Abstract
BACKGROUND:
Childhood brain tumor survivors (BT) experience persisting health concerns across the lifespan. We evaluated changes in symptom burden over the course of 12 months, using pediatric Patient Reported Outcomes Measurement Information System (PROMIS) measures.
METHODS:
Data from 202 BTs, aged 8–21yrs, and 262 parents of BT aged 5–21yrs) were analyzed. All completed a PROMIS Cognition short-form, and computerized adaptive tests (CATs) of pediatric Anxiety, Depressive Symptoms, Fatigue, Mobility, Upper Extremity Function, and Peer Relationships. About half (223: 97 BT; 126 parents) completed 12-month follow-up. Linear mixed-effects models (LMEM) evaluated group-level symptoms over time. Cox proportional hazard models explored whether symptoms predicted survival, and latent class growth analysis (LCGA) investigated patterns of individual-level symptom change over time.
RESULTS:
LMEMs showed patient-reported Cognition and parent-reported Anxiety worsened over time. LCGA results indicated that patient and parent reports diverged, both in the number of classes identified, and in the trends of these classes. Parents and patients reported similar patterns of depression over time. For the other areas, parents were either more likely to see different patterns (peer relationships, mobility), or less likely to see different patterns (upper extremity function, cognition, anxiety, fatigue). Baseline patient-reported Mobility and Upper Extremity Function were associated with survival.
CONCLUSIONS:
Childhood brain tumor survivors demonstrated different trajectory patterns of symptom burden. Along with baseline functioning status and days since treatment, patient-reported Mobility and Upper Extremity Function were associated with survival, suggesting a possible role of PROs in clinical care, especially individualized, tailored assessments such as PROMIS.
Keywords: Symptom burden, Patient-reported outcomes, Children, Brain tumor, CAT
Precis
Childhood brain tumor survivors and their parents reported different and variable symptom and function patterns over time, and the infleuntial factors associated with each pattern also varied. Baseline patient-reported Mobility and Upper Extremity Function were associated with survival, suggesting a possible role of this information in clinical care.
INTRODUCTION
Despite recent increases in overall survival, survivors of childhood brain tumors often experience detrimental, persistant health effects and brain tumor-related treatment across the lifespan,1–6 Adult survivors of childhood brain tumor, compared to other pediatric cancer survivors, are more likely to experience functional impairment,4,5 treatment-related adverse events,6 and to have lower educational achievement, full-time employment, income, and likelihood of marriage.7 Hovén et al8 found that 40% of childhood brain tumor survivors required more medical care, illness education and psychosocial services than the general population. Accordingly, it is recommended that children with cancer and their family should receive systematic assessments of their psychosocial health care needs.9 Additionally, children with brain tumors and those who received neurotoxic treatments should also be monitored for neuropsychological deficits during and after treatment.10
There is considerable variability across studies in defining and measuring symptom burden in this population.11 Accurate information on symptom burden over time depends upon psychometrically sound and individually-tailored measurement tools to detect change. The Patient Reported Outcome Measurement Information System (PROMIS®)12,13 meets this need. All PROMIS measures were developed using a rigorous mixed-methods approach, including both qualitative and quantitative approaches. These measures demonstrated satisfactory psychometric properties, which were evaluated using factor analytic approaches and item response models (IRT),14,15 and were validated on children with cancer.16–18 Its measures can be administered as computerized adaptive tests (CATs), wherein respondents only receive the most informative items, selected by an algorithm, around the estimated scores based on their responses to previous items. As a result, individualized, tailored and precise estimation of symptom scores can be achieved with brief assessment.19–21
Taking advantage of CATs to better understand the extent of symptom burden experienced by pediatric patients with brain tumors, this study evaluated changes in patient-reported outcomes (PROs) over the course of 12 months using pediatric PROMIS Anxiety, Depressive Symptoms, Fatigue, Mobility, Upper Extremity Function, and Peer Relationships CATs, and Cognition brief, fixed-form. Unlike previous studies that examined symptom burden differences at the group level, our primary goal was to examine patterns of PRO changes reported by individual patients and demographic and clinical characteristics associated with these patterns. Additionally, we examined symptom burden trajectories, and evaluated the concordance between patient- and parent-reported symptom burden. Finally, since studies22–24 in adult cancer patients showed symptom burden was associated with survival and that routine assessment of patient-reported outcomes could increase survial rates,25 we also explored whether symptom burden reported by patients and parents predicted patient survival.
