Abstract
The membrane proteins gI and gE of Pseudorabies virus (PRV) are required for viral invasion and spread through some neural pathways of the rodent central nervous system. Following infection of the rat retina with wild-type PRV, virus replicates in retinal ganglion neurons and anterogradely spreads to infect all visual centers in the brain. By contrast, gI and gE null mutants do not infect a specific subset of the visual centers, e.g., the superior colliculus and the dorsal lateral geniculate nucleus. In previous experiments, we suggested that the defect was not due to inability to infect projection-specific retinal ganglion cells, because mixed infection of a gE deletion mutant and a gI deletion mutant restored the wild-type phenotype (i.e., genetic complementation occurred). In the present study, we provide direct evidence that gE and gI function to promote the spread of infection after entry into primary neurons. We used stereotaxic central nervous system injection of a fluorescent retrograde tracer into the superior colliculus and subsequent inoculation of a PRV gI-gE double null mutant into the eye of the same animal to demonstrate that viral antigen and fluorescent tracer colocalize in retinal ganglion cells. Furthermore, we demonstrate that direct injection of a PRV gI-gE double null mutant into the superior colliculus resulted in robust infection followed by retrograde transport to the eye and replication in retinal ganglion neuron cell bodies. These experiments provide additional proof that the retinal ganglion cells projecting to the superior colliculus are susceptible and permissive to gE and gI mutant viruses. Our studies confirm that gI and gE specifically facilitate anterograde spread of infection by affecting intracellular processes in the primary infected neuron such as anterograde transport in axons or egress from axon terminals.
Alphaherpesviruses, like Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), Varicella-zoster virus, and Pseudorabies virus (PRV), exhibit a predilection to infect neurons in the central nervous system (CNS) and peripheral nervous system (PNS) of an animal host (9a). During natural infections, virus replicates in nonneuronal cells and then undergoes retrograde spread of virus into afferent (e.g., sensory) or efferent (e.g., motor) nerve fibers innervating the infected tissue. Typically, this viral infection is limited to the PNS, where a latent infection is established in neurons directly innervating the peripheral site. Reactivation from latency is associated with virus replication in the primary neuron followed by anterograde transport of newly synthesized virus particles from the neuronal cell body back to the innervated tissue. Although less frequent, virus may also spread from the PNS to the CNS following primary infection or reactivation; this often has fatal consequences for the host. Hence, alphaherpesviruses are likely to encode mechanisms that facilitate travel in either direction within a neural circuit and thus may regulate the choice of direction at different stages of the virus life cycle (9a).
Insight that glycoproteins gE and gI are potential viral gene products involved in these mechanisms comes from studies demonstrating reduced infection of CNS after peripheral infection. For example, after intravitreal inoculation of the rat eye with wild-type PRV, virus replicates in retinal ganglion neurons and then undergoes anterograde spread to all visual nuclei in the CNS (6, 7, 31). By contrast, gI and gE mutant viruses replicate well in the eye but do not spread to second-order CNS neurons in a major subset of visual centers such as the superior colliculus and the dorsal lateral geniculate nucleus (dLGN). A similar inability to infect a subset of cardiac neurons in the rat brain stem has been reported following inoculation of gE mutants into the heart (26, 27). Similar defects in directional spread of infection have been noted after infection of mice by PRV gE and gI mutants (1). Importantly, similar defects have been seen after nasal infection by PRV gE and gI mutants of swine, the natural host (11, 13, 14, 18, 19). Like PRV, gE and gI deletion mutants of HSV-1 showed reduced neuroinvasion of the CNS in mice from peripherally infected sites (2, 21). While PRV gI and gE are required for efficient anterograde spread through most neuronal circuits tested, they are not required for retrograde spread (4, 5, 9a).
Enquist et al. (9) suggested that failure of gI- and gE-negative viruses to produce a productive infection in second-order neurons in the rat visual system was not due to an inability of these mutants to enter and replicate in the first-order retinal ganglion cells. They demonstrated that coinfection of the retina with separate gE- and gI-defective strains resulted in a phenotypically wild-type infection with virus replication in all visual nuclei. This result suggested that the mutants complemented each other, which could occur only after initial cellular entry (9). Thus, the defect in anterograde spread must occur in the primary infected neuron. One or more steps of virus replication and spread may require the presence of gE and gI: (i) virus is produced in the neuronal cell body but transport into the axon does not occur; (ii) virus reaches the axon terminal but fails to exit; (iii) viral egress from the retinal ganglion cell axon is successful but virus fails to cross the synapse; (iv) virus receptor-mediated entry of the postsynaptic neuron fails; or (v) virus penetrates the second-order neuron, but this postsynaptic neuron is nonpermissive to mutant virus replication.
The studies presented in this report were designed to determine the stage where gI- and gE-deficient viruses are blocked from infecting second-order superior colliculus neurons following intravitreal inoculation. First, to confirm and extend the conclusions of the coinfection experiments discussed above (9), we determined directly whether gI-gE double null mutant viruses were capable of infecting retinal ganglion cells that send axons to neurons in the superior colliculus. Second, we determined if gE and gI mutant viruses could replicate in superior colliculus neurons. Finally, we tested whether retinal ganglion neurons that project to the superior colliculus were competent to replicate gE and gI mutants by direct CNS injection and retrograde transport of virus back to the retina.
(Portions of this work were taken from the undergraduate Senior Thesis of T.K.)
MATERIALS AND METHODS
Model system: retinorecipient neuronal circuitry.
