Abstract
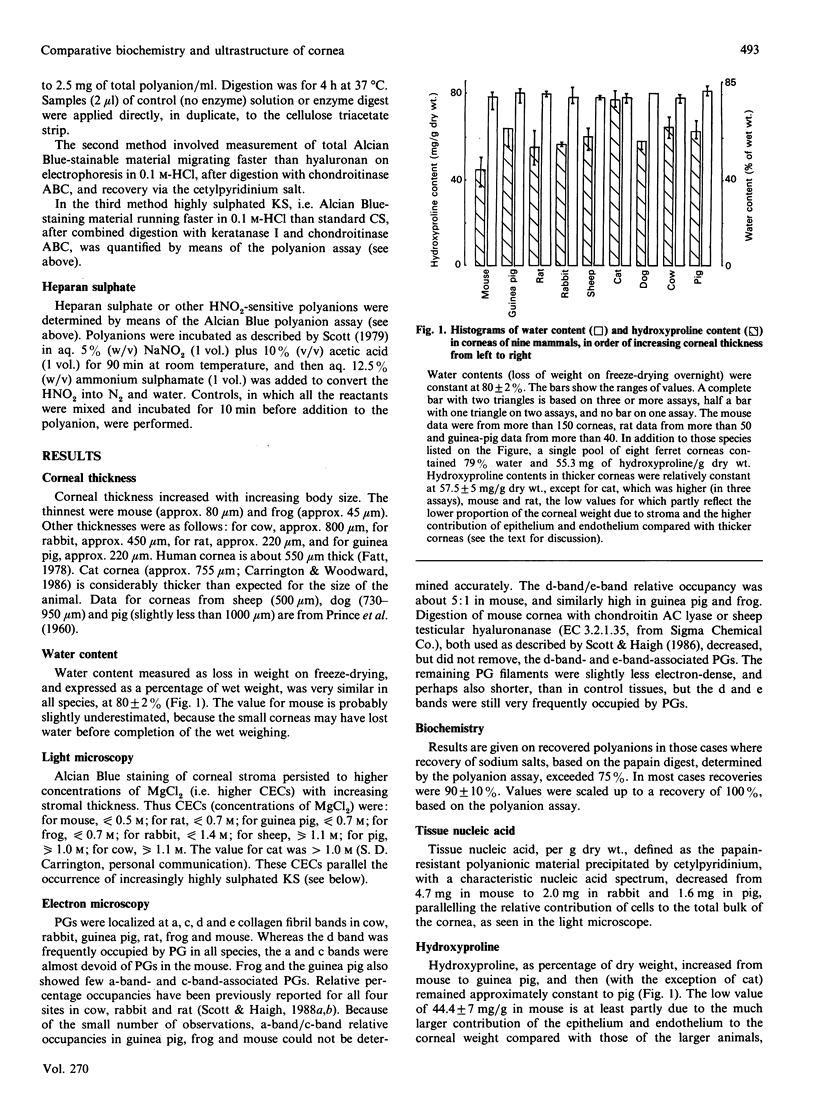
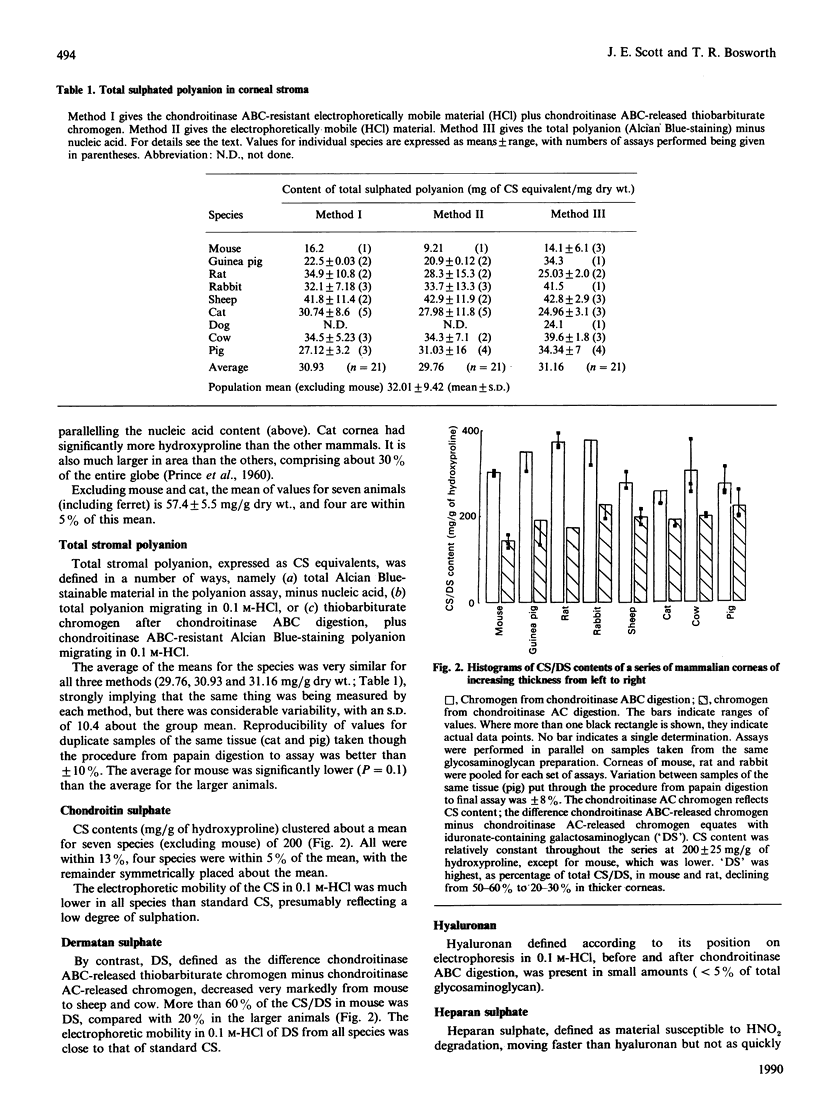
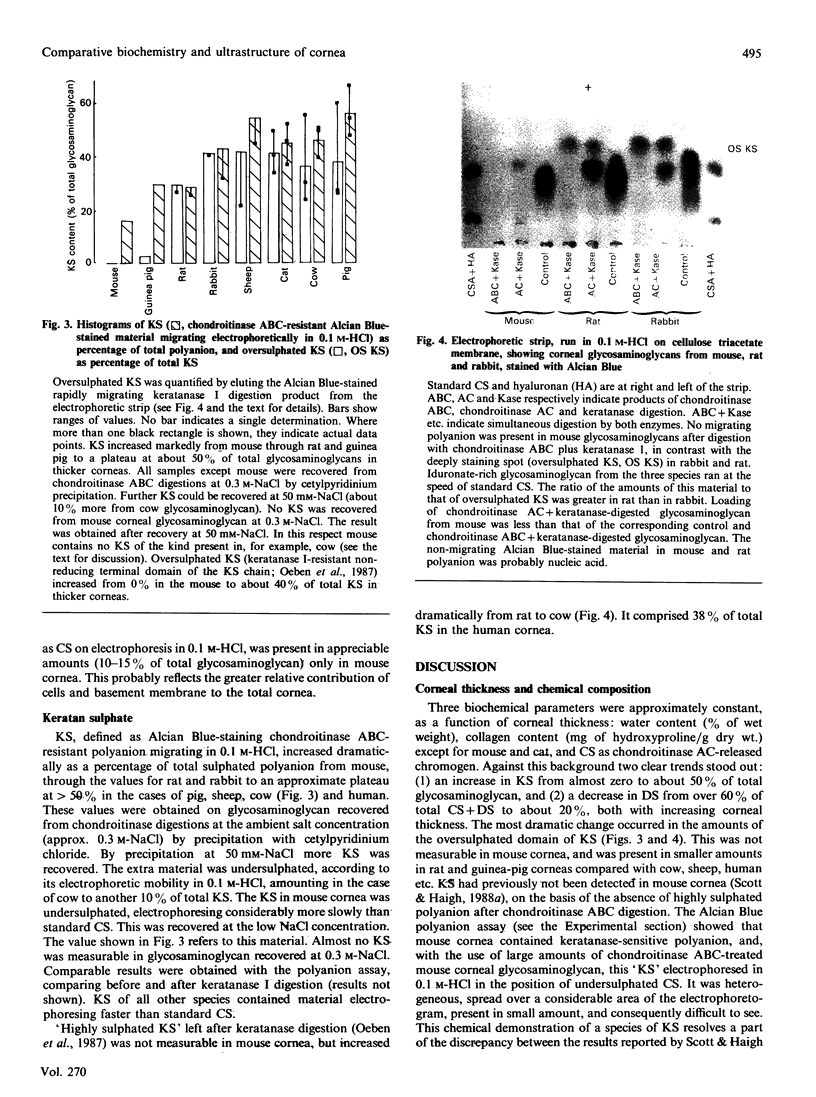
1. Corneas of mouse, rat, guinea pig, rabbit, sheep, cat, dog, pig and cow were quantitatively analysed for water, hydroxyproline, nucleic acid, total sulphated polyanion, chondroitin sulphate/dermatan sulphate and keratan sulphate, several samples or pools of tissue from each species being used. Ferret cornea was similarly analysed for water and hydroxyproline on one pool of eight corneas. Pooled frog (38) and ferret (eight) corneas and a single sample of human cornea were qualitatively examined for keratan sulphate and chondroitin sulphate/dermatan sulphate by electrophoresis on cellulose acetate membranes. Nine species (mouse, frog, rat, guinea pig, rabbit, sheep, cat, pig and cow) were examined by light microscopy and six (mouse, frog, rat, guinea pig, rabbit and cow) by electron microscopy, with the use of Alcian Blue or Cupromeronic Blue in critical-electrolyte-concentration (CEC) methods to stain proteoglycans. 2. Water (% of wet weight), hydroxyproline (mg/g dry wt.) and chondroitin sulphate (mg/g of hydroxyproline) contents were approximately constant across the species, except for mouse. 3. Keratan sulphate contents (mg/g of hydroxyproline) increased with corneal thickness, whereas dermatan sulphate contents decreased. The oversulphated domain of keratan sulphate was absent from mouse and frog corneas, increasing as percentage of total keratan sulphate with increasing corneal thickness. Sulphation of dermatan sulphate was essentially complete (i.e. one sulphate group per disaccharide unit). 