METHODS
Institutional Review Boards (IRB) at each recruitment site approved this study. All participants provided informed consent (parents and patients with ages 18 years or older) or assent (ages varied depending on each IRB’s requirements) prior to participation in this study.
Participants and Procedures
Patients and one of their parents were recruited from the Ann and Robert H. Lurie Children’s Hospital of Chicago (including Northwestern Medicine Chicago Proton Center and Marianjoy Rehabilitation Hospital), Boston Children’s Hospital, and Maryland Proton Treatment Center. Inclusion criteria were: diagnosis of a brain tumor, between 5 and 22 years of age, at any stage of the treatment continuum (including long-term survival), and undergoing or have undergone any type of cancer treatment. Patients and parents were excluded from the study if they were unable to read and understand consent/assent forms in English or respond to the questions. Once participants signed consent/assent forms, patients and one of their parents completed baseline assessments in oncology clinics, using an iPad. They were then asked to complete 6-month (on-therapy patients only) and 12-month follow-up assessments during their clinical visits or at home using any device with internet access.
Instruments
Patients completed the following assessments: Symptom Distress Scale (SDS), PROMIS pediatric Anxiety, Depressive Symptoms, Fatigue, Mobility, Upper Extremity Function, and Peer Relationships CATs, and Cognition short-form (aka, Pediatric Perceived Cognitive Function, pedsPCF). Parents provided sociodemographic information at baseline, and completed parent proxy versions of the pediatric self-report measures, and a single item about their child’s quality of life (excellent, very good, good, fair, poor). All PROMIS scores were reported on a T-score metric, with a mean of 50 referenced to the norming sample and a standard deviation of 10. Higher scores represent either better functioning (Cognition, Mobility, Upper Extremity Function, and Peer Relationships) or more symptomatic (Anxiety, Depressive Symptoms, and Fatigue).
Statistical Analyses
We evaluated patients’ symptoms over time using three sets of analyses. First, we used linear mixed-effects models, as implemented in SAS 9.4 (Cary, NC), to evaluate symptom changes over time at the group level.26,27 This model allows for missing data across timepoints,28,29 which is needed for our study as about half the sample did not complete all time-points. Least squares means, standard errors and 95% confidence intervals were estimated from the models. Linear mixed models were also performed on SDS reported by patients and parents.
Secondly, we used latent class growth analysis (LCGA), as implemented in R package lcmm, to investigate changes at the individual level by determining whether patient- or parent-reported outcome trajectories over time fell into statistically defined groups, or classes. A linear time trend was used and models fitting 2 to 5 classes were investigated. Bayesian information criterion (BIC) was used to select the best-fitting number of classes for each model. The best fitting model was identified as that with the lowest BIC that did not result in any classes with very small sample sizes (n<5). Once the best-fit classes were identifed, we explored predictors of class membership using chi-square tests (for categorical variables) or analysis of variance (for continuous variables).
Finally, Cox proportional hazard models were used to explore potential symptom predictors of survival. Survival time was calculated as the time from the study baseline assessment to date of death. For surviving patients, survival time was censored at the time of the last completed study follow-up date. In addition to patient- and parent-reported PROMIS measures, patient age, gender, race/ethnicity, time since diagnosis, treatment modalities experienced (radiation, surgery, chemotherapy), and performance status were modeled because they were significantly associated with baseline symptom burden.18 Due to low event rates, each predictor was examined in a separate Cox model without adjustment and predictors with p<0.05 are described in the results.