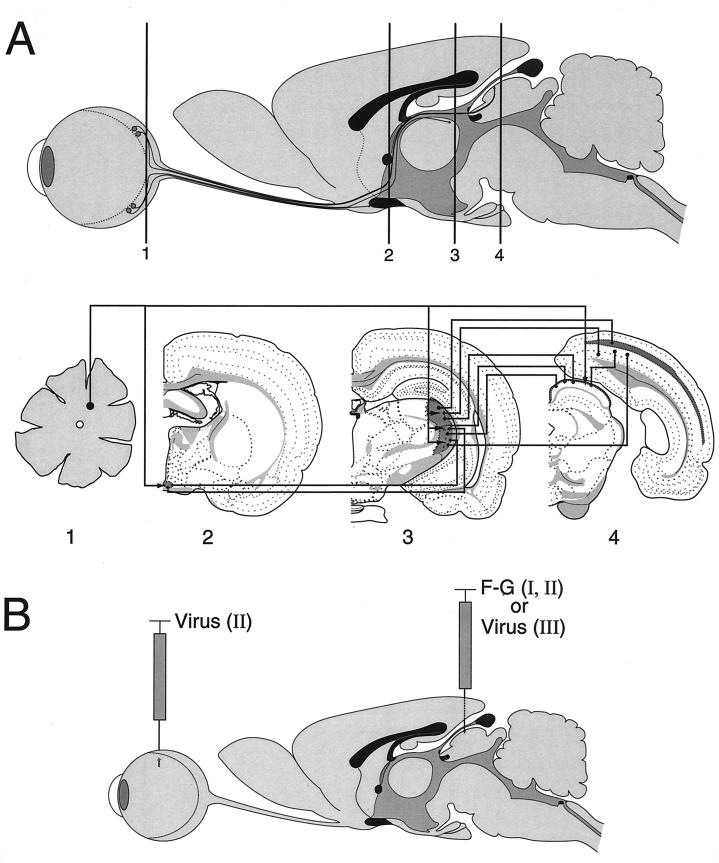
The experiments described in this report were performed using the rat eye infection model developed by Card et al. (7) and illustrated in Fig. 1A. The well-defined neuronal circuitry of this system has been used previously to assess the neuroinvasive and neurovirulent properties of various PRV mutant strains (see, e.g., references 12 and 29). Injection of PRV into the vitreous body of one eye leads to infection of retinal ganglion neurons on the vitreal surface of the retina. Following productive viral replication in retinal ganglion neurons (the first-order neurons), infection spreads anterogradely to retinorecipient neurons in the fore- and midbrain (the second-order neurons). An anterograde infection is operationally defined here as spread of virus from the cell body to the axon terminal followed by transneuronal passage to synaptically linked neurons. The direction of anterograde virus spread is the same as that of the nerve impulse. Conversely, retrograde infection is defined as spread of virus from the axon terminals to the neuronal cell body and is directionally opposite to movement of the nerve impulse. The organization of the central visual pathways in the rodent has been studied in extensive detail (reviewed in references 3, 17, and 25).
FIG. 1.
Visual nuclear neuronal circuitry and experimental design. (A) Sagittal image of a rat eye, optic nerve, and brain with coronal positions (levels 1 to 4) through the retina and major visual centers illustrated. Axons of retinal ganglion cells (level 1) project, via the optic nerve, to terminate in subcortical visual nuclei of the rat brain including the SCN (level 2), LGN (level 3), and superior colliculus (level 4), also known as the optic tectum. Visual cortical areas (level 4) of the occipital cortex are innervated by projections from subcortical visual centers. In coronal sections, visual nuclei are indicated by areas of darkened shading. Retinal axon projections and interconnections between central visual nuclei are indicated by arrowed lines. Lines with arrowheads at both ends represent commissural connections. For illustration clarity, retinal axon terminal arbors are shown as branches of a single fiber. However, this is not meant to imply that all visual nuclei are innervated by branches of retinocollicular axons. Retinal projection is contralateral to retinorecipient nuclei; interconnections between visual nuclei are ipsilateral. The coronal templates used in this figure were modified from reference 28. In the rat eye infection paradigm, PRV strains expressing gI and gE can spread to and infect central visual projection fields located in the forebrain, such as the hypothalamus (SCN) and the thalamus (LGN, including the dorsal [dLGN] and ventral [vLGN] nuclei, as well as the IGL), in addition to a midbrain target, the superior colliculus. Viruses lacking gI, gE, or both are able to infect only a subset of these retinorecipient regions, including the SCN and the IGL but not the dLGN or superior colliculus. (B) A colocalization experiment was used to examine whether a gI/gE-deleted PRV strain was able to infect retinal ganglion cells which project to the superior colliculus. Superior colliculus-projecting retinal ganglion neurons were identified by stereotaxic injection of the fluorescent tracer F-G into the left superior colliculus of the rat brain (experiment I; F-G alone). Following retrograde axonal transport within retinotectal projections, F-G tracer accumulates in ganglionic neuron cell bodies and processes in the contralateral retina. Intravitreal inoculation of PRV results in viral infection of retinal ganglion neurons. Colocalization of viral antigen and F-G tracer would identify retinotectal projecting neurons as permissive to viral infection (experiment II; F-G and virus). In separate independent experiments, individual PRV strains were inoculated into the left superior colliculus by stereotaxy and virus infection of central visual nuclei and the retina were examined (experiment III; virus alone).
In addition to the pathways illustrated in Fig. 1A, other recipient nuclei and their projections are well characterized and were infected by PRV in our studies. In all cases, the pattern of infection in all recipient nuclei was consistent with established projections and strict transsynaptic, rather than lytic, nonspecific passage of virus. However, for simplicity of analysis and clarity of presentation, only the subset of visual nuclei (the superior colliculus, the LGN, and the suprachiasmatic nucleus [SCN]) are highlighted in our analysis.
Virus strains and cells.
