criteria in Phase III Randomized Controlled Trials of Drug Therapy in Heart Failure With Preserved Ejection Fraction: The Critical Play-Off Between a “Pure” Patient Phenotype and the Generalizability of Trial Findings. Journal of Cardiac Failure . 2017;23:517–524. doi: 10.1016/j.cardfail.2017.04.006. [DOI] [PubMed] [Google Scholar]
- [10].Wintrich J, Kindermann I, Ukena C, Selejan S, Werner C, Maack C, et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clinical Research in Cardiology . 2020;109:1079–1098. doi: 10.1007/s00392-020-01633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal . 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- [12].Vaishnav J, Sharma K. A Stepwise Guide to the Diagnosis and Treatment of Heart Failure With Preserved Ejection Fraction. Journal of Cardiac Failure . 2022;28:1016–1030. doi: 10.1016/j.cardfail.2021.12.013. [DOI] [PubMed] [Google Scholar]
- [13].Ho JE, Redfield MM, Lewis GD, Paulus WJ, Lam CSP. Deliberating the Diagnostic Dilemma of Heart Failure With Preserved Ejection Fraction. Circulation . 2020;142:1770–1780. doi: 10.1161/CIRCULATIONAHA.119.041818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Čulić V. Heart Failure with Preserved Ejection Fraction. The New England Journal of Medicine . 2017;376:896. doi: 10.1056/NEJMc1615918. [DOI] [PubMed] [Google Scholar]
- [15].Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. European Journal of Heart Failure . 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- [16].Severino P, D’Amato A, Prosperi S, Dei Cas A, Mattioli AV, Cevese A, et al. Do the Current Guidelines for Heart Failure Diagnosis and Treatment Fit with Clinical Complexity? Journal of Clinical Medicine . 2022;11:857. doi: 10.3390/jcm11030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Severino P, D’Amato A, Prosperi S, Myftari V, Canuti ES, Labbro Francia A, et al. Heart Failure Pharmacological Management: Gaps and Current Perspectives. Journal of Clinical Medicine . 2023;12:1020. doi: 10.3390/jcm12031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fayol A, Wack M, Livrozet M, Carves JB, Domengé O, Vermersch E, et al. Aetiological classification and prognosis in patients with heart failure with preserved ejection fraction. ESC Heart Failure . 2022;9:519–530. doi: 10.1002/ehf2.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation . 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC. Heart Failure. . 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kyodo A, Kanaoka K, Keshi A, Nogi M, Nogi K, Ishihara S, et al. Heart failure with preserved ejection fraction phenogroup classification using machine learning. ESC Heart Failure . 2023;10:2019–2030. doi: 10.1002/ehf2.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Palazzuoli A, Tramonte F, Beltrami M. Laboratory and Metabolomic Fingerprint in Heart Failure with Preserved Ejection Fraction: From Clinical Classification to Biomarker Signature. Biomolecules . 2023;13:173. doi: 10.3390/biom13010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography . 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- [24].Ünlü S, Özden Ö, Çelik A. Imaging in Heart Failure with Preserved Ejection Fraction: A Multimodality Imaging Point of View. Cardiac Failure Review . 2023;9:e04. doi: 10.15420/cfr.2022.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circulation. Heart Failure. . 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shin SH, Claggett B, Inciardi RM, Santos ABS, Shah SJ, Zile MR, et al. Prognostic Value of Minimal Left Atrial Volume in Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association . 2021;10:e019545. doi: 10.1161/JAHA.120.019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hoit BD. Left atrial size and function: role in prognosis. Journal of the American College of Cardiology . 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- [28].Boe E, Smiseth OA. Left atrial strain imaging: ready for clinical implementation in heart failure with preserved ejection fraction. European Heart Journal. Cardiovascular Imaging. . 