Additionally, we conducted descriptive examination of the association between patient and parent reports by calculating change scores for PROMIS Cognition, Anxiety, Depressive Symptoms, Fatigue, Mobility, Upper Extremity Function, and Peer Relationships from baseline to each participant’s last assessment time-point. We then examined Pearson product moment correlations and estimated intra-class correlation coefficients (ICCs) (two-way mixed effects models) between patient and parent change scores for each domain. To interpret the magnitude of correlations, Cohen’s cut-offs were used: small: 0.10< r<0.243; medium: 0.243 < r <0.371; large: r > 0.371.30 For ICC’s the following criteria were used to interpret magnitude: excellent, > 0.75; good, 0.40–0.75; marginal, <0.40.31
RESULTS
Participants
A total of 382 dyads were approached. Of these, 52 refused to participate. The remaining 330 patient-parent dyads provided informed consent/assent: 250 patients aged 8–22 years and 317 parents of patients aged 5–22 years. Of these, 202 patients and 262 parents provided valid data at baseline. Over the course of the study, 25 participants (13 parents and 12 patients) withdrew from the study after completing the baseline assessment. Sixty-seven participants (25 patients and 42 parents) and 223 participants (96 patients and 127 parents) completed the 6- and 12-month follow-up, respectively. Due to continuing enrollment beyond the time that would allow for complete follow-up, some patients were only followed for 6 months. Twenty-four patients were lost to follow-up due to death. Average survival by the end of the study was 346.4 days (SD=256.7; min=38 and max=966).
Participant Characteristics
Table 1 shows participant demographic and clinical information. Patients were on average age 12.4 years (SD=4.7), 54.5% were male, and 78.6% were White. Most patients (93%) attended school and 49.6% attended regular classrooms without any individualized or special educational program (IEP). Most patients had had a surgical procedure performed (70.8%), chemotherapy (84.6%) or radiation (60.1%) and 75.4% received more than one treatment modality. Patients with medulloblastoma and other embryonal tumors or with glioneuronal tumor were more likely to complete a 12-month follow up, while those with high grade glioma were less likely to complete a 12-month follow-up. Patients who completed a 12-month follow-up were more likely to be newly diagnosed, and received chemotherapy or radiation more than one year at baseline.
Table 1.
Participant demographic and clinical information
| All patients (N=289) | Patients with vs. without 12-month Follow-up |
||||
|---|---|---|---|---|---|
| Without (n=150) | With (n=139) | ||||
|
| |||||
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | p | |
|
| |||||
| Age (patients) | 12.4 (4.7) | 12.2 (4.6) | 12.4 (4.8) | 0.714 | |
|
| |||||
| Age (parents) | 43.0 (7.0) | 42.6 (6.9) | 43.3 (7.2) | 0.391 | |
|
| |||||
| Year since the most recent treatment | 0.39 (1.2) | 0.41 (1.3) | 0.35 (1.1) | 0.531 | |
|
| |||||
| Variable | Categories | % | % | % | |
|
| |||||
| Gender (Patients) | Male | 54.5 | 54.7 | 54.7 | 0.999 |
| Female | 45.6 | 45.3 | 45.3 | ||
