Abstract
The mechanisms underlying the hepatotropism of hepatitis A virus (HAV) and the relapsing courses of HAV infections are unknown. In this report, we show for a mouse hepatocyte model that HAV-specific immunoglobulin A (IgA) mediates infection of hepatocytes with HAV via the asialoglycoprotein receptor, which binds and internalizes IgA molecules. Proof of HAV infection was obtained by detection of HAV minus-strand RNA, which is indicative for virus replication, and quantification of infectious virions. We demonstrate that human hepatocytes also ingest HAV–anti-HAV IgA complexes by the same mechanism, resulting in infection of the cells, by using the HepG2 cell line and primary hepatocytes. The relevance of this surrogate receptor mechanism in HAV pathogenesis lies in the fact that HAV, IgA, and antigen-IgA complexes use the same pathway within the organism, leading from the gastrointestinal tract to the liver via blood and back to the gastrointestinal tract via bile fluid. Therefore, HAV-specific IgA antibodies produced by gastrointestinal mucosa-associated lymphoid tissue may serve as carrier and targeting molecules, enabling and supporting HAV infection of IgA receptor-positive hepatocytes and, in the case of relapsing courses, allowing reinfection of the liver in the presence of otherwise neutralizing antibodies, resulting in exacerbation of liver disease.
Hepatitis A virus (HAV), a hepatotropic picornavirus (for a review, see reference 15), causes acute viral hepatitis in humans by an immunopathogenetic mechanism (41). The HAV infection is characterized by a short, self-limited disease and does not lead to chronic cases. However, after initial improvement in symptoms and liver test values, one or more relapses of the disease are described for up to 20% of patients (14, 40). These relapses occur between 30 and 90 days after the primary episode, when high titers of neutralizing antibodies are already detectable (12). HAV is transmitted by the fecal-oral route, but the mechanism by which the virus first enters the bloodstream and reaches the liver as well as the pathogenetic mechanism leading to a relapsing disease remains unclear.
Kaplan et al. (17) reported that a mucin-like class I integral membrane glycoprotein which was identified on African green monkey kidney cells acts as an attachment molecule for HAV. It was demonstrated that the human homolog is a binding receptor for HAV; it has been suggested that it is also a functional receptor (10). Although cell lines originating from tissues other than liver, such as fibroblasts and kidney cells, are also susceptible to HAV infection (9, 11) and although HAV antigen and the putative receptor for HAV could be detected in different organs, such as kidney, spleen, and gastrointestinal tract (2, 6, 10, 18), no extrahepatic sites of HAV replication have been clearly identified. The data on the ubiquitous expression of a receptor for HAV and the ability of HAV to replicate in a number of nonliver cells in cell cultures, but obviously not in the organism, suggest that HAV may be targeted to the liver by a particular mechanism.
Data from several laboratories showed that HAV virions are partially associated with immunoglobulin A (IgA) molecules (19, 22) and other host organism-derived materials, such as fibronectin or α2-macroglobulin (21, 23, 24, 35, 43). As viruses may find entry into host cells via receptors specific for molecules of the host organism ligated to the virion (13, 16, 20, 26) and as the liver plays a central role in IgA metabolism by eliminating IgA as well as antigen-IgA complexes (4), we wondered if HAV-specific IgA ligated to HAV supports the targeting of HAV to the liver and is able to mediate the entry of HAV into hepatocytes via receptors specific for the IgA molecule and if such a carrier-mediated mechanism may result in viral infection. This mechanism, by which a molecule normally designed to neutralize viral infectivity is recruited to arrange HAV infection of the liver, can explain still-unanswered questions about HAV pathogenesis, such as the lack of extrahepatic sites of replication and the relapsing courses of HAV infection in the presence of otherwise neutralizing antibodies (12, 14, 40). Therefore, our studies were designed to examine binding to and uptake into hepatocytes of HAV–anti-HAV IgA immunocomplexes and the following viral replication. We also investigated whether HAV–anti-HAV IgA complexes may play a role in the oral transmission of HAV.
MATERIALS AND METHODS
Cells.
The murine hepatocellular cell line NCTC clone 1469 (ATCC CCL 9.1) was used to investigate the IgA-assisted entry of HAV into hepatocytes. The cells were maintained as continuous cultures in Dulbecco modified Eagle medium (DMEM) supplemented with 1% fetal calf serum. In order to split the cells weekly at a ratio of 1:2, they were detached from the tissue culture plate with Versene and cultivated with DMEM–10% FCS as the growth medium.
FRhK-4, HepG2, and Ltk− cells were cultivated as described previously (9).
Human primary hepatocytes were kindly provided by B. Flehmig, Tübingen, Germany. The cells were grown in 24-well culture plates using minimum essential medium (MEM) supplemented with Hank's and Earl's salt solutions, 5% fetal calf serum, 1% human serum, 20 μg of ornithine per ml, 50 μg of ascorbic acid per ml, 25 μg of insulin per ml, and 0.7 μl of BME-vitamin solution (GIBCO) per ml.
Virus.
HAV was prepared by triple freeze-thaw cycles and removal of cellular debris (9) from FRhK-4 and HepG2 cells infected with a tissue culture-adapted variant of strain HM175, which was recovered from persistently infected cultures (7).
The 50% tissue culture infective dose (TCID50) titer was determined 2 weeks after inoculation by indirect immunofluorescence using the monoclonal anti-HAV IgG antibody 7E7 (Mediagnost, Tübingen, Germany) and calculated by the Kärber method (3).
Preparation of virion RNA and transfection.
HAV replication was investigated with cells not able to be infected with the virus after transfection with genomic RNA phenol extracted from purified virions (9). Virions were purified from viral stocks by incubation for 2 h at room temperature with a mixture of detergents (0.5% sodium dodecyl sulfate [SDS], 0.4% sodium deoxycholate, 1% Nonidet P-40), extraction with chloroform until the interface was clear, and pelleting of the virions through a 40% sucrose–0.5% Sarkosyl cushion by centrifugation at 40,000 rpm for 5 h at 4°C in an SW40 rotor.