Strain PRV-Becker (PRV Be) is the parental strain for the gE and gI mutants used in this report. Isogenic strains PRV 91, PRV 98, and PRV 99 carry deletions of gE, gI, and gI-gE, respectively (31). All viruses were propagated on PK15 (porcine kidney) cells in Dulbecco's modified Eagle's medium supplemented with 2% heat-inactivated fetal bovine serum. PK15 cells were maintained by consecutive passage in Dulbecco's modified Eagle's medium plus 10% heat-inactivated fetal bovine serum.
Animals.
Adult male Sprague-Dawley albino rats (Taconic, Germantown, N.Y.), weighing 230 to 260 g, were used in this study (see Table 1). Animals were housed in a Biosafety Level 2/3 facility with controlled temperature (22°C) and constant photoperiod (12 h of light, 12 h of dark; light on at 0700). The rats were maintained in individual cages with food and water available ad libitum throughout the experiment. They were allowed to acclimate to the animal facility for at least 7 days prior to any experimental procedure. All experimental protocols were approved by the Princeton University Animal Welfare Committee and were consistent with regulations stipulated by the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (Public Law 99-198).
TABLE 1.
Tracer and virus colocalization experimental groups
| Virus | No. of animals | Infection time (h)a | Dye transport time (h)b |
|---|---|---|---|
| PRV 99 | 2 | 48 | 48 |
| 3 | 48 | 96 | |
| 2 | 48 | 144 | |
| 3 | 60 | 60 | |
| 3 | 72 | 72 | |
| 8 | 96 | 96 | |
| PRV Be | 3 | 48 | 48 |
| 4 | 48 | 96 |
Infection time is the duration from the time of eye infection to the time of euthanasia.
Dye transport time is the duration from the time of F-G injection to the time of euthanasia.
Experimental paradigm.
The animals were anesthetized with intramuscular xylazine (13 mg/kg) and ketamine (86 mg/kg) and placed into a sterotaxic frame (Kopf Instruments, Tujunga, Calif.) to secure the cranium. An incision was made on the midline of the scalp, cranial suture reference points (bregma and lambda) were equalized in the dorsal/ventral and medial/lateral axes, and a hole was surgically drilled through the skull above the injection site. Stereotaxic coordinates for injection into the left superior colliculus were calculated from bregma, as described previously (20). Two different injection paradigms were employed, specific to the tracer used in the experimental design detailed in Fig. 1B. For PRV, a single site injection was performed using stereotaxic coordinates, AP = −6.2, ML = +1.3, DV = −3.5 from the surface of the dura (AP, anteroposterior; ML, mediolateral; DV, dorsoventral). For Fluoro-Gold (F-G) (Fluorochrome Inc., Englewood, N.J.), a double injection was made using two sets of stereotaxic coordinates, (i) AP = −5.8, ML = +1.3, DV = −3.5 from the surface of the dura, and (ii) AP = −6.8, ML = +1.3, DV = −3.1 from the surface of the dura. Virus (200 nl/animal) or F-G (2% solution in isotonic saline; 300 nl/site) was injected through a 1-μl Hamilton syringe at a rate of 20 nl/min. The tip of the syringe was left in situ for an additional 5 min to reduce reflux of the inoculum up the needle tract. Following completion of the injection, the needle was slowly removed, bone wax was placed in the hole in the skull, the scalp was closed with suture and wound clips, and the animal was returned to its cage.
For intraocular injections, anesthetized animals were intravitreally inoculated into the right eye with 2.5 μl virus at a rate of 100 nl/min. All virus stocks used for intracranial and intraocular injections were clarified of cell debris by centrifugation (5,000 × g for 5 min) following sonication. So that similar amounts of virus were inoculated during all experiments, virus stocks were diluted to the same titer (2.5 × 108 PFU/ml; plaque titer was determined on PK15 cells). All injections were performed during the light phase of the photoperiod.
Animals were closely monitored for signs of infection (lethargy; hunched posture; piloerection; labored breathing; hardarian gland secretion; sensory irritation; hyperresponsiveness to touch; oral, nasal and/or ocular discharge; and hyperkinesis) throughout the course of the experiment and sacrificed after the appropriate postinoculation interval (36, 48, 60, 72, or 96 h) or when moribund.
Perfusion and tissue preparation.
Experimental animals were deeply anesthetized with xylazine and ketamine and exsanguinated by transcardiac perfusion of isotonic (0.9%) saline followed by 1% paraformaldehyde containing lysine and sodium metaperiodate (PLP) at 4°C (16). After cessation of the perfusion to enucleate both ocular orbits, the animals were reperfused with 4% PLP at 4°C. Whole retinas were immediately dissected from each eye, and fiducial marks were made on each retina to identify retinal hemifields. The retinas were stored in 4% paraformaldehyde at 4°C for up to 12 h. The brains were removed, postfixed in 4% paraformaldehyde for 12 to 24 h at 4°C, and cryoprotected in phosphate-buffered 30% sucrose solution at 4°C for 72 h. Coronal sections of brain tissue (30 μm thick) were cut with a freezing rotary microtome and stored in a cryopreservative (30) at −20°C prior to being used for epifluorescence analysis or immunohistochemical localization of viral antigen.
Antisera.
Rabbit polyclonal antiserum, Rb133, prepared against acetone-inactivated wild-type PRV Be virions has been previously described (5). Biotinylated goat anti-rabbit immunoglobulin G (IgG) and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG were purchased from Vector Laboratories (Burlingame, Calif.) and Jackson ImmunoResearch Laboratories (West Grove, Pa.), respectively.
Immunohistochemistry and indirect immunofluorescence.
Free-floating brain sections, separated by distances of 120 μm (every fourth section), or whole retinas were incubated in primary antibody Rb133 (1:5,000) for 72 h. Immunohistochemical processing of brain tissue was completed using the avidin-biotin modification of the immunoperoxidase procedure (10) (reagents from Vector Laboratories). Detailed protocols for this procedure have been previously published (8). Indirect-immunofluorescence localization of viral antigen in retinal tissue was performed using TRITC-conjugated goat anti-rabbit IgG (1:200) and incubation at room temperature for 4 h.