4. Chondroitin sulphate/dermatan sulphate proteoglycans were present at the d bands of the collagen fibrils of all species examined, orthogonally arrayed, with high frequency, and occasionally at the e bands. Keratan sulphate proteoglycans were present at the a and c bands of all species examined, but with far higher frequency in the thicker corneas, where keratan sulphate contents were high. 5. Alcian Blue CEC staining showed much higher sulphation of keratan sulphate in thick corneas, e.g. that of cow, than in thin corneas, e.g. that of mouse, in keeping with biochemical analyses. 6. It is suggested that the constancy of interfibrillar volumes is regulated via the swelling and osmotic pressure of the interfibrillar polyanions, by adjustment of the extent of sulphation in two independent proteoglycan populations, to achieve an 'average sulphation' of the total polyanion similar to that of fully sulphated chondroitin sulphate/dermatan sulphate. 7. The balance of synthesis of the two kinds of proteoglycans may be determined by the O2 supply to the avascular cornea. O2 supply may also determine the conversion of chondroitin sulphate into dermatan sulphate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrington S. D., Woodward E. G. Corneal thickness and diameter in the domestic cat. Ophthalmic Physiol Opt. 1986;6(4):385–389. doi: 10.1111/j.1475-1313.1986.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Craig A. S., Parry D. A. Collagen fibrils of the vertebrate corneal stroma. J Ultrastruct Res. 1981 Feb;74(2):232–239. doi: 10.1016/s0022-5320(81)80081-0. [DOI] [PubMed] [Google Scholar]
- Gregory J. D., Cöster L., Damle S. P. Proteoglycans of rabbit corneal stroma. Isolation and partial characterization. J Biol Chem. 1982 Jun 25;257(12):6965–6970. [PubMed] [Google Scholar]
- HEDBLOM E. E. The role of polysaccharides in corneal swelling. Exp Eye Res. 1961 Sep;1:81–91. doi: 10.1016/s0014-4835(61)80012-2. [DOI] [PubMed] [Google Scholar]