2022;23:1169–1170. doi: 10.1093/ehjci/jeac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Welsh P, Campbell RT, Mooney L, Kimenai DM, Hayward C, Campbell A, et al. Reference Ranges for NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) and Risk Factors for Higher NT-proBNP Concentrations in a Large General Population Cohort. Circulation. Heart Failure. . 2022;15:e009427. doi: 10.1161/CIRCHEARTFAILURE.121.009427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maisel AS, Duran JM, Wettersten N. Natriuretic Peptides in Heart Failure: Atrial and B-type Natriuretic Peptides. Heart Failure Clinics . 2018;14:13–25. doi: 10.1016/j.hfc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- [31].Morfino P, Aimo A, Castiglione V, Vergaro G, Emdin M, Clerico A. Biomarkers of HFpEF: Natriuretic Peptides, High-Sensitivity Troponins and Beyond. Journal of Cardiovascular Development and Disease . 2022;9:256. doi: 10.3390/jcdd9080256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Severino P, D’Amato A, Prosperi S, Fanisio F, Birtolo LI, Costi B, et al. Myocardial Tissue Characterization in Heart Failure with Preserved Ejection Fraction: From Histopathology and Cardiac Magnetic Resonance Findings to Therapeutic Targets. International Journal of Molecular Sciences . 2021;22:7650. doi: 10.3390/ijms22147650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chamsi-Pasha MA, Zhan Y, Debs D, Shah DJ. CMR in the Evaluation of Diastolic Dysfunction and Phenotyping of HFpEF: Current Role and Future Perspectives. JACC. Cardiovascular Imaging. . 2020;13:283–296. doi: 10.1016/j.jcmg.2019.02.031. [DOI] [PubMed] [Google Scholar]
- [34].Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, et al. Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects. JACC. Cardiovascular Imaging. . 2018;11:577–585. doi: 10.1016/j.jcmg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- [35].Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, et al. Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. International Journal of Cardiology . 2015;191:314–319. doi: 10.1016/j.ijcard.2015.05.048. [DOI] [PubMed] [Google Scholar]
- [36].Palazzuoli A, Buono MGD, Ruocco G, Caravita S, Abbate A, Lavie CJ. The Conundrum of HFpEF Definition: Non-Invasive Assessment Uncertainties and Alternative Diagnostic Strategies. Current Problems in Cardiology . 2023;48:101433. doi: 10.1016/j.cpcardiol.2022.101433. [DOI] [PubMed] [Google Scholar]
- [37].Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) European Heart Journal . 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- [38].Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation . 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Uijl A, Savarese G, Vaartjes I, Dahlström U, Brugts JJ, Linssen GCM, et al. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. European Journal of Heart Failure . 2021;23:973–982. doi: 10.1002/ejhf.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gouda P, Alemayehu W, Rathwell S, Ian Paterson D, Anderson T, Dyck JRB, et al. Clinical Phenotypes of Heart Failure across the spectrum of Ejection Fraction: A Cluster Analysis. Current Problems in Cardiology . 2022;47:101337. doi: 10.1016/j.cpcardiol.2022.101337. [DOI] [PubMed] [Google Scholar]
- [41].Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, et al. Echocardiographic Features of Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction. Journal of the American College of Cardiology . 2019;74:2858–2873. doi: 10.1016/j.jacc.2019.09.063. [DOI] [PubMed] [Google Scholar]
- [42].Solomon SD, Vaduganathan M, L Claggett B, Packer M, Zile M, Swedberg K, et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation . 2020;141:352–361. doi: 10.1161/CIRCULATIONAHA.119.044586. [DOI] [PubMed] [Google Scholar]
- [43].Palazzuoli A, Ruocco G. Different Phenotype Characterization in PARAGON-HF: An Unresolved Puzzle in Patients With HFpEF. Journal of the American College of Cardiology . 2020;75:1500–1501. doi: 10.1016/j.jacc.2020.01.029. [DOI] [PubMed] [Google Scholar]