Immunohistochemically processed brain sections were mounted on gelatin-coated glass slides, dehydrated in graded ethanol concentrations, cleared in xylene, and coverslipped with DPX mountant (Fluka, Milwaukee, Wis.). Brain or whole retinal tissue for epifluorescence analysis was mounted on gelatin-coated glass slides and coverslipped with 90% glycerol–10% phosphate-buffered saline.
Data analysis and presentation.
Bright-field photomicrographs of immunohistochemically processed brain sections were taken using a Zeiss Axiolab microscope, Acroplan phase 10× objective, and Kodak T-Max 100 film. Epifluorescence images were captured by either confocal laser microscopy using a Nikon Optiphot II microscope, Plan Fluor 5× objective, and Bio-Rad MRC600 argon-krypton laser or standard fluorescence microscopy using a Nikon Optiphot II microscope, Plan Fluor 10×, 20×, or 40× objective, excitation/emission filter sets for F-G (323/408 nm) and TRITC (550/570 nm), and Kodak Royal Gold 1000 film. Photographic film images were digitized using a Nikon slide scanner. Digital-image composites were arranged using Adobe Photoshop 4.0 computer software.
RESULTS
Rationale.
The deduction that gE and gI mutants could enter and replicate in retinal ganglion cells that send axons to the superior colliculus was made after a phenotypically wild-type CNS infection was observed after coinfection of the rat eye by gE and gI mutants (9). We determined directly if a gI-gE-deficient PRV strain was capable of infecting retinal ganglion neurons that are in synaptic contact with second-order neurons in the superior colliculus. We did so by colocalization of virus-infected cells in the retina with an inert, fluorescent tracer injected in the superior colliculus to mark the retinal ganglion cells that send axons to the superior colliculus (Fig. 1B). Such an experiment is facilitated by the fact that more than 90% of retinal ganglion neurons in the adult rat project axons to the contralateral superior colliculus (15). Therefore, injection of the inert retrograde tracer F-G into the left superior colliculus was expected to label large numbers of neurons in the retinal ganglion cell layer of the right retina. Since the F-G dye fails to diffuse from the cell soma, cross synapses, or be taken up by intact fibers of passage (23, 24), labeling would be strictly limited to neurons that send axons to the superior colliculus. After F-G injection, retinal ganglion neurons were infected in situ with a PRV gI-gE null mutant and viral antigen was localized with anti-virion and fluorochrome-conjugated secondary antibodies. Dually fluorochrome-labeled cells, indicating colocalization of both tracer and virus, would provide direct evidence that gE and gI mutants can enter and replicate in retinal ganglion neurons that send axons to the superior colliculus.
F-G labeling of retina and visual center nuclei.
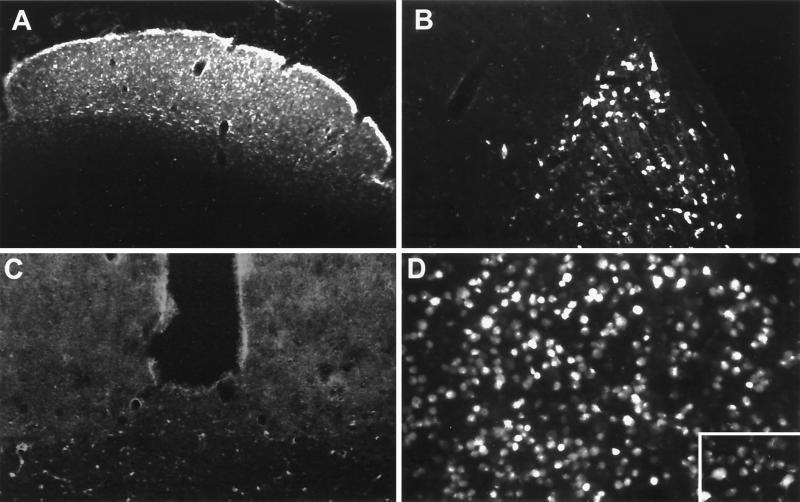
Experimental parameters for F-G injection were empirically determined (data not shown). By injecting two sites in the superior colliculus, limiting the maximum injected volume to 300 nl per site, and separating the two sites by 1 mm in the anterior/posterior axis, we were able to limit nonspecific labeling of other brain regions due to leakage of tracer into the third ventricle. By examining animals at various times after F-G injection, we determined that maximum intracellular labeling of retinal ganglion cells occurred 48 h after injection (Fig. 2). Extensive labeling was evident in superficial and deep layers of the superior colliculus (Fig. 2A), the intergeniculate leaflet (IGL) and the ventral LGN (vLGN) (Fig. 2B), and retinal ganglion cell bodies in the retina (Fig. 2D) and their cellular processes (Fig. 2D inset). Neuronal labeling was equivalent across the entire retina (data not shown). No labeling was observed in the dLGN (Fig. 2B) or the SCN (Fig. 2C), as predicted from the lack of any known projections of axons from the SCN or dLGN to the superior colliculus (Fig. 1A). In some animals, while F-G labeling was well localized, it was not completely restricted to the superior colliculus and we observed limited staining in ependymal cells lining ventricles (data not shown).
FIG. 2.
Pattern of F-G labeling in CNS following superior colliculus injection. The retrograde tracer F-G was injected by stereotaxy into the left superior colliculus of rat brains, and the animals were sacrificed 96 h postinoculation. The figure displays the pattern of retrograde neuronal labeling with F-G in coronal brain sections through the superior colliculus (A), LGN (B), SCN (C), and retina in whole mount (D with inset). Brain sections and retina are ipsilateral and contralateral, respectively, to the site of injection. Photomicrographs of epifluorescence in the retina (D and inset; ×170 and ×340, respectively) were taken at higher magnification than those of brain sections (panels A to C; ×85) using standard fluorescence optics.