Three to four micrograms of RNA was used to transfect cells, which were cultivated on 6-cm dishes, using DEAE-dextran. HAV replication was examined by slot blot analysis of total cell RNA as described previously (9). Briefly, total cell RNA was prepared by extraction with phenol-chloroform–1% SDS 2 weeks after transfection from the transfected cells and after amplification in FRhK-4 cells, which were inoculated with one-third of the cell lysate prepared from the transfected cells and then were incubated for 10 days. The RNA was blotted onto nitrocellulose, baked at 80°C for 2 h, and hybridized with full-length 32P-labeled HAV cDNA.
IgA binding studies.
For IgA binding experiments, cells were incubated with 20 to 200 μg of polyclonal mouse anti-trinitrophenyl IgA antibody MOPC 315 (Sigma) per ml for 30 min at 4°C. After three washes, the cells were incubated with fluorescein-labeled goat anti-mouse IgA antibody (Kirkegaard & Perry) for 30 min at 4°C and then washed again three times. Binding was analyzed by flow cytometry using an EPICS XL instrument (Coulter). All antibody dilutions and washes were performed with medium 199. Electrophoretic analysis of IgA MOPC 315 showed that the MOPC 315 line contains monomeric as well as polymeric IgA.
In order to characterize the IgA receptor molecules present on NCTC 1469 cells, the following substances were added to the incubation reaction alternately: 10 mM Ca2+, 1 mM Mn2+, 0.45 M sucrose, 10 mM acetic acid, and 50 ng of phorbol myristate acetate (PMA) per ml.
Preparation of HAV immunocomplexes.
HAV–anti-HAV IgA complexes were prepared by incubating HAV (105 to 106 TCID50/ml) with 20 μg of monoclonal mouse anti-HAV IgA antibody 1.193 (28) per ml or isolated human anti-HAV IgA for 2 h at room temperature in DMEM. As these antibodies neutralize HAV upon infection of FRhK-4 cells, complex formation was assayed by infection inhibition on FRhK-4 cells. Inhibition was determined by titration on FRhK-4 cells and compared with that obtained with HAV alone in amounts equal to those in the samples with antibodies. The data obtained correspond to the inoculum values reported in Results. As determined by polyacrylamide gel electrophoresis, IgA antibody 1.193 contains monomeric and polymeric IgA molecules in nearly equal amounts.
For IgA supplementation, one-half of the culture medium of infected cells was removed and treated with IgA as described above.
HAV–anti-HAV IgG complexes were prepared as described for IgA complex formation using human anti-HAV IgG.
Isolation and characterization of human IgA and IgG.
Human IgA was purified from sera of HAV patients by ammonium sulfate precipitation followed by affinity chromatography using jacalin (Pierce) (30) to isolate IgA, elution with 0.1 M melibiose, and gel filtration through Sephadex G-200 (Pharmacia) to separate monomeric IgA and dimeric IgA. The IgG fraction was obtained after the removal of IgA with jacalin. The fractions were analyzed by SDS-polyacrylamide gel electrophoresis and radial immunodiffusion (Behring Diagnostics), which also allowed quantification of IgA and IgG, respectively. The HAV specificity of the antibodies was determined by an HAV neutralization assay using FRhK-4 cells.
Influence of pH on HAV–anti-HAV IgA complex stability.
HAV–anti-HAV IgA complexes prepared as described above were incubated for different times at 37°C in 0.1 M glycine-HCl buffer of pHs 1.5, 2.5, 3.5, and 7.0. After pH neutralization with 1 M NaOH, the reaction mixture was diluted 1:10 with DMEM. Detection of infectious HAV was performed by titration using FRhK-4 cells. Twofold dilutions were incubated with the cells for 2 weeks at 34°C, and HAV replication was detected by indirect immunofluorescence. The TCID50 titer was calculated by the Kärber method.
Detection of HAV uptake, release from the immunocomplexes, and replication.
IgA-assisted infection with HAV was examined by incubating cells cultivated in six-well plates with 500 μl of HAV–anti-HAV IgA complexes in the presence of 10 mM Ca2+ for 2 h at 34°C. After five washes, the cells were incubated at 34°C. Cell lysates were prepared by triple freeze-thaw cycles; alternatively, cRNA was prepared by guanidinium-acid-phenol extraction (5) at various times. Substances influencing the binding and uptake of the immunocomplexes were preincubated for 1 h with the cells (200 μg of nonspecific IgA MOPC 315 per ml and anti-human asialoglycoprotein receptor [ASGPR] antibody) or added during the incubation period (0.45 M sucrose). The anti-human ASGPR antibody was kindly provided by U. Treichel, Mainz, Germany (39). Variations in the experimental conditions are indicated in Results.
HAV minus-strand RNA was detected by the hybrid detection assay (HDA) as described previously (34). Briefly, RNA extracts were hybridized with a specific biotinylated single-stranded DNA probe. The RNA-DNA hybrids were captured onto streptavidin-coated microwells, incubated with an anti-hybrid antibody conjugated to alkaline phosphatase (Digene Diagnostics, Silver Spring, Md.), and detected with a chemiluminescent substrate by use of a luminometer.
Detection of infectious HAV was performed by titration. Twofold dilutions of cell lysates were incubated with FRhK-4 cells cultivated in 96-well plates for 2 weeks at 34°C, and HAV replication was detected by indirect immunofluorescence. The TCID50 titer was calculated by the Kärber method.
RESULTS
IgA-mediated HAV infection in a mouse hepatocyte model. (i) Characteristics of NCTC 1469 cells.
In order to examine IgA-mediated HAV infection of hepatocytes, a cell line is needed which does not allow detectable replication of infectious virus after infection with HAV but which provides an internal cell environment supporting viral replication after bypassing the steps of viral entry mediated through the cognate virus receptor and which expresses an IgA-specific receptor. As human and primate cell lines normally used for studying HAV do not meet these requirements, we attempted to identify a cell line with suitable characteristics.