- [44].Peters AE, Tromp J, Shah SJ, Lam CSP, Lewis GD, Borlaug BA, et al. Phenomapping in heart failure with preserved ejection fraction: insights, limitations, and future directions. Cardiovascular Research . 2023;118:3403–3415. doi: 10.1093/cvr/cvac179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, et al. Patient selection in heart failure with preserved ejection fraction clinical trials. Journal of the American College of Cardiology . 2015;65:1668–1682. doi: 10.1016/j.jacc.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Palazzuoli A, Caravita S, Paolillo S, Ghio S, Tocchetti CG, Ruocco G, et al. Current gaps in HFpEF trials: Time to reconsider patients’ selection and to target phenotypes. Progress in Cardiovascular Diseases . 2021;67:89–97. doi: 10.1016/j.pcad.2021.03.007. [DOI] [PubMed] [Google Scholar]
- [47].Smit MD, Moes ML, Maass AH, Achekar ID, Van Geel PP, Hillege HL, et al. The importance of whether atrial fibrillation or heart failure develops first. European Journal of Heart Failure . 2012;14:1030–1040. doi: 10.1093/eurjhf/hfs097. [DOI] [PubMed] [Google Scholar]
- [48].Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JGF, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet . 2014;384:2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- [49].Packer M, O’Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. The New England Journal of Medicine . 1996;335:1107–1114. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- [50].Cohn JN, Ziesche S, Smith R, Anand I, Dunkman WB, Loeb H, et al. Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Vasodilator-Heart Failure Trial (V-HeFT) Study Group. Circulation. . 1997;96:856–863. doi: 10.1161/01.cir.96.3.856. [DOI] [PubMed] [Google Scholar]
- [51].IONA Study Group Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet . 2002;359:1269–1275. doi: 10.1016/S0140-6736(02)08265-X. [DOI] [PubMed] [Google Scholar]
- [52].Kanamasa K, Hayashi T, Kimura A, Ikeda A, Ishikawa K. Long-term, continuous treatment with both oral and transdermal nitrates increases cardiac events in healed myocardial infarction patients. Angiology . 2002;53:399–408. doi: 10.1177/000331970205300405. [DOI] [PubMed] [Google Scholar]
- [53].Wilson SR, Scirica BM, Braunwald E, Murphy SA, Karwatowska-Prokopczuk E, Buros JL, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. Journal of the American College of Cardiology . 2009;53:1510–1516. doi: 10.1016/j.jacc.2009.01.037. [DOI] [PubMed] [Google Scholar]
- [54].Omar W, Pandey A, Haykowsky MJ, Berry JD, Lavie CJ. The Evolving Role of Cardiorespiratory Fitness and Exercise in Prevention and Management of Heart Failure. Current Heart Failure Reports . 2018;15:75–80. doi: 10.1007/s11897-018-0382-z. [DOI] [PubMed] [Google Scholar]
- [55].Löfman I, Szummer K, Evans M, Carrero JJ, Lund LH, Jernberg T. Incidence of, Associations With and Prognostic Impact of Worsening Renal Function in Heart Failure With Different Ejection Fraction Categories. The American Journal of Cardiology . 2019;124:1575–1583. doi: 10.1016/j.amjcard.2019.07.065. [DOI] [PubMed] [Google Scholar]
- [56].Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine . 2021;203:24–36. doi: 10.1164/rccm.202009-3533SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. The European Respiratory Journal . 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- [58].Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. European Heart Journal . 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- [59].Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet . 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- [60].Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. The New England Journal of Medicine . 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- [61].Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. The New England Journal of Medicine . 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- [62].Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation . 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. The New England Journal of Medicine . 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- [64].van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) Journal of the American College of Cardiology . 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- [65].Dalal J, Chandra P, Ray S, Hazra PK, Hiremath J, Kumar V, et al. Practical Recommendations for the Use of Angiotensin Receptor-Neprilysin Inhibitors (ARNI) in Heart Failure: Insights from Indian Cardiologists. Cardiology and Therapy . 2023;12:445–471. doi: 10.1007/s40119-023-00323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jia R, Ji Y, Sun D. Progress and prospects of Sacubitril/Valsartan: Based on heart failure with preserved ejection fraction. Biomedicine & Pharmacotherapy . 2022;155:113701. doi: 10.1016/j.biopha.2022.113701. [DOI] [PubMed] [Google Scholar]