Colocalization of F-G and viral antigen in retinal ganglion neurons.
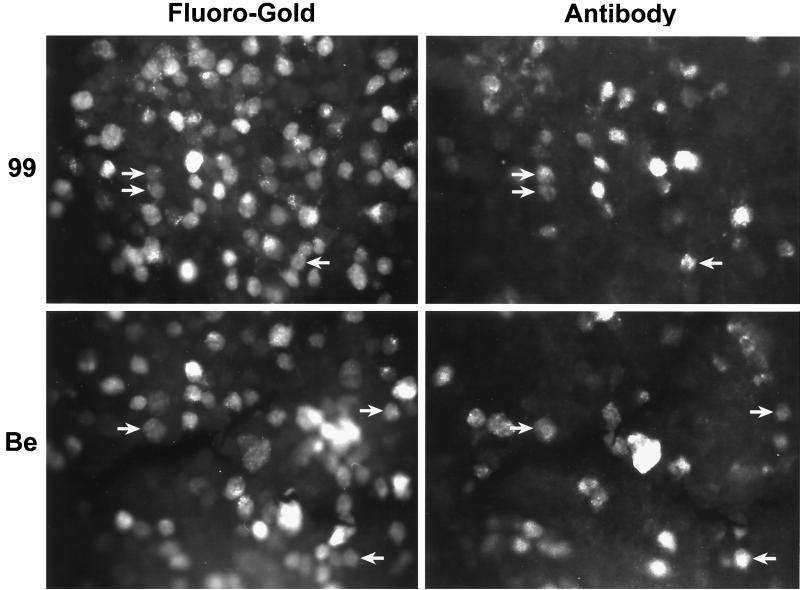
We determined the optimal time interval between F-G injection and virus infection of the eye (Table 1). Briefly, F-G was injected either concurrently with or after virus infection. Such studies were critical because of the differences in the times that animals could survive infection by various PRV mutants (e.g., the typical average survival times were 96 h for PRV 99 and other gE and gI mutants compared to less than 72 h for PRV Be). We also noted that the number of infected retinal ganglion cells continued to increase with time after infection for all viruses (data not shown). Retinal ganglion neurons that contained both the F-G tracer and viral antigens were observed at every time point examined following infection by either PRV 99 or PRV Be (Fig. 3). However, the largest numbers of double-labeled cells were present in animals infected for extended times. F-G-labeled and viral antigen-positive retinal ganglion neurons were present across the entire retina after infection by either strain. The numbers of F-G-labeled and viral antibody-labeled retinal ganglion cells, counted from photomicrographic images of identical fields, were compared for PRV 99 and PRV Be (Table 2). Using matched F-G-transport and virus infection times, roughly equivalent numbers of double-labeled neurons, expressed as a percentage of total F-G- or antigen-positive cells, were found following infection by either strain. Approximately 16% of PRV 99-infected retinal ganglion neurons contained F-G label, compared to 6.7% of PRV Be-infected neurons. Given the sample size, we consider the difference to be insignificant. Our interpretation of these data is that both PRV 99 (gI− gE−) and PRV Be (gI+ gE+) infect retinal ganglion neurons that project to the superior colliculus with equivalent efficiencies. Therefore, the inability of PRV 99 (and other gE or gI mutants by inference) to infect the superior colliculus after eye infection results from a defect(s) other than inability to infect retinal ganglion cells that are connected to the superior colliculus.
FIG. 3.
Colocalization of virus and tracer in retinotectally projecting retinal ganglion neurons. F-G was injected into the left superior colliculus; this was followed by intravitreal inoculation of either PRV 99 (gI− gE−) or PRV Be (gI+ gE+) into the contralateral (right) eye. F-G inoculation preceded PRV Be injection by 48 h; F-G and PRV 99 inoculations were concurrent. At maximal postinfection survival times (PRV 99, 96 h; PRV Be, 48 h), the animals were sacrificed and their retinas were isolated. Retinal tissue was reacted with rabbit polyclonal antibody Rb133 and TRITC-conjugated secondary antibody. Immunoreacted retinas were whole mounted and visualized by fluorescence microscopy. Each panel doublet (F-G or antibody) represents the same microscopic field. For each virus, results from one representative animal are presented. Retinal ganglion neurons displaying colocalization of viral antigen and tracer are indicated by arrows.
TABLE 2.
Cell counts of single- and double-labeled retinal ganglion neurons
| Virus strain | PRV infection time (h)a | F-G transport time (h)b | No. of fieldsc | Total no. of F-G+ cells | Total no. of PRV+ cells | Total no. of F-G+ plus PRV+ cells | F-G+ plus PRV+/F-G+ cells (%) | F-G+ plus PRV+/PRV+ cells (%) |
|---|---|---|---|---|---|---|---|---|
| 99 | 48 | 48 | 8 | 1,328 | 92 | 15 | 1.1 | 16.3 |
| 96 | 96 | 8 | 985 | 284 | 13 | 1.3 | 4.6 | |
| Be | 48 | 48 | 8 | 1,170 | 150 | 10 | 0.85 | 6.7 |
Infection time is the duration from the time of eye infection to the time of euthanasia.
Dye transport time is the duration from the time of F-G injection to the time of euthanasia.
Fields were examined at a magnification of ×200.
Direct superior colliculus infection and virus spread to synaptically connected CNS nuclei.