In a previous study, it was shown that infection of mouse hepatocellular NCTC 1469 cells with HAV did not result in the production of progeny virus, a finding which was demonstrated by slot blot analysis of viral RNA, radioimmunoassay, and infectivity titers (9). The result that NCTC 1469 cells are resistant to HAV infection was confirmed by using the HDA (34), showing that no HAV minus-strand RNA was detectable after inoculation with HAV (Table 1); therefore, no detectable HAV replication occurred. However, transfection of NCTC 1469 cells with naked HAV virion RNA resulted in the production of infectious virus, a finding which was demonstrated by inoculation of HAV-permissive FRhK-4 cells with lysates prepared from NCTC 1469 cells 2 weeks after transfection (data not shown). These results show that infection of NCTC 1469 cells with HAV cannot be demonstrated, whereas direct delivery of viral RNA into the cytoplasm results in the recovery of infectious virus.
TABLE 1.
Proof of HAV negative-strand RNA in the murine hepatocyte cell line NCTC 1469 after inoculation with HAV, HAV–anti-HAV IgA, and HAV–anti-HAV IgA plus anti-HAV IgA supplementation at day 3 postinfectiona
| Day(s) postinfection | Mean LCPSb/105 (SD) after treatment with:
|
||
|---|---|---|---|
| HAV | HAV–anti-HAV IgA | HAV–anti-HAV IgA + IgA supplementation | |
| 1 | 0 | 2,134 (25) | 2,103 (169) |
| 5 | 0 | 610 (33) | 1,167 (68) |
Cells were inoculated for 2 h at 34°C with HAV–anti-HAV IgA complexes or HAV in amounts equivalent to those in the complex inoculum (215 TCID50/ml) and incubated at 34°C. In addition, anti-HAV IgA (30 μg/ml) was added at day 3 after infection with HAV–anti-HAV IgA in a separate experiment. On the days indicated, cRNA was extracted and HAV minus-strand RNA was determined by the HDA. Each datum point is an average value obtained from two separate experiments and is adjusted by deduction of the value of the negative control.
LCPS, luminescence counts per second.
IgA binding studies using the murine IgA antibody MOPC 315 revealed that NCTC 1469 cells bind isotype-specific IgA and internalize bound IgA by receptor-mediated endocytosis, a finding which was demonstrated using the endocytosis inhibitors sucrose and acetic acid (data not shown). In order to further characterize the IgA receptor present on NCTC 1469 cells, we examined the influence of certain substances or conditions on IgA binding (8, 13, 29, 31, 32, 33, 36, 38) by flow cytometry. We could exclude the involvement of the Fcα-like receptor, because PMA inhibited IgA binding, as well as that of the polymeric immunoglobulin receptor, because treatment of the cells with trypsin had no influence on the affinity for IgA (data not shown). The findings that 10 mM Ca2+ enhanced IgA binding capacity, PMA reduced the affinity for IgA, and the pH optimum of binding was between 6.5 and 8.0 are indicative for the ASGPR. We also found that 1,4-β-galactosyltransferase is present on NCTC 1469 cells, because 1 mM Mn2+ significantly increased IgA binding capacity, but that endocytotic IgA uptake is mediated by the ASGPR only (data not shown).
(ii) Transfer of HAV into the cytoplasm of NCTC 1469 cells by IgA.
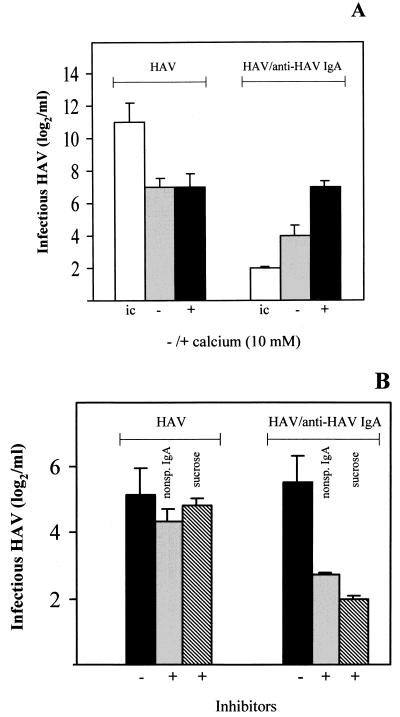
In order to determine whether HAV-specific IgA can transmit HAV virions into the cytoplasm of NCTC 1469 cells via the ASGPR, we inoculated the cells with HAV–anti-HAV IgA complexes using the neutralizing monoclonal HAV-specific IgA antibody 1.193. Titration of the cell lysates, which were prepared after inoculation of the cells with the complexes for 2 h at 34°C, on FRhK-4 cells revealed that virions were released from the complexes in the presence of 10 mM Ca2+. In the complex inoculum, which was also incubated for 2 h at 34°C, HAV remained nearly neutralized (Fig. 1A). Inoculation of the cells with HAV alone resulted in the attachment of HAV, but this was not influenced by Ca2+ (Fig. 1A).
FIG. 1.
HAV release from the HAV–anti-HAV IgA complexes during interaction with the murine hepatocyte cell line NCTC 1469. NCTC 1469 cells were inoculated with HAV–anti-HAV IgA immunocomplexes for 2 h at 34°C. As controls, cells were inoculated with HAV in amounts equivalent to those in the immunocomplex inoculum (211 TCID50/ml). HAV titers were determined by titration of the cell lysates using FRhK-4 cells. (A) Inoculation with and without Ca2+. ic, inocula without cell contact. (B) Preincubation with 200 μg of nonspecific (nonsp.) IgA MOPC 315 per ml or inoculation in the presence of 0.45 M sucrose. The data represent means from two independent experiments, and error bars indicate standard deviations.
Applying the same approach, nonspecific IgA antibody MOPC 315, used as a competitive inhibitor for binding of the HAV–anti-HAV IgA complexes to the ASGPR, as well as the endocytosis inhibitor sucrose inhibited the release of HAV from the complexes by 90% (Fig. 1B). Attachment of HAV alone was not influenced under these conditions (Fig. 1B). These results show that HAV–anti-HAV IgA immunocomplexes bind to NCTC 1469 cells via the Ca2+-dependent ASGPR and are internalized by endocytosis and that HAV is released from the complexes.
(iii) IgA-mediated infection of NCTC 1469 cells with HAV.