- [67].Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation . 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- [68].Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet . 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- [69].Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, et al. Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial. JAMA . 2021;326:1919–1929. doi: 10.1001/jama.2021.18463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. The New England Journal of Medicine . 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- [71].Mentz RJ, Ward JH, Hernandez AF, Lepage S, Morrow DA, Sarwat S, et al. Angiotensin-Neprilysin Inhibition in Patients With Mildly Reduced or Preserved Ejection Fraction and Worsening Heart Failure. Journal of the American College of Cardiology . 2023;82:1–12. doi: 10.1016/j.jacc.2023.04.019. [DOI] [PubMed] [Google Scholar]
- [72].Kuno T, Ueyama H, Fujisaki T, Briasouli A, Takagi H, Briasoulis A. Meta-Analysis Evaluating the Effects of Renin-Angiotensin-Aldosterone System Blockade on Outcomes of Heart Failure With Preserved Ejection Fraction. The American Journal of Cardiology . 2020;125:1187–1193. doi: 10.1016/j.amjcard.2020.01.009. [DOI] [PubMed] [Google Scholar]
- [73].Vaduganathan M, Mentz RJ, Claggett BL, Miao ZM, Kulac IJ, Ward JH, et al. Sacubitril/valsartan in heart failure with mildly reduced or preserved ejection fraction: a pre-specified participant-level pooled analysis of PARAGLIDE-HF and PARAGON-HF. European Heart Journal . 2023;44:2982–2993. doi: 10.1093/eurheartj/ehad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Aimo A, Senni M, Barison A, Panichella G, Passino C, Bayes-Genis A, et al. Management of heart failure with preserved ejection fraction: from neurohormonal antagonists to empagliflozin. Heart Failure Reviews . 2023;28:179–191. doi: 10.1007/s10741-022-10228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Peh ZH, Dihoum A, Hutton D, Arthur JSC, Rena G, Khan F, et al. Inflammation as a therapeutic target in heart failure with preserved ejection fraction. Frontiers in Cardiovascular Medicine . 2023;10:1125687. doi: 10.3389/fcvm.2023.1125687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jadhav U, Nair T, Mohanan P, Chopra V, Kerkar P, Das Biswas A, et al. Impact of Mineralocorticoid Receptor Antagonists in the Treatment of Heart Failure: Targeting the Heart Failure Cascade. Cureus . 2023;15:e45241. doi: 10.7759/cureus.45241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA . 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- [78].Mitter SS, Shah SJ. Spironolactone for Management of Heart Failure with Preserved Ejection Fraction: Whither to After TOPCAT? Current atherosclerosis reports . 2015;17:64. doi: 10.1007/s11883-015-0541-6. [DOI] [PubMed] [Google Scholar]
- [79].Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation . 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- [80].Lavall D, Jacobs N, Mahfoud F, Kolkhof P, Böhm M, Laufs U. The non-steroidal mineralocorticoid receptor antagonist finerenone prevents cardiac fibrotic remodeling. Biochemical Pharmacology . 2019;168:173–183. doi: 10.1016/j.bcp.2019.07.001. [DOI] [PubMed] [Google Scholar]
- [81].Derosa G, Maffioli P, Scelsi L, Bestetti A, Vanasia M, Cicero AFG, et al. Canrenone on cardiovascular mortality in congestive heart failure: CanrenOne eFFects on cardiovascular mortality in patiEnts with congEstIve hearT failure: The COFFEE-IT study. Pharmacological Research . 2019;141:46–52. doi: 10.1016/j.phrs.2018.11.037. [DOI] [PubMed] [Google Scholar]
- [82].Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction (SPIRRIT) 2017. [(Accessed: 21 May 2017)]. Available at: https://clinicaltrials.gov/ct2/show/NCT02901184. NLM Identifier: NCT02901184.
- [83].Spironolactone In The Treatment of Heart Failure (SPIRIT-HF) Available at: https://clinicaltrials.gov/study/NCT04727073 NLM Identifier: NCT04727073.