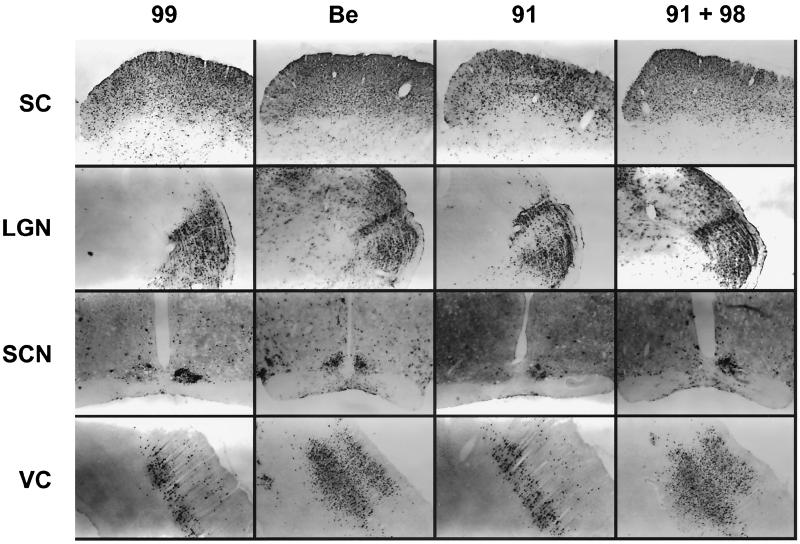
PRV Be and PRV gE-gI mutants replicated efficiently when directly injected into the superior colliculus (Fig. 4). Immunohistochemical localization of virus in coronal brain sections demonstrated robust infection of retinorecipient neurons in the superior colliculus with PRV Be, trans-complemented (coinfection by PRV 91 and PRV 98), gE-negative (PRV 91), and gI-gE-deleted (PRV 99) strains (top row). Both PRV Be and gE-gI null mutants were capable of progressive horizontal and vertical spread of neuronal infection in laminae of the superior colliculus, as well as in other visual centers (data not shown). It is noteworthy that we could not see any viral antigen at the injection site when animals were sacrificed at 4 h after injection. Therefore, the presence of viral antigen at later time points must reflect newly replicated virus and not the original inoculum. We conclude that gI and gE mutants can enter and replicate in superior colliculus neurons.
FIG. 4.
Differential patterns of infection following virus inoculation into the superior colliculus. PRV 99 (gI− gE−; n = 4), PRV Be (gI+ gE+; n = 4), PRV 91 (gE−; n = 2), or PRV 91 plus PRV 98 (1:1, gI+ + gE+; n = 2) (200 nl; 5 × 104 PFU total) was inoculated into the left superior colliculus of the rat brain. At maximal postinoculation survival times (PRV 99 and PRV 91, 60 h; PRV Be and PRV 91 plus PRV 98, 48 h), the animals were sacrificed and their brain tissue was isolated. Coronal brain sections were immunoreacted for viral antigen. Vertical columns (PRV 99, PRV Be, PRV 91, and PRV 91 plus PRV 98) present data from a single, representative virus-infected animal. Each horizontal row represents a specific recipient nucleus: superior colliculus (SC), LGN, SCN, and occipital cortex (VC). All panel images are ipsilateral relative to the site of injection. Images from identical regions were produced at the same magnification and settings.
PRV gE and gI are required for anterograde spread of infection in the CNS.
As further proof that the PRV strains could replicate in superior colliculus neurons, we mapped the spread of virus infection to other areas of the CNS known to be in synaptic contact with the superior colliculus (Fig. 4). Marked differences in spread were observed for infections with gE and gI mutants compared to infection with PRV Be or mixed infection by gE and gI mutants. Notably, the dLGN (Fig. 4, second row) could not be infected by gE and gI mutants alone whereas PRV Be and mixed injections with PRV 91 plus PRV 98 strains produced a robust infection of the dLGN. By contrast, infection of the IGL and vLGN was robust and indistinguishable after injection of all four strains. Similarly, all four virus injections gave rise to marked infections of the subdivisions of the SCN (Fig. 4, third row), as well as visual cortical areas of the occipital cortex (Fig. 4, VC, bottom row). Animals inoculated with PRV 99 or PRV 91 exhibited reduced infection of the occipital cortex relative to that in animals infected with PRV 91 plus PRV 98 or with PRV Be. Whereas PRV Be and mixed injection of PRV 91 plus PRV 98 showed extensive spread of virus by 48 h, spread of gI- and gE-null mutants was considerably more restricted within the same region even at later times after CNS injection. Time course analysis of spread to the occipital cortex following PRV 99 injection revealed only sparse neuronal staining by 48 h after injection and no viral infection was observed at the 36-h time point (data not shown). All PRV strains replicated in superior colliculus neurons and were transported equally through known retrograde connections (e.g., to the IGL, vLGN, and SCN). These patterns of labeling are consistent with known neural circuitry involving the superior colliculus if we assume that gE and gI are required for efficient anterograde (but not retrograde) spread of infection.
Retrograde infection of retinal ganglion neurons after direct injection of virus into the superior colliculus.