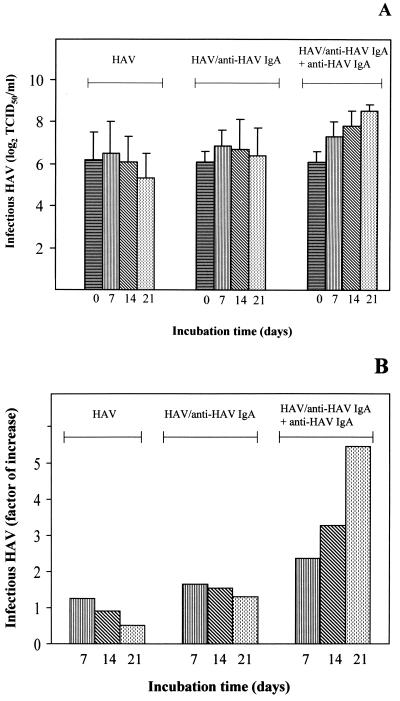
In order to investigate whether HAV replication takes place after release of the virus from the immunocomplexes following endocytotic uptake, the cells were incubated for different times after inoculation with the HAV–anti-HAV IgA complexes, and the TCID50 titer of the cell lysates was determined with FRhK-4 cells. After inoculation of NCTC 1469 cells with HAV alone in amounts equivalent to those in the complexes, the amount of nonspecific bound virus remained stable for 14 days and decreased slightly at day 21 postinfection (Fig. 2). After inoculation with the HAV–anti-HAV IgA complexes, which contained a remaining amount of infectious HAV of 25 TCID50/ml, we did detect a slight increase in the amount of infectious virus, which remained stable over the course of 21 days (Fig. 2). However, using the HDA, we detected HAV negative-sense RNA between days 1 and 5 after inoculation with the HAV–anti-HAV IgA complexes but not after inoculation with HAV alone (Table 1). Therefore, replication of HAV seems to have occurred after inoculation with the immunocomplexes.
FIG. 2.
Proof of IgA-mediated infection of the murine hepatocyte cell line NCTC 1469 with HAV. NCTC 1469 cells were inoculated for 2 h at 34°C with HAV–anti-HAV IgA immunocomplexes as well as with HAV as a control in amounts equivalent to those in the immunocomplex inoculum (216 TCID50/ml) and incubated further for various times. Additionally, HAV-specific IgA was used as a supplement at days 4, 8, 12, and 16 after infection with HAV–anti-HAV IgA. (A) HAV titers were determined by titration of the cell lysates using FRhK-4 cells. Day 0 represents infectious HAV after inoculation for 2 h. (B) Factors by which infectious HAV increased during the course of the incubation in comparison to the virus detected after inoculation for 2 h (day 0). The data are means obtained from two separate experiments, and error bars indicate standard deviations.
In order to consider the possibility that infectious HAV also is newly generated and released from the cells after inoculation with the immunocomplexes but that this effect is strongly limited by the lack of adaptation to NCTC 1469 cell-specific conditions and because newly generated virus released from the cells is not able to infect new cells, we removed 1 ml of the supernatant of the cells inoculated with the immunocomplexes, added anti-HAV IgA 1.193 to generate immunocomplexes, and returned the supernatant to the cells. This procedure, which allows new rounds of infection by released progeny virus, was performed at days 4, 8, 12, and 16 after the first inoculation. We then detected increasing amounts of infectious virus depending on the incubation time, with TCID50 titers of 1 × 26/ml 2 h after inoculation and 1.5 × 28/ml at day 21 (Fig. 2: HAV–anti-HAV IgA plus anti-HAV IgA). In comparison with the titers obtained after inoculation with the complexes and without the supplemental addition of IgA (Fig. 2: HAV–anti-HAV IgA), these titers increased continually over the course of the experiment and were significantly higher. An increased amount of HAV minus-strand RNA was also detected 5 days after the initial inoculation with the immunocomplexes and IgA supplementation at day 4 compared to the results obtained in the experiment without IgA supplementation (Table 1). This result shows that incubation of NCTC 1469 cells with HAV–anti-HAV IgA complexes results in HAV infection and that the addition of HAV-specific IgA leads to the formation of immunocomplexes with newly generated virus, resulting in amplification of the infection.
Therefore, our finding that the lack of susceptibility of NCTC 1469 cells to HAV infection can be circumvented by introducing HAV into the cells with the assistance of IgA molecules and via IgA receptors shows that HAV may gain access to hepatocytes by an IgA-mediated mechanism.
IgA-mediated infection of human hepatocytes with HAV. (i) IgA-mediated infection of HepG2 cells with HAV.
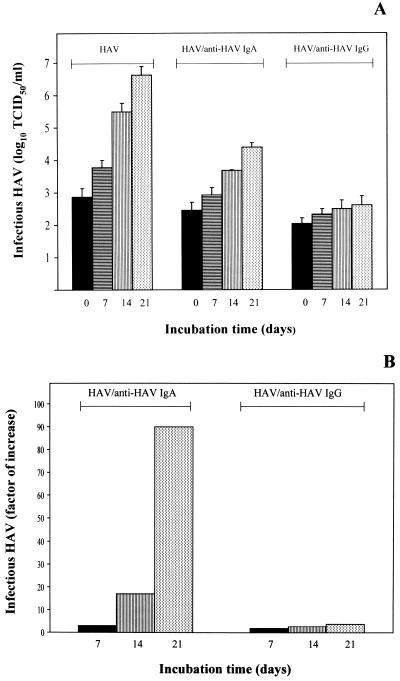
In order to investigate whether the findings obtained with the mouse hepatocyte model are transferable to human hepatocytes, we used the HepG2 cell line, which expresses the ASGPR (36) as well as an HAV receptor molecule. HepG2 cells were inoculated for 2 h at 34°C with HAV–anti-HAV IgA complexes using human anti-HAV IgA isolated from HAV patient serum. The amount of infectious virus remaining in the complex inoculum was 6 × 101 TCID50/ml. After several washing steps, the cells were incubated for different times, and the TCID50 titers were determined with FRhK-4 cells. At 2 h after inoculation, the amount of infectious virus was slightly increased compared to that in the complex inoculum (Fig. 3A: HAV–anti-HAV IgA, day 0). At day 21 postinfection, the amount of infectious HAV was amplified significantly (Fig. 3). However, inoculation of the cells with HAV–human anti-HAV IgG complexes with a remaining TCID50 titer of 102/ml did not result in a significant increase in the amount of infectious HAV over the course of 21 days (Fig. 3). This result shows that neutralization of HAV to a titer of 102/ml does not result in significant virus replication under these experimental conditions and that IgA molecules are therefore able to mediate uptake and infection of HepG2 cells with HAV via the ASGPR. These results were supported by the finding that nonspecific IgA as well as anti-human ASGPR antibodies, which inhibit IgA binding to the human ASGPR competitively, reduced HAV uptake and replication by 50% after inoculation with HAV–human anti-HAV IgA complexes (data not shown). Infection of the cells with HAV alone in amounts equivalent to those in the complex inoculum (Fig. 3A) was not influenced by the presence of the inhibitors.