- [84].Kintscher U, Edelmann F. The non-steroidal mineralocorticoid receptor antagonist finerenone and heart failure with preserved ejection fraction. Cardiovascular Diabetology . 2023;22:162. doi: 10.1186/s12933-023-01899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. The New England Journal of Medicine . 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- [86].Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. The New England Journal of Medicine . 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- [87].Shah M, Awad AS, Abdel-Rahman EM. Nonsteroidal Mineralocorticoid Receptor Antagonist (Finerenone) in Cardiorenal Disease. Journal of clinical medicine . 2023;12:6285. doi: 10.3390/jcm12196285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Correale M, Petroni R, Coiro S, Antohi EL, Monitillo F, Leone M, et al. Paradigm shift in heart failure treatment: are cardiologists ready to use gliflozins? Heart Failure Reviews . 2022;27:1147–1163. doi: 10.1007/s10741-021-10107-8. [DOI] [PubMed] [Google Scholar]
- [89].Liang B, Liang Y, Gu N. Pharmacological mechanisms of sodium-glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction. BMC Cardiovascular Disorders . 2022;22:261. doi: 10.1186/s12872-022-02693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Correale M, Lamacchia O, Ciccarelli M, Dattilo G, Tricarico L, Brunetti ND. Vascular and metabolic effects of SGLT2i and GLP-1 in heart failure patients. Heart Failure Reviews . 2023;28:733–744. doi: 10.1007/s10741-021-10157-y. [DOI] [PubMed] [Google Scholar]
- [91].Gronda E, Colivicchi F, Zuccalà G. Letter by Gronda et al Regarding Article, “Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial”. Circulation . 2022;145:e839–e840. doi: 10.1161/CIRCULATIONAHA.121.057530. [DOI] [PubMed] [Google Scholar]
- [92].Butler J, Usman MS, Filippatos G, Ferreira JP, Böhm M, Brueckmann M, et al. Safety and Efficacy of Empagliflozin and Diuretic Use in Patients with Heart Failure and Preserved Ejection Fraction: A Post Hoc Analysis of the EMPEROR-Preserved Trial. JAMA Cardiology . 2023;8:640–649. doi: 10.1001/jamacardio.2023.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Madonna R. Exploring the mechanisms of action of gliflozines in heart failure and possible implications in pulmonary hypertension. Vascular Pharmacology . 2021;138:106839. doi: 10.1016/j.vph.2021.106839. [DOI] [PubMed] [Google Scholar]
- [94].Pandey AK, Bhatt DL, Pandey A, Marx N, Cosentino F, Pandey A, et al. Mechanisms of benefits of sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction. European Heart Journal . 2023;44:3640–3651. doi: 10.1093/eurheartj/ehad389. [DOI] [PubMed] [Google Scholar]
- [95].Zhang X, Wang N, Fu P, An Y, Sun F, Wang C, et al. Dapagliflozin Attenuates Heart Failure With Preserved Ejection Fraction Remodeling and Dysfunction by Elevating β-Hydroxybutyrate-activated Citrate Synthase. Journal of Cardiovascular Pharmacology . 2023;82:375–388. doi: 10.1097/FJC.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mone P, Lombardi A, Kansakar U, Varzideh F, Jankauskas SS, Pansini A, et al. Empagliflozin Improves the MicroRNA Signature of Endothelial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Diabetes. The Journal of Pharmacology and Experimental Therapeutics . 2023;384:116–122. doi: 10.1124/jpet.121.001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Correale M, Mazzeo P, Fortunato M, Paradiso M, Furore A, Fanizzi AI, et al. Switch to gliflozins and biventricular function improvement in patients with chronic heart failure and diabetes mellitus. Clinical Physiology and Functional Imaging . 2024;44:112–117. doi: 10.1111/cpf.12857. [DOI] [PubMed] [Google Scholar]
- [98].Zhang T, Wang X, Wang Z, Zhai J, He L, Wang Y, et al. Canagliflozin Ameliorates Ventricular Remodeling through Apelin/Angiotensin-Converting Enzyme 2 Signaling in Heart Failure with Preserved Ejection Fraction Rats. Pharmacology . 2023;108:478–491. doi: 10.1159/000533277. [DOI] [PubMed] [Google Scholar]