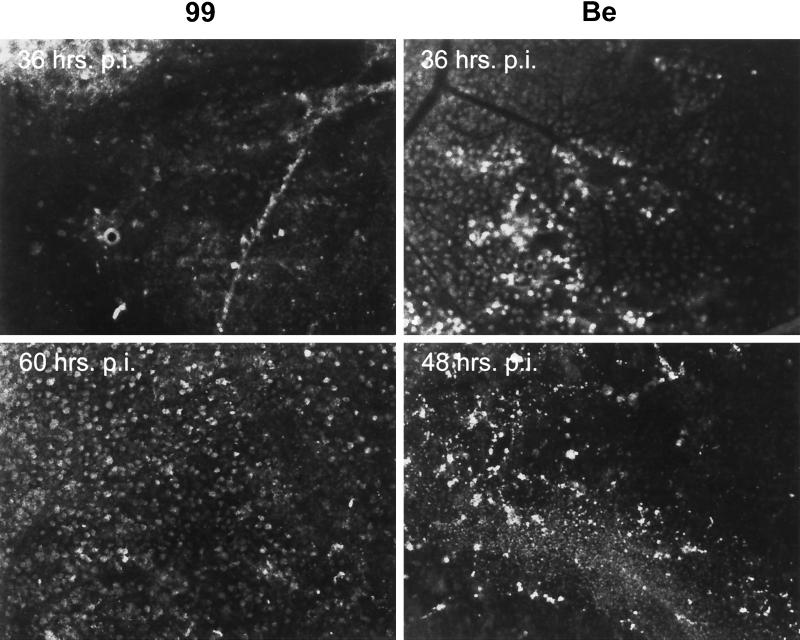
In the previous experiments, we demonstrated that gE and gI mutant viruses could replicate in retinal ganglion neurons in synaptic contact with neurons in the superior colliculus by colocalization of an inert tracer and virus antigen. In this experiment, we demonstrate that the vast majority of retinal ganglion cells that send axons to the superior colliculus can be infected by wild-type and gE-gI mutant viruses. To do so, we injected PRV 99 or PRV Be directly into the superior colliculus and then determined if virus would spread back to the retina by retrograde mechanisms (Fig. 5). With both virus strains, extensive infection of retinal ganglion neurons was observed across the entire contralateral retina, indicating that all viruses were capable of retrograde transport and replication in retinal ganglion cells that project axons to the superior colliculus. Interestingly, whereas both viruses displayed infection of these retinal ganglion neurons at the earliest time points examined (36 h postinjection), wild-type virus infected more retinal ganglion cells earlier than did PRV 99. At later times, both viruses infected approximately the same numbers of retinal ganglion cells.
FIG. 5.
Infection of retinal ganglion neurons after PRV injection directly into the superior colliculus. PRV Be or PRV 99 was inoculated into the left superior colliculus of the rat brain, as described in the legend to Fig. 4. Animals were sacrificed at the indicated times postinfection, and their retinas were isolated. Retinas were processed by immunohistochemistry for viral antigen, mounted on slides, and visualized by fluorescence microscopy. The images show results from retinas contralateral to the site of injection in the CNS.
DISCUSSION
By directly injecting PRV into the CNS, we were able to confirm and extend our previous suggestions that the gE and gI genes of PRV are required for efficient anterograde transport in the rodent visual system. Following stereotaxic injection of F-G, an inert, fluorescent, retrograde tracer, into the superior colliculus and subsequent intravitreal inoculation of a gI-gE double-null mutant (PRV 99), we found that viral antigen and fluorescent tracer colocalized in the same retinal ganglion cells in the rat retina.
This direct-injection paradigm proved that second-order CNS neurons in the superior colliculus that receive information from the retinal ganglion cells were susceptible and permissive to both wild-type and gE-gI null viruses based on the presence of extensive viral antigen. Therefore, as we deduced indirectly from mixed-infection studies (9), our data confirm that PRV gI-gE are not required either for entry into or replication in the functionally distinct set of retinal ganglion cells that project to the superior colliculus. Moreover, the use of PRV 99, a gI-gE double-null virus, prevented any possible production of complemented virus or wild-type recombinants.
The absence of gI and gE had little effect on the kinetics of infection, as shown by the colocalization of viral antigen and tracer by equivalent numbers of cells as early as 48 h after injection with either wild-type or gI-gE-deficient viruses. However, the relatively small number of virus-infected retinal ganglion cells that contained F-G tracer was somewhat unexpected, given that 90% or more of the million retinal ganglion cells in each retina are in synaptic contact with the superior colliculus. However, LaVail et al. (14a) demonstrated that F-G inhibited the growth of HSV-1 by 50% in the trigeminal ganglion after corneal infection. We believe that F-G also has an inhibitory effect on PRV replication in our experiments since the number of double-labeled cells was significantly larger when PRV was allowed to replicate in retinal ganglion cells before F-G was injected. Thus, while we cannot draw any conclusions about the absolute number of double-labeled cells, we can conclude that there was no significant difference between wild-type virus and gE-gI mutant virus in the ability to enter and replicate in retinal neurons that are in synaptic contact with the superior colliculus.
We also found that neurons in the superior colliculus are equally susceptible and permissive to direct infection by wild-type and mutant viruses despite the observation that they cannot be infected after retinal infection. Indeed, all viruses replicated well and to similar a extent in all areas of the superior colliculus as determined by robust staining with polyvalent antibody specific for PRV structural proteins. Therefore, the inability of gE and gI mutants to infect the superior colliculus after eye infection is due to a defect prior to infection of the postsynaptic neuron in the superior colliculus.
After direct injection of PRV into the superior colliculus, the virus spreads throughout this region and then to other areas of the CNS in synaptic contact. We noted that both wild-type and gI-gE null mutants infected the IGL and vLGN in the thalamus and the SCN in the hypothalamus, whose neurons are known to send axons directly to the superior colliculus. Their infection is consistent with retrograde spread of virus. The retrograde connection of these neurons to the superior colliculus was confirmed by their labeling when the retrograde tracer F-G was injected into the superior colliculus. However, the dLGN in the same region of the thalamus was not infected by gI- gE-deficient mutants (PRV 91 or PRV 99) and was not labeled by F-G. It is well known that a set of neurons in the superior colliculus send axonal projections to the dLGN. We suggest that the inability of gE and gI mutants (and F-G tracer) to label these neurons reflects the general inability of these viruses and tracers to spread by anterograde mechanisms (e.g., from presynaptic to postsynaptic cells) (Fig. 1) (22). This suggestion was reinforced by the finding that coinjection of a 1:1 mixture of PRV 91 and PRV 98 into the superior colliculus produced a robust infection of the dLGN with regard to both infection pattern and virulence. For such functional complementation to occur, coinfection of neuron cell bodies in the superior colliculus and subsequent passage of virus through efferent axons in synaptic contact with neurons in the dLGN is required. The exceedingly sparse infection of the dLGN at the longest survival intervals with PRV 99 and PRV 91 may reflect the passage of virus through a multiorder, retrograde circuit via the ipsilateral occipital cortex or contralateral retina.