- Haigh M., Scott J. E. A method of processing tissue sections for staining with cu-promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30(4):479–486. [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Humphries D. E., Silbert C. K., Silbert J. E. Sulphation by cultured cells. Cysteine, cysteinesulphinic acid and sulphite as sources for proteoglycan sulphate. Biochem J. 1988 May 15;252(1):305–308. doi: 10.1042/bj2520305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAURICE D. M. The structure and transparency of the cornea. J Physiol. 1957 Apr 30;136(2):263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton D. J., Scott J. E., Whiteman P. The estimation of acid glycosaminoglycan-Alcian blue complexes eluted from electrophoretic strips. Anal Biochem. 1974 Nov;62(1):268–273. doi: 10.1016/0003-2697(74)90386-8. [DOI] [PubMed] [Google Scholar]
- Oeben M., Keller R., Stuhlsatz H. W., Greiling H. Constant and variable domains of different disaccharide structure in corneal keratan sulphate chains. Biochem J. 1987 Nov 15;248(1):85–93. doi: 10.1042/bj2480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Scott J. E. A reaction for the simple sensitive fluorimetric assay of heparin and 2-amino sugars. Biochem J. 1979 Oct 1;183(1):91–97. doi: 10.1042/bj1830091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. 'Small'-proteoglycan:collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the 'a' and 'c' bands. Biosci Rep. 1985 Sep;5(9):765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Identification of specific binding sites for keratan sulphate proteoglycans and chondroitin-dermatan sulphate proteoglycans on collagen fibrils in cornea by the use of cupromeronic