- [99].Ma S, He LL, Zhang GR, Zuo QJ, Wang ZL, Zhai JL, et al. Canagliflozin mitigates ferroptosis and ameliorates heart failure in rats with preserved ejection fraction. Naunyn-Schmiedeberg’s Archives of Pharmacology . 2022;395:945–962. doi: 10.1007/s00210-022-02243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Borlaug BA, Reddy YNV, Braun A, Sorimachi H, Omar M, Popovic D, et al. Cardiac and Metabolic Effects of Dapagliflozin in Heart Failure With Preserved Ejection Fraction: The CAMEO-DAPA Trial. Circulation . 2023;148:834–844. doi: 10.1161/CIRCULATIONAHA.123.065134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Packer M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nature Reviews. Cardiology. . 2023;20:443–462. doi: 10.1038/s41569-022-00824-4. [DOI] [PubMed] [Google Scholar]
- [102].Bayes-Genis A, Iborra-Egea O, Spitaleri G, Domingo M, Revuelta-López E, Codina P, et al. Decoding empagliflozin’s molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Scientific Reports . 2021;11:12025. doi: 10.1038/s41598-021-91546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovascular Research . 2021;117:495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- [104].Zhang N, Feng B, Ma X, Sun K, Xu G, Zhou Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovascular Diabetology . 2019;18:107. doi: 10.1186/s12933-019-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Guan XQ, Wang CH, Cheng P, Fu LY, Wu QJ, Cheng G, et al. Effects of Empagliflozin on Gut Microbiota in Heart Failure with a Preserved Ejection Fraction: The Design of a Pragmatic Randomized, Open-Label Controlled Trial (EMPAGUM) Drug Design, Development and Therapy . 2023;17:1495–1502. doi: 10.2147/DDDT.S404479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. The New England Journal of Medicine . 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- [107].Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. European Heart Journal . 2022;43:416–426. doi: 10.1093/eurheartj/ehab798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. The New England Journal of Medicine . 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- [109].Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, et al. Dapagliflozin in Patients Recently Hospitalized With Heart Failure and Mildly Reduced or Preserved Ejection Fraction. Journal of the American College of Cardiology . 2022;80:1302–1310. doi: 10.1016/j.jacc.2022.07.021. [DOI] [PubMed] [Google Scholar]
- [110].Chatur S, Cunningham JW, Vaduganathan M, Mc Causland FR, Claggett BL, Desai AS, et al. Renal and blood pressure effects of dapagliflozin in recently hospitalized patients with heart failure with mildly reduced or preserved ejection fraction: Insights from the DELIVER trial. European Journal of Heart Failure . 2023;25:1170–1175. doi: 10.1002/ejhf.2915. [DOI] [PubMed] [Google Scholar]
- [111].Wang Y, Gao T, Meng C, Li S, Bi L, Geng Y, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: an updated systematic review and meta-analysis. European Journal of Medical Research . 2022;27:314. doi: 10.1186/s40001-022-00945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. The Lancet. Diabetes & Endocrinology. . 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovascular Diabetology . 2021;20:154. doi: 10.1186/s12933-021-01344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet . 2018;391:2430–2440. doi: 10.1016/S0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- [115].Nodari S, Fioretti F, Barilla F. Redefining diabetes mellitus treatments according to different mechanisms beyond hypoglycaemic effect. Heart Failure Reviews . 2023;28:607–625. doi: 10.1007/s10741-021-10203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovascular Diabetology . 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. Journal of the American College of Cardiology . 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circulation. Heart Failure. . 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. European Heart Journal . 2015;36:1718–1718. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- [120].Ferreira JP, Sharma A, Butler J, Packer M, Zannad F, Vasques-Nóvoa F, et al. Glucagon-Like Peptide-1 Receptor Agonists Across the Spectrum of Heart Failure. The Journal of Clinical Endocrinology and Metabolism . 2023;109:4–9. doi: 10.1210/clinem/dgad398. [DOI] [PubMed] [Google Scholar]
- [121].Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nature Reviews. Cardiology. . 2023;20:463–474. doi: 10.1038/s41569-023-00849-3. [DOI] [PubMed] [Google Scholar]