FIG. 3.
Proof of IgA-mediated infection of the human hepatocyte cell line HepG2 with HAV. HepG2 cells were inoculated for 2 h at 34°C with HAV–human anti-HAV IgA as well as with HAV–human anti-HAV IgG. As controls, cells were inoculated with HAV in amounts equivalent to those in the immunocomplex inocula (3 × 106 TCID50/ml). (A) HAV titers were determined by titration of the cell lysates using FRhK-4 cells. Day 0 represents infectious HAV after inoculation for 2 h. (B) Factors by which infectious HAV increased during the course of the incubation in comparison to the virus detected after inoculation for 2 h (day 0). The data represent means from two independent experiments, and error bars indicate standard deviations.
(ii) Inhibitors of IgA binding to the human ASGPR diminish HAV infection of human primary hepatocytes by HAV–anti-HAV IgA complexes.
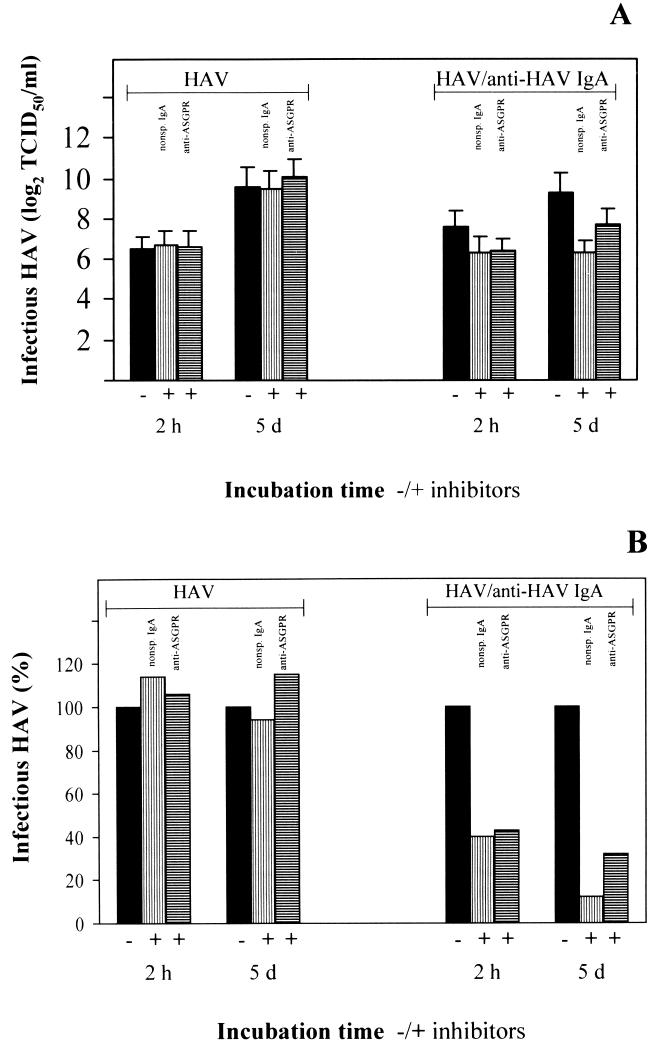
We inoculated cultured human primary hepatocytes with HAV–anti-HAV IgA complexes prepared with polyclonal human anti-HAV IgA antibodies. As human hepatocytes express cellular receptors for HAV and as the ASGPR is a major glycoprotein on the surface of hepatocytes (36), we incubated the cells, respectively, with nonspecific IgA antibody MOPC 315 molecules and polyclonal rabbit anti-human ASGPR antibodies for 1 h before inoculation with the immunocomplexes to differentiate between viral uptake via HAV receptor molecules and IgA receptors. The TCID50 titer was determined after inoculation for 2 h and 5 days after inoculation. We found that HAV was released from the complexes and that replication occurred over the course of 5 days (Fig. 4A). In the presence of the inhibitors, HAV uptake via the ASGPR and HAV replication were inhibited significantly (Fig. 4). Incubation with HAV alone in amounts equivalent to those in the complex inoculum resulted in infection of the hepatocytes as well. However, this infection was influenced by preincubation neither with nonspecific IgA nor with anti-ASGPR antibodies (Fig. 4).
FIG. 4.
Influence of inhibitors of IgA binding on IgA-mediated HAV infection of primary human hepatocytes. Cultured human primary hepatocytes preincubated for 1 h with 200 μg of nonspecific (nonsp.) IgA MOPC 315 (▥) per ml or anti-human ASGPR antibody (▤) were inoculated with HAV–human anti-HAV IgA complexes. As controls, cells were inoculated with HAV in amounts equivalent to those in the immunocomplex inoculum (217 TCID50/ml). (A) HAV titers were determined after inoculation for 2 h and 5 days (d) after inoculation by titration of the cell lysates using FRhK-4 cells. (B) TCID50 per milliliter as a percentage of the titers obtained without inhibitors (black bars) for a better view of the inhibitory effect. The data represent means from two separate experiments, and error bars indicate standard deviations.
These results show that HAV can be introduced into human hepatocytes through the assistance of IgA molecules via the ASGPR and that HAV may gain access to hepatocytes via an IgA-mediated mechanism in vivo.
Stability of HAV–anti-HAV IgA complexes under conditions simulating the gastrointestinal environment.