blue in 'critical-electrolyte-concentration' techniques. Biochem J. 1988 Jul 15;253(2):607–610. doi: 10.1042/bj2530607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Keratan sulphate and the ultrastructure of cornea and cartilage: a 'stand-in' for chondroitin sulphate in conditions of oxygen lack? J Anat. 1988 Jun;158:95–108. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-collagen interactions in intervertebral disc. A chondroitin sulphate proteoglycan associates with collagen fibrils in rabbit annulus fibrosus at the d-e bands. Biosci Rep. 1986 Oct;6(10):879–888. doi: 10.1007/BF01116241. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Histochemistry of Alcian blue. 3. The molecular biological basis of staining by Alcian blue 8GX and analogous phthalocyanins. Histochemie. 1972;32(3):191–212. doi: 10.1007/BF00306028. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan histochemistry--a valuable tool for connective tissue biochemists. Coll Relat Res. 1985 Dec;5(6):541–575. doi: 10.1016/s0174-173x(85)80008-x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert J. E., Palmer M. E., Humphries D. E., Silbert C. K. Formation of dermatan sulfate by cultured human skin fibroblasts. Effects of sulfate concentration on proportions of dermatan/chondroitin. J Biol Chem. 1986 Oct 15;261(29):13397–13400. [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Observations on the acid glycosaminoglycan (mucopolysaccharide) content of the matrix of aging cartilage. Ann Rheum Dis. 1965 Jul;24(4):341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn G., Mason R. M. Absence of keratan sulphate from skeletal tissues of mouse and rat. Biochem J. 1985 Jun 1;228(2):443–450. doi: 10.1042/bj2280443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]