- [122].Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation . 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- [123].Drucker DJ. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metabolism . 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- [124].McLean BA, Wong CK, Kaur KD, Seeley RJ, Drucker DJ. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight . 2021;6:e153732. doi: 10.1172/jci.insight.153732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kosiborod MN, Bhatta M, Davies M, Deanfield JE, Garvey WT, Khalid U, et al. Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes, Obesity & Metabolism . 2023;25:468–478. doi: 10.1111/dom.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hasegawa Y, Hori M, Nakagami T, Harada-Shiba M, Uchigata Y. Glucagon-like peptide-1 receptor agonists reduced the low-density lipoprotein cholesterol in Japanese patients with type 2 diabetes mellitus treated with statins. Journal of Clinical Lipidology . 2018;12:62–69. doi: 10.1016/j.jacl.2017.11.006. e1. [DOI] [PubMed] [Google Scholar]
- [127].Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation . 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- [128].Almutairi M, Gopal K, Greenwell AA, Young A, Gill R, Aburasayn H, et al. The GLP-1 Receptor Agonist Liraglutide Increases Myocardial Glucose Oxidation Rates via Indirect Mechanisms and Mitigates Experimental Diabetic Cardiomyopathy. The Canadian Journal of Cardiology . 2021;37:140–150. doi: 10.1016/j.cjca.2020.02.098. [DOI] [PubMed] [Google Scholar]
- [129].Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. The Lancet. Diabetes & Endocrinology. . 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- [130].Ferreira JP, Sharma A, Vasques-Nóvoa F, Angélico-Gonçalves A, Leite AR, Borges-Canha M, et al. Albiglutide in patients with type 2 diabetes and heart failure: a post-hoc analysis from Harmony Outcomes. European Journal of Heart Failure . 2022;24:1792–1801. doi: 10.1002/ejhf.2660. [DOI] [PubMed] [Google Scholar]
- [131].Branch KRH, Dagenais GR, Avezum A, Basile J, Conget I, Cushman WC, et al. Dulaglutide and cardiovascular and heart failure outcomes in patients with and without heart failure: a post-hoc analysis from the REWIND randomized trial. European Journal of Heart Failure . 2022;24:1805–1812. doi: 10.1002/ejhf.2670. [DOI] [PubMed] [Google Scholar]
- [132].Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical Research Ed.) . 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. European Journal of Heart Failure . 2021;23:1858–1871. doi: 10.1002/ejhf.2337. [DOI] [PubMed] [Google Scholar]
- [134].Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. European Journal of Heart Failure . 2022;24:1346–1356. doi: 10.1002/ejhf.2513. [DOI] [PubMed] [Google Scholar]
- [135].Venkateshvaran A, Faxen UL, Hage C, Michaëlsson E, Svedlund S, Saraste A, et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: insights from the PROMIS-HFpEF study. European Journal of Heart Failure . 2022;24:2251–2260. doi: 10.1002/ejhf.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Pocock SJ, McMurray JJV, Dobson J, Yusuf S, Granger CB, Michelson EL, et al. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. European Heart Journal . 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- [137].Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA . 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, et al. Body Weight Change During and After Hospitalization for Acute Heart Failure: Patient Characteristics, Markers of Congestion, and Outcomes: Findings From the ASCEND-HF Trial. JACC. Heart Failure. . 2017;5:1–13. doi: 10.1016/j.jchf.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Miranda WR, Batsis JA, Sarr MG, Collazo-Clavell ML, Clark MM, Somers VK, et al. Impact of bariatric surgery on quality of life, functional capacity, and symptoms in patients with heart failure. Obesity Surgery . 2013;23:1011–1015. doi: 10.1007/s11695-013-0953-8. [DOI] [PubMed] [Google Scholar]
- [140].Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet . 2019;394:39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- [141].Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric Surgery and Emergency Department Visits and Hospitalizations for Heart Failure Exacerbation: Population-Based, Self-Controlled Series. Journal of the American College of Cardiology . 2016;67:895–903. doi: 10.1016/j.jacc.2015.12.016. [DOI] [PubMed] [Google Scholar]