Findings from several laboratories show that HAV is partly shedded as IgA immunocomplexes (19, 22). In order to investigate whether these complexes may play a role in oral transmission from person to person, reach the intestine, and lead to HAV infection, we studied the stability of HAV–anti-HAV IgA complexes under the harsh conditions of the stomach. The pH range to which the HAV–anti-HAV IgA complexes are exposed lies between 1.5 and 5.0 and is dependent on the food ingested. The time of exposure, which is between minutes and 4 h, also depends on different parameters, such as the sort of food and whether it is solid or liquid.
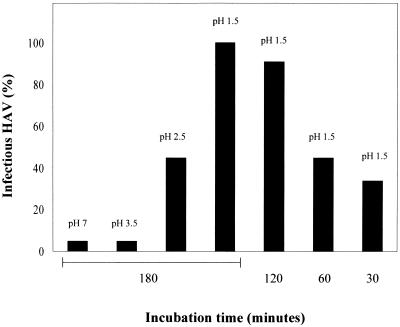
In order to investigate the stability of the HAV–anti-HAV IgA complexes under these conditions, HAV opsonized with anti-HAV IgA antibody 1.193 was incubated at pHs 1.5, 2.5, 3.5, and 7.0 for 180 min at 37°C as well as at pH 1.5 for 30, 60, and 120 min at 37°C, and the TCID50 titer was determined with FRhK-4 cells. The titration revealed that complex stability depended on the pH and the incubation time (Fig. 5). At pH 1.5, 34, 45, and 90% of infectious viruses were recovered from the neutralizing complexes after incubation times of 30, 60, and 120 min, respectively. After incubation for 180 min at pH 1.5, 100% of infectious virus was rescued. At pHs 2.5 and 3.5, 55 and 100% of the complexes, respectively, remained stable for 180 min. Incubation of HAV alone in amounts equivalent to those in the complexes and under the same conditions did not result in a loss of infectivity, and HAV remained neutralized during incubation of HAV immunocomplexes at pH 7.0 for 180 min. To exclude the possibility that after pH neutralization of the incubation reaction reassociation of HAV and IgA occurred, virus incubated under the same conditions as the complexes was supplemented with IgA after neutralization, and titration showed that no complex formation occurred over the time course between neutralization and titration. These results show that depending on the actual conditions existing in the stomach, HAV–anti-HAV IgA complexes can pass the stomach undamaged and gain access to the intestinal tract after oral uptake.
FIG. 5.
Stability of HAV–anti-HAV IgA complexes at different pHs. HAV–anti-HAV IgA complexes were incubated in 0.1 M glycine-HCl buffer at pHs 7, 3.5, 2.5, and 1.5 for 180 min and at pH 1.5 for 30, 60, and 120 min at 37°C. After pH neutralization, infectious virus was determined by titration with FRhK-4 cells. As a control, HAV in amounts equivalent to those in the complexes (105 TCID50/ml) was treated in the same way. Shown are the TCID50 per milliliter as a percentage of the titer obtained with HAV without treatment.
DISCUSSION
Our study examined the mechanism underlying the hepatotropism of HAV and the pathogenetic principle of relapsing infections. HAV is transmitted by the fecal-oral route, but at present it is not known how HAV finds its way to the liver (15). Based on the data that no extrahepatic sites for HAV replication could be clearly identified until now (2, 6, 18), although the putative receptor for HAV is expressed ubiquitously (10) and HAV has the ability to infect a number of nonhepatic cells (9, 11), we suggest that a certain fraction of HAV is targeted to the liver by a liver-directed carrier mechanism.
Using a mouse hepatocyte model which does not allow infection with HAV and therefore allows investigation of carrier-mediated HAV entry into host cells without interference from HAV receptors, we show that HAV is taken up by hepatocytes as an immunocomplex with HAV-specific IgA by endocytosis via the ASGPR, followed by viral RNA replication and production of infectious virus. We demonstrate that human hepatocytes can be infected by the same mechanism, lending credence to the assumption that HAV-specific IgA mediates infection of hepatocytes with HAV via the ASGPR in vivo.
The ASGPR is a major glycoprotein present on human hepatocytes and is located at the basolateral surface facing the capillaries (36). After endocytosis, the intraluminal milieu of the endosome allows escape of HAV into the cytoplasm and release of the genomic RNA, resulting in virus replication, as demonstrated in our experiments. For a growing number of viruses, it has been demonstrated that IgG-coated virions find entry into cells via Fcγ receptors (reviewed in reference 16); these include foot-and-mouth disease virus (25) and poliovirus (1) which, like HAV, are members of the picornavirus family. These data on IgG-mediated infection by foot-and-mouth disease virus and poliovirus correlate with our finding of IgA-mediated infection by HAV with regard to the fact that with HAV, no specific conformational change of the capsid, which is triggered by binding to the viral receptor, is necessary for the release of genomic RNA. Also, IgA-mediated viral infections of cells bearing the polymeric immunoglobulin receptor or the Fcα receptor have been described for Epstein-Barr virus, influenza virus, mouse mammary tumor virus, and human immunodeficiency virus (13, 20, 26, 42).
Based on the fact that IgA molecules and antigen-IgA complexes are eliminated from the blood by liver functions, IgA molecules not only may assist HAV entry into hepatocytes but also may direct HAV to the liver. This mechanism may play a role in the course of relapsing HAV infections. In such a situation, anti-HAV IgA which is synthesized and released into the intestinal tract during the first encounter with the virus may associate with HAV virions which are released from the liver to the gastrointestinal tract during the course of the primary infection and may serve as carrier molecule to mediate transport of the virus back to the liver and reinfection of IgA receptor-positive hepatocytes. This mechanism, which may depend on the strength of the IgA response during the first contact with the virus (37), would protect the virus from the neutralizing activities of low-avidity IgM and IgG antibodies present in this early phase of the clinical course of the infection and would thereby enable reinfection of the liver, resulting in exacerbation of liver disease. However, in the case of relapsing infections, HAV is eliminated from the organism eventually. A possible explanation for the interruption of the reinfection cascade would be the production of high-avidity anti-HAV IgG molecules in a later phase by maturation of the immune response, resulting in displacement of the IgA molecules in the HAV immunocomplexes by neutralizing IgG molecules.
