Abstract
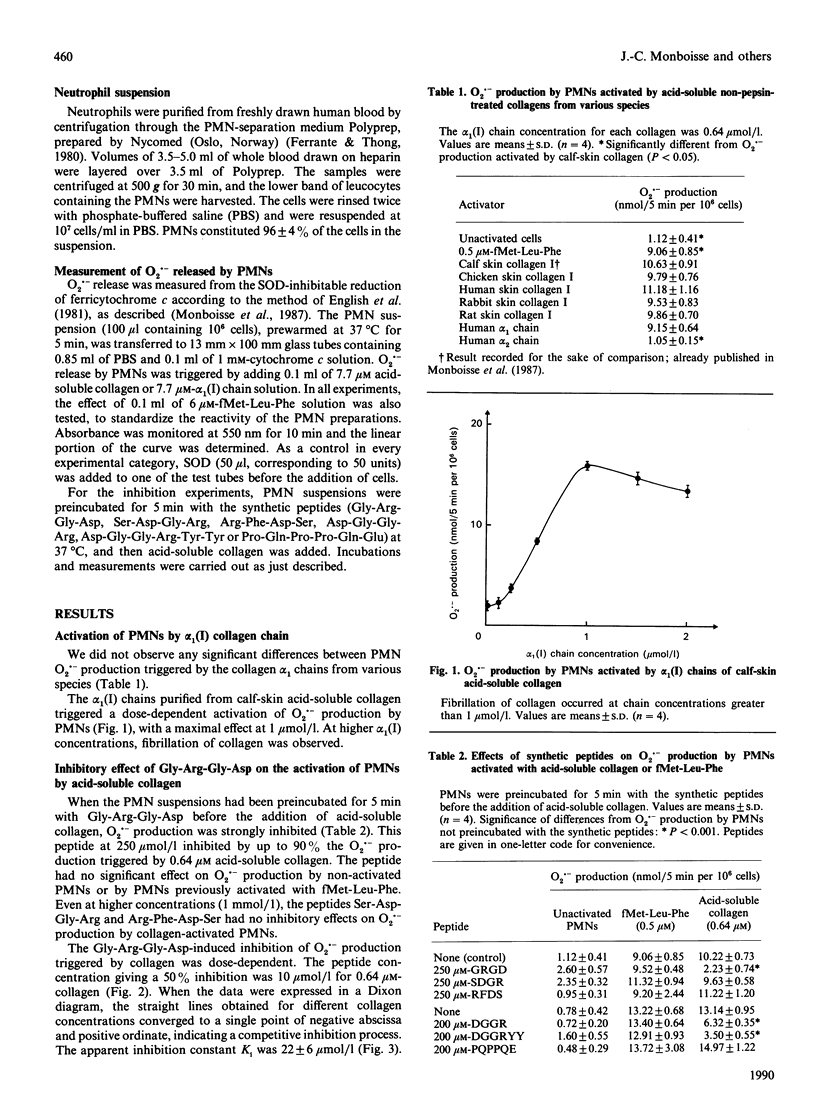
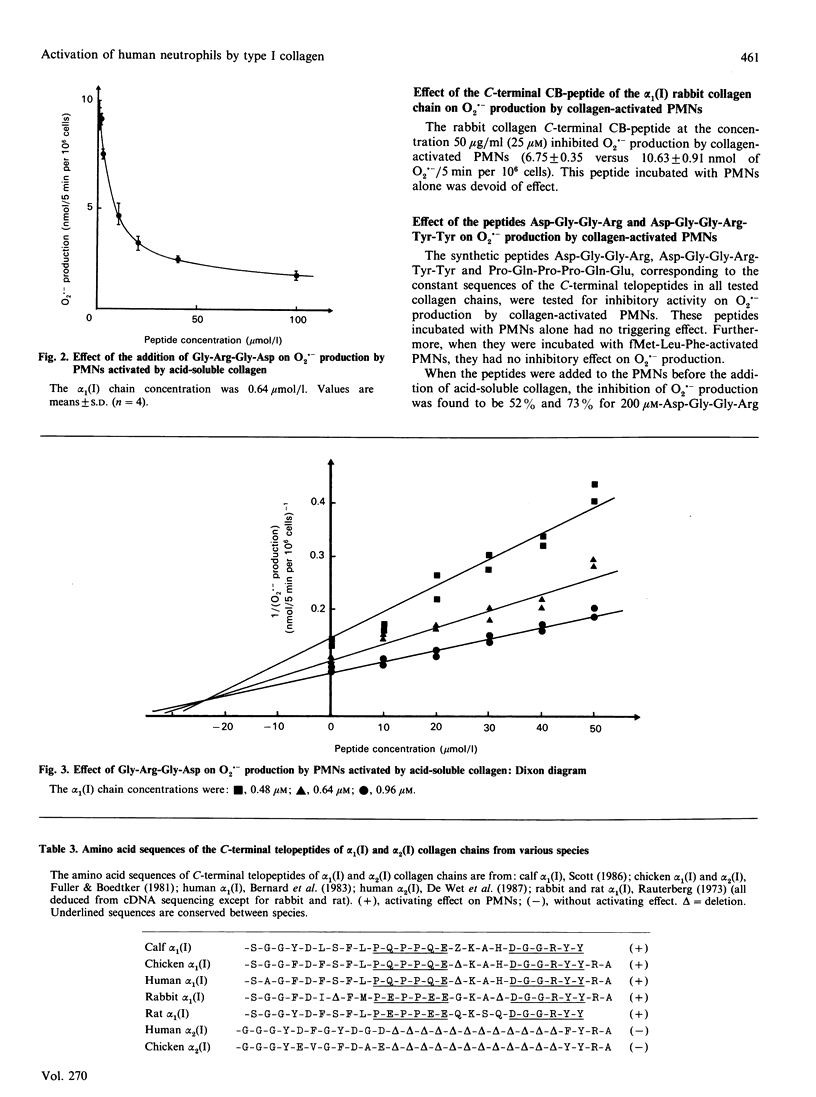
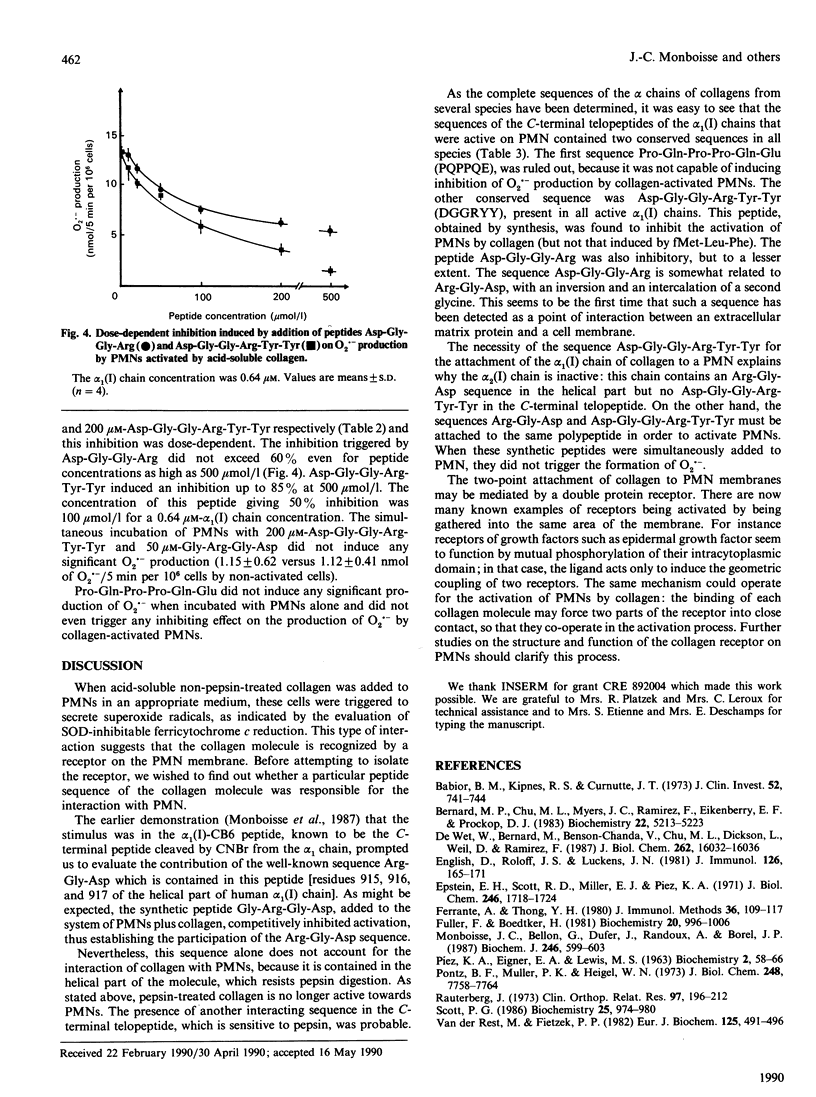
Contact between type I collagen purified from several species and human polymorphonuclear neutrophils (PMNs) triggers the production of O2.- by these cells. The activity of collagen is located in the alpha 1(I)-CB6 cyanogen bromide-cleaved (CB)-peptide, which is the C-terminal CB-peptide of the alpha 1(I) chain. Experiments based on the competitive inhibition of O2.- production by simultaneous incubation of PMNs with type I collagen and synthetic peptides identical to the conserved sequences of this collagen demonstrated that the binding of collagen to PMNs and the subsequent activation of these cells depend on the simultaneous presence of two sequences: Arg-Gly-Asp [residues 915, 916 and 917 of the complete alpha 1(I) chain, located in the helical part] Asp-Gly-Gly-Arg-Tyr-Tyr (residues 1034-1039, located in the C-terminal non-helical telopeptide).