- [142].Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. The Lancet. Diabetes & Endocrinology. . 2018;6:275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- [143].Kamisaka K, Kamiya K, Iwatsu K, Iritani N, Imoto S, Adachi T, et al. Impact of weight loss in patients with heart failure with preserved ejection fraction: results from the FLAGSHIP study. ESC Heart Failure . 2021;8:5293–5303. doi: 10.1002/ehf2.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care . 2018;41:258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- [145].Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) Diabetes & Metabolism. . 2020;46:100–109. doi: 10.1016/j.diabet.2019.101117. [DOI] [PubMed] [Google Scholar]
- [146].Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. The New England Journal of Medicine . 2023;389:1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- [147].Borlaug BA, Kitzman DW, Davies MJ, Rasmussen S, Barros E, Butler J, et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nature Medicine . 2023;29:2358–2365. doi: 10.1038/s41591-023-02526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kosiborod MN, Verma S, Borlaug BA, Butler J, Davies MJ, Jon Jensen T, et al. Effects of Semaglutide on Symptoms, Function, and Quality of Life in Patients With Heart Failure With Preserved Ejection Fraction and Obesity: A Prespecified Analysis of the STEP-HFpEF Trial. Circulation . 2024;149:204–216. doi: 10.1161/CIRCULATIONAHA.123.067505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine . 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- [150].Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. The New England Journal of Medicine . 2023;389:2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- [151].Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Christensen L, Davies M, et al. Design and Baseline Characteristics of STEP-HFpEF Program Evaluating Semaglutide in Patients With Obesity HFpEF Phenotype. JACC. Heart Failure. . 2023;11:1000–1010. doi: 10.1016/j.jchf.2023.05.010. [DOI] [PubMed] [Google Scholar]
- [152].Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. European Heart Journal . 2023;44:4043–4140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- [153].ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care . 2023;46:S140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Patoulias D, Dimosiari A, Fragakis N. Tirzepatide for the treatment of heart failure in Type 2 diabetes mellitus: (SUR)PASS, or not? Future Cardiology . 2023;19:301–312. doi: 10.2217/fca-2022-0112. [DOI] [PubMed] [Google Scholar]
- [155].De Block C, Bailey C, Wysham C, Hemmingway A, Allen SE, Peleshok J. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes, obesity & metabolism . 2023;25:3–17. doi: 10.1111/dom.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Gheorghiade M, Marti CN, Sabbah HN, Roessig L, Greene SJ, Böhm M, et al. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Failure Reviews . 2013;18:123–134. doi: 10.1007/s10741-012-9323-1. [DOI] [PubMed] [Google Scholar]
- [157].Vannuccini F, Campora A, Barilli M, Palazzuoli A. Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications. Biomedicines . 2022;10:2471. doi: 10.3390/biomedicines10102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Emdin M, Aimo A, Castiglione V, Vergaro G, Georgiopoulos G, Saccaro LF, et al. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure: JACC Review Topic of the Week. Journal of the American College of Cardiology . 2020;76:1795–1807. doi: 10.1016/j.jacc.2020.08.031. [DOI] [PubMed] [Google Scholar]
- [159].Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. European Heart Journal . 2017;38:1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O’Connor CM, et al. Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA . 2020;324:1512–1521. doi: 10.1001/jama.2020.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Kaul P, Rathwell S, Lam CSP, Westerhout CM, Spertus JA, Anstrom KJ, et al. Patient-Reported Frailty and Functional Status in Heart Failure With Preserved Ejection Fraction: Insights From VITALITY-HFpEF. JACC. Heart Failure. . 2023;11:392–403. doi: 10.1016/j.jchf.2022.11.015. [DOI] [PubMed] [Google Scholar]
- [162].Böhm M, Bewarder Y, Kindermann I. Ejection fraction in heart failure revisited- where does the evidence start? European Heart Journal . 2020;41:2363–2365. doi: 10.1093/eurheartj/ehaa281. [DOI] [PubMed] [Google Scholar]