The targeting function of IgA in HAV infection may also play a role in primary infection if the HAV-specific IgA response in the gastrointestinal tract takes place fast after oral uptake, allowing rapid immunocomplex formation, or if HAV already is transmitted as an HAV–anti-HAV IgA complex. Several laboratories have presented data that support both hypotheses; i.e., HAV would be neutralized by IgA for infection of IgA receptor-negative cells, so that no extrahepatic site of HAV replication could be identified, but not for infection of IgA receptor-positive hepatocytes.
HAV-specific serum IgA can be detected up to 5 years after disease in humans (27), a fact which is amazing, because IgA molecules, which have a half-life of 5 days, are catabolized fast by the liver. This information could indicate that IgA production is strongly stimulated, after ingestion of HAV, in the gastrointestinal tract by contact of the virus with the mucosa-associated lymphoid tissue. This notion is supported by the detection of HAV in epithelial cells of the intestinal crypts and in cells of the lamina propria of the small intestine 3 days after oral infection of owl monkeys (2), indicating that accumulation of HAV occurs in the gastrointestinal tract. Immunocomplex formation would then occur in the submucosa, and a certain fraction of invading HAV could be transported to the liver via lymph fluid and blood. Because the HAV-specific IgG response reaches its maximum in the reconvalescence phase, which represents a remarkable delay, IgA does not compete with IgG in the early and acute phase of disease.
It was also demonstrated that HAV is partly shedded in HAV–anti-HAV IgA complexes (19, 22). The speculation that these complexes are involved in transmission and the targeting mechanism means that they have to endure gastrointestinal tract passage. In our experiments on complex stability, we showed that HAV–anti-HAV IgA complexes indeed may pass through the harsh environment of the stomach. It remains to be shown if these complexes enter the pathway leading from the gastrointestinal tract to the liver by existing transport activities of the intestinal tract (42).
ACKNOWLEDGMENTS
We thank Bertram Flehmig and Richard Viebahn, University of Tübingen, Tübingen, Germany, for providing the human primary hepatocytes; Ulrich Treichel, University of Mainz, Mainz, Germany, for providing the anti-human ASGPR antibody; and Mediagnost, Tübingen, Germany, for providing the monoclonal anti-HAV antibody 7E7.
This work was supported by the Tönjes-Vagt-Stiftung, Bremen, Germany.
REFERENCES
- 1.Arita M, Horie H, Arita M, Nomoto A. Interaction of poliovirus with its receptor affords a high level of infectivity to the virion in poliovirus infections mediated by the Fc receptor. J Virol. 1999;73:1066–1074. doi: 10.1128/jvi.73.2.1066-1074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher L V, Binn L N, Mensing T L, Marchwicki R H, Vassell R A, Young G D. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus) J Med Virol. 1995;47:260–268. doi: 10.1002/jmv.1890470312. [DOI] [PubMed] [Google Scholar]
- 3.Brack K, Frings W, Dotzauer A, Vallbracht A. A cytopathogenic, apoptosis-inducing variant of hepatitis A virus. J Virol. 1998;72:3370–3376. doi: 10.1128/jvi.72.4.3370-3376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown T A, Russel M W, Mestecky J. Elimination of intestinally absorbed antigen into the bile by IgA. J Immunol. 1984;132:780–782. [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J I, Feinstone S M, Purcell R H. Hepatitis A virus infection in a chimpanzee: duration of viremia and detection of virus in saliva and throat swabs. J Infect Dis. 1989;160:887–890. doi: 10.1093/infdis/160.5.887. [DOI] [PubMed] [Google Scholar]
- 7.Cromeans T, Sobsey M D, Fields H A. Development of a plaque assay for a cytopathic, rapidly replicating isolate of hepatitis A virus. J Med Virol. 1987;22:45–56. doi: 10.1002/jmv.1890220107. [DOI] [PubMed] [Google Scholar]
- 8.Daniels C K, Schmucker D L, Jones A L. Hepatic asialoglycoprotein receptor-mediated binding of human polymeric IgA. Hepatology. 1989;9:229–234. doi: 10.1002/hep.1840090211. [DOI] [PubMed] [Google Scholar]
- 9.Dotzauer A, Feinstone S M, Kaplan G. Susceptibility of nonprimate cell lines to hepatitis A virus infection. J Virol. 1994;68:6064–6068. doi: 10.1128/jvi.68.9.6064-6068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan G. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flehmig B, Vallbracht A, Wurster G. Hepatitis A virus in cell culture. III. Propagation of hepatitis A virus in human embryo kidney cells and human embryo fibroblast strain. Med Microbiol Immunol. 1981;170:83–89. doi: 10.1007/BF02122672. [DOI] [PubMed] [Google Scholar]
- 12.Flehmig B, Zahn J, Vallbracht A. Levels of neutralizing and binding antibodies to hepatitis A virus after onset of icterus: a comparison. J Infect Dis. 1984;150:461. doi: 10.1093/infdis/150.3.461. [DOI] [PubMed] [Google Scholar]
- 13.Gan Y, Chodosh J, Morgan A, Sixbey J W. Epithelial cell polarization is a determinant in the infectious outcome of immunoglobulin A-mediated entry by Epstein-Barr virus. J Virol. 1997;71:519–526. doi: 10.1128/jvi.71.1.519-526.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D. Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine. 1992;71:14–23. doi: 10.1097/00005792-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Gust I D, Feinstone S M. Hepatitis A. Boca Raton, Fla: CRC Press, Inc.; 1988. [Google Scholar]
- 16.Halstead S B. Antibody-dependent enhancement of infection: a mechanism for indirect virus entry into cells. In: Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 493–516. [Google Scholar]