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N. Regulation of human polymorphonuclear leukocyte superoxide release by cellular responses to chemotactic peptides. J Immunol. 1981 Jan;126(1):165–171. [PubMed] [Google Scholar]
- Epstein E. H., Jr, Scott R. D., Miller E. J., Piez K. A. Isolation and characterization of the peptides derived from soluble human and baboon skin collagen after cyanogen bromide cleavage. J Biol Chem. 1971 Mar 25;246(6):1718–1724. [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Fuller F., Boedtker H. Sequence determination and analysis of the 3' region of chicken pro-alpha 1(I) and pro-alpha 2(I) collagen messenger ribonucleic acids including the carboxy-terminal propeptide sequences. Biochemistry. 1981 Feb 17;20(4):996–1006. doi: 10.1021/bi00507a054. [DOI] [PubMed] [Google Scholar]
- Monboisse J. C., Bellon G., Dufer J., Randoux A., Borel J. P. Collagen activates superoxide anion production by human polymorphonuclear neutrophils. Biochem J. 1987 Sep 15;246(3):599–603. doi: 10.1042/bj2460599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauterberg J. The C-terminal non-helical portion of the collagen molecule. Clin Orthop Relat Res. 1973 Nov-Dec;(97):196–212. doi: 10.1097/00003086-197311000-00027. [DOI] [PubMed] [Google Scholar]
- Scott P. G. Spectroscopic study of environment-dependent changes in the conformation of the isolated carboxy-terminal telopeptide of type I collagen. Biochemistry. 1986 Mar 11;25(5):974–980. doi: 10.1021/bi00353a005. [DOI] [PubMed] [Google Scholar]
- de Wet W., Bernard M., Benson-Chanda V., Chu M. L., Dickson L., Weil D., Ramirez F. Organization of the human pro-alpha 2(I) collagen gene. J Biol Chem. 1987 Nov 25;262(33):16032–16036. [PubMed] [Google Scholar]
- van der Rest M., Fietzek P. P. A comprehensive approach to the study of collagen primary structure based on high-performance liquid chromatography. Eur J Biochem. 1982 Jul;125(3):491–496. doi: 10.1111/j.1432-1033.1982.tb06709.x. [DOI] [PubMed] [Google Scholar]