PRV Be and the gE-gI null mutants spread to the retina after direct injection into the superior colliculus. The prominent infection of retinal ganglion cells after all infections at the earliest times examined (36 h) is consistent with uptake of virus at axon terminals in the superior colliculus followed by retrograde spread of virus back to the retina, where replication occurs in the cell bodies of retinal ganglion cells. Consistent with previous data obtained with other circuits, gI and gE were not required for retrograde transport or for replication in retinal ganglion cells. Interestingly, we observed that gE-gI mutants had a perceptibly different rate of appearance in retinal ganglion cells after central injection. Similar differences in retrograde neuronal circuit spread have been observed with the attenuated vaccine strain PRV Bartha compared to wild-type strains (26). The molecular mechanism for this effect is not known.
In summary, we have confirmed and extended our previous work with the rat eye infection model that indicated that gE and gI gene expression in primary infected cells is required for efficient anterograde spread of virus to second-order neurons. PRV gI and gE proteins may affect the targeting of critical proteins to axons, transport of important viral proteins (or intracellular virus) within axons, or targeting of viral proteins to sites of viral egress at or near axon terminals. Importantly, these mechanisms are likely to function not only after infection of the retina but also after direct injection into the CNS (see also reference 2a). We suggest that the expression of gE and gI by PRV and perhaps other alphaherpesviruses is essential to regulate the choice of direction for virus spread at different stages of the virus life cycle.
ACKNOWLEDGMENTS
We gratefully thank Patrick Card (University of Pittsburgh) for helpful comments and constructive criticisms during the design of the described experiments and for a review of the manuscript.
This work was supported by National Institute of Neurological Diseases and Stroke grant 1RO133506 to L.W.E.
REFERENCES
- 1.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 2.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 2a.Card J P, Levitt P, Enquist L W. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol. 1998;72:4434–4441. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Card J P, Moore R Y. The organization of visual circuits influencing the circadian activity of the suprachiasmatic nucleus. In: Klein D C, Moore R Y, Reppert S M, editors. Suprachiasmatic nucleus. The mind's clock. New York, N.Y: Oxford University Press, Inc.; 1991. pp. 51–106. [Google Scholar]
- 4.Card J P, Rinaman L, Lynn R B, Lee B-H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two α-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 8.Enquist L W, Card J P. Pseudorabies virus: a tool for tracing neuronal connections. In: Lowenstein P R, Enquist L W, editors. Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. Chichester, England: John Wiley & Sons Ltd.; 1996. pp. 333–348. [Google Scholar]
- 9.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1999;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 10.Hsu S-M, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 11.Kimman T G, de Wind N, Oei-Lie N, Pol J M A, Berns A J M, Gielkens A L J. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpes type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 12.Knapp A C, Husak P J, Enquist L W. The gE and gI homologs from two alphaherpesviruses have conserved and divergent neuroinvasive properties. J Virol. 1997;71:5820–5827. doi: 10.1128/jvi.71.8.5820-5827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kritas S K, Nauwynck H J, Pensaert M B. Dissemination of wild-type and gC-, gE-, and gI-deleted mutants of Aujeszky's disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995;76:2063–2066. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- 14.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63, and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 14a.LaVail J H, Carter S R, Topp K S. The retrograde tracer Fluoro-Gold interferes with the infectivity of herpes simplex virus. Brain Res. 1993;625:57–62. doi: 10.1016/0006-8993(93)90137-c. [DOI] [PubMed] [Google Scholar]
- 15.Linden R, Perry V H. Massive retinotectal projection in rats. Brain Res. 1983;272:145–149. doi: 10.1016/0006-8993(83)90371-2. [DOI] [PubMed] [Google Scholar]
- 16.McLean I W, Nakane P K. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 17.Morin L P. The circadian visual system. Brain Res Rev. 1994;67:102–127. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 18.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulder W A M, Jacobs L, Priem J, Kok G L, Wagenaar F, Kimman T G, Pol J M A. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1986. [Google Scholar]
- 21.Rajcáni J, Herget U, Kaerner H C. Spread of herpes simplex virus (HSV) strains SC16, ANG, ANGpath and its GlyC minus and GlyE minus mutants in DBA-2 mice. Acta Virol. 1990;34:305–320. [PubMed] [Google Scholar]
- 22.Reese B E. The projection from the superior colliculus to the dorsal lateral geniculate nucleus in the rat. Brain Res. 1984;305:162–168. doi: 10.1016/0006-8993(84)91133-8. [DOI] [PubMed] [Google Scholar]
- 23.Richmond F J R, Gladdy R, Creasy J L, Kitamura S, Smits E, Thomson D B. Efficacy of seven retrograde tracers, compared in multiple-labelling studies of feline motoneurones. J Neurosci Methods. 1994;53:35–46. doi: 10.1016/0165-0270(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 24.Schmued L C, Fallon J H. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- 25.Sefton A J, Dreher B. Visual system. In: Paxinos G, editor. The rat nervous system. 2nd edition. San Diego, Calif: Academic Press, Inc.; 1995. pp. 833–898. [Google Scholar]
- 26.Standish A, Enquist L W, Escardo J A, Schwaber J S. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Standish A, Enquist L W, Schwaber J S. Innervation of the heart and its central medullary origin defined by viral tracing. Science. 1994;263:232–234. doi: 10.1126/science.8284675. [DOI] [PubMed] [Google Scholar]
- 28.Swanson L W. Brain maps: structure of the rat brain. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1992. [Google Scholar]
- 29.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson R E, Wiegand S T, Clough R W, Hoffman G E. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 31.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H-J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]