- 17.Kaplan G, Totsuka A, Thompson P, Akatsuka T, Moritsugu Y, Feinstone S M. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 18.Karayiannis P, Jowett T, Enticott M, Moore D, Pagnatelli M, Brenes F, Scheuer P J, Thomas H C. Hepatitis A virus replication in tamarins and host immune response in relation to pathogenesis of liver cell damage. J Med Virol. 1986;18:261–276. doi: 10.1002/jmv.1890180308. [DOI] [PubMed] [Google Scholar]
- 19.Karayiannis P, McGarvey M J, Fry M A, Thomas H C. Detection of hepatitis A virus RNA in tissues and faeces of experimentally infected tamarins by cDNA-RNA hybridisation. In: Zuckerman A J, editor. Viral hepatitis and liver disease. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 117–120. [Google Scholar]
- 20.Kozlowski P A, Black K P, Shen L, Jackson S. High prevalence of serum IgA HIV-1 infection-enhancing antibodies in HIV-infected persons. J Immunol. 1995;154:6163–6173. [PubMed] [Google Scholar]
- 21.Lemon S M, Binn L N. Incomplete neutralization of hepatitis A virus in vitro due to lipid-associated virions. J Gen Virol. 1985;66:2501–2505. doi: 10.1099/0022-1317-66-11-2501. [DOI] [PubMed] [Google Scholar]
- 22.Locarnini S A, Coulepis A G, Kaldor J, Gust I D. Coproantibodies in hepatitis A: detection by enzyme-linked immunosorbent assay and immune electron microscopy. J Clin Microbiol. 1980;11:710–716. doi: 10.1128/jcm.11.6.710-716.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolis H S, Nainan O V. Identification of virus components in circulating immune complexes isolated during hepatitis A virus infection. Hepatology. 1990;11:31–37. doi: 10.1002/hep.1840110107. [DOI] [PubMed] [Google Scholar]
- 24.Margolis H S, Nainan O V, Krawczynski K, Bradley D W, Ebert J W, Spelbring J, Fields H A, Maynard J E. Appearance of immune complexes during experimental hepatitis A infection in chimpanzees. J Med Virol. 1988;26:315–326. doi: 10.1002/jmv.1890260311. [DOI] [PubMed] [Google Scholar]
- 25.Mason P W, Baxt B, Brown F, Harber J, Murdin A, Wimmer E. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology. 1993;192:568–577. doi: 10.1006/viro.1993.1073. [DOI] [PubMed] [Google Scholar]
- 26.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A antihemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naudet G C. Ph.D. thesis. Tübingen, Germany: Medical Faculty of the University of Tübingen; 1988. [Google Scholar]
- 28.Ping L-H, Lemon S M. Antigenic structure of human hepatitis A virus defined by analysis of escape mutants selected against murine monoclonal antibodies. J Virol. 1992;66:2208–2216. doi: 10.1128/jvi.66.4.2208-2216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao T D, Maghazachi A A, Gonzales A V, Phillips-Quagliata J M. A novel IgA receptor expressed on murine B cell lymphoma. J Immunol. 1992;149:143–153. [PubMed] [Google Scholar]
- 30.Roque-Barreira M C, Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985;134:1740–1743. [PubMed] [Google Scholar]
- 31.Sancho J, Gonzales E, Egido J. The importance of the Fc receptors for IgA in the recognition of IgA by mouse liver cells: its comparison with the carbohydrate and secretory component receptors. Immunology. 1986;57:37–42. [PMC free article] [PubMed] [Google Scholar]
- 32.Sancho J, Gonzales E, Rivera F, Escanero J F, Egido J. Hepatic and kidney uptake of soluble monomeric and polymeric IgA aggregates. Immunology. 1984;52:161–167. [PMC free article] [PubMed] [Google Scholar]
- 33.Schiff J M, Fisher M M, Jones A L, Underdown B J. Human heterovalent ligand: switching from the asialoglycoprotein receptor to secretory component during transport across the rat hepatocyte. J Cell Biol. 1986;102:920–931. doi: 10.1083/jcb.102.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz G, Dotzauer A. Proof of hepatitis A virus negative-sense RNA by RNA/DNA-hybrid detection: a method for specific detection of both viral negative- and positive-strand RNA species. Nucleic Acids Res. 1998;26:5230–5232. doi: 10.1093/nar/26.22.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seelig R, Pott G, Seelig H P, Liehr H, Metzger P, Waldherr R. Virus-binding activity of fibronectin: masking of hepatitis A virus. J Virol Methods. 1984;8:335–347. doi: 10.1016/0166-0934(84)90071-5. [DOI] [PubMed] [Google Scholar]
- 36.Spiess M. The asialoglycoprotein receptor: a model for endocytic transport receptors. Biochemistry. 1990;29:10009–10018. doi: 10.1021/bi00495a001. [DOI] [PubMed] [Google Scholar]
- 37.Stapleton J T, Lange D K, LeDuc J W, Binn L N, Jansen R W, Lemon S M. The role of secretory immunity in hepatitis A virus infection. J Infect Dis. 1991;163:7–11. doi: 10.1093/infdis/163.1.7. [DOI] [PubMed] [Google Scholar]
- 38.Tomana M, Zikan J, Moldoveanu Z, Kulhavy R, Bennett J C, Mestecky J. Interactions of cell-surface galactosyltransferase with immunoglobulins. Mol Immunol. 1993;30:277–286. doi: 10.1016/0161-5890(93)90055-g. [DOI] [PubMed] [Google Scholar]
- 39.Treichel U, Meyer zum Büschenfelde K-H, Stockert R J, Poralla T, Gerken G. The asialoglycoprotein receptor mediates hepatic binding and uptake of natural hepatitis B virus particles derived from viraemic carriers. J Gen Virol. 1994;75:3021–3029. doi: 10.1099/0022-1317-75-11-3021. [DOI] [PubMed] [Google Scholar]
- 40.Vallbracht A, Gabriel P, Zahn J, Flehmig B. Hepatitis A virus infection and the interferon system. J Infect Dis. 1985;152:211–213. doi: 10.1093/infdis/152.1.211. [DOI] [PubMed] [Google Scholar]
- 41.Vallbracht A, Maier K, Stierhof Y-D, Wiedmann K H, Flehmig B, Fleischer B. Liver-derived cytotoxic T cells in hepatitis A virus infection. J Infect Dis. 1989;160:209–217. doi: 10.1093/infdis/160.2.209. [DOI] [PubMed] [Google Scholar]
- 42.Weltzin R, Lucia-Jandris P, Fields B N, Kraehenbuhl J P, Neutra M R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108:1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zajac A J, Amphlett E M, Rowlands D J, Sanger D V. Parameters influencing the attachment of hepatitis A virus to a variety of continuous cell lines. J Gen Virol. 1991;72:1667–1675. doi: 10.1099/0022-1317-72-7-1667. [DOI] [PubMed] [Google Scholar]