|
| |||||
| Gender (Parents) | Male | 17.4 | 19.9 | 15.1 | 0.315 |
| Female | 82.6 | 80.2 | 84.9 | ||
|
| |||||
| Race | White | 78.6 | 75.2 | 83.5 | 0.492 |
| Black or African-American | 7.1 | 10.1 | 3.3 | ||
| Asian | 3.2 | 3.1 | 3.3 | ||
|
| |||||
| Does your child go to school? | Yes | 93.0 | 90.8 | 96.0 | 0.098 |
| No | 7.0 | 9.2 | 4.0 | ||
|
| |||||
| Type of classroom attending | Mainstream classroom, no IEP | 49.6 | 50.0 | 48.3 | 0.432 |
| Mainstream classroom, with IEP | 35.3 | 35.6 | 35.6 | ||
| Special education classroom within a regular school | 7.1 | 5.9 | 8.5 | ||
| Special education school | 1.3 | 2.5 | 0.0 | ||
| Other | 6.7 | 5.9 | 7.6 | ||
|
| |||||
| How do you rate your child’s quality of life in general? | Poor | 1.2 | 2.3 | 0.0 | 0.197 |
| Fair | 11.4 | 12.4 | 10.6 | ||
| Good | 27.5 | 23.3 | 31.7 | ||
| Very good | 37.3 | 41.1 | 33.3 | ||
| Excellent | 22.8 | 20.9 | 24.4 | ||
|
| |||||
| Histology | Low grade glioma | 23.5 | 25.7 | 21.7 | 0.044 |
| Medulloblastoma & other embryonal tumors | 22.8 | 18.9 | 26.8 | ||
| Glioneuronal tumor | 11.1 | 5.4 | 16.7 | ||
| Ependymoma | 7.3 | 6.8 | 8.0 | ||
| Germinoma | 6.9 | 6.1 | 7.3 | ||
| High grade glioma | 5.5 | 7.4 | 3.6 | ||
|
| |||||
| Current Status of Tumor | Initial diagnosis only | 86.3 | 81.5 | 91.3 | 0.017 |
| Recurrent | 13.7 | 18.5 | 8.7 | ||
|
| |||||
| Treatments received | None | 4.5 | 4.1 | 5.1 | 0.222 |
| 1 of 3 possible treatments | 24.2 | 27.7 | 19.6 | ||
| 2 of 3 possible treatments | 33.2 | 35.1 | 31.9 | ||
| Chemo+radiation+surgery | 38.1 | 33.1 | 43.5 | ||
|
| |||||
| Treatments | Radiation (missing=3) | 0.018 | |||
| No radiation | 39.5 | 39.2 | 39.9 | ||
| <=1 year | 29.4 | 35.8 | 22.5 | ||
| > 1 year | 31.1 | 25.0 | 37.7 | ||
| Chemotherapy (missing=6) | <0.001 | ||||
| No chemotherapy | 25.5 | 34.7 | 15.4 | ||
| <=1 year | 37.8 | 38.1 | 37.5 | ||
| > 1 year | 36.7 | 27.2 | 47.1 | ||
| Surgery (missing n=5) | 0.866 | ||||
| No surgery | 28.9 | 28.4 | 29.2 | ||
| <=1 year | 21.3 | 22.3 | 19.7 | ||
| > 1 year | 49.8 | 49.3 | 51.1 | ||
|
| |||||
| Type of radiation received | Photon | 44.7 | 51.1 | 38.8 | 0.271 |
| Proton | 52.9 | 46.6 | 58.8 | ||
| Both photon and proton | 2.4 | 2.3 | 2.5 | ||
|
| |||||
| Years since last treatment | <= 1 year | 83.9 | 84.1 | 83.5 | 0.886 |
| > 1 year | 16.1 | 15.9 | 16.5 | ||
|
| |||||
| Performance Status Rating | 50 | 0.7 | 0.7 | 0.8 | 0.121 |
| 60 | 2.2 | 2.9 | 1.5 | ||
| 70 | 2.2 | 3.6 | 0.8 | ||
| 80 | 11.0 | 15.0 | 6.9 | ||
| 90 | 29.3 | 29.3 | 29.2 | ||
| 100 | 54.6 | 48.6 | 60.8 | ||
Analysis Results
Group-Level Symptom Changes over Time
Means of PROMIS measures reported by patients and parents across time are shown on Figure 1. No significant differences were found between baseline and 12-month scores. We did not include 6-month data in this figure due to its significantly smaller sample size. Parents reported worse or similar symptom scores (Anxiety, Depressive Symptoms, Fatigue) and worse functioning scores (Mobility, Upper Extremity Function, Peer relationship, and Cognition) than the norms. However, patients reported worse or similar symptom and better functioning on all domains across all time-points.
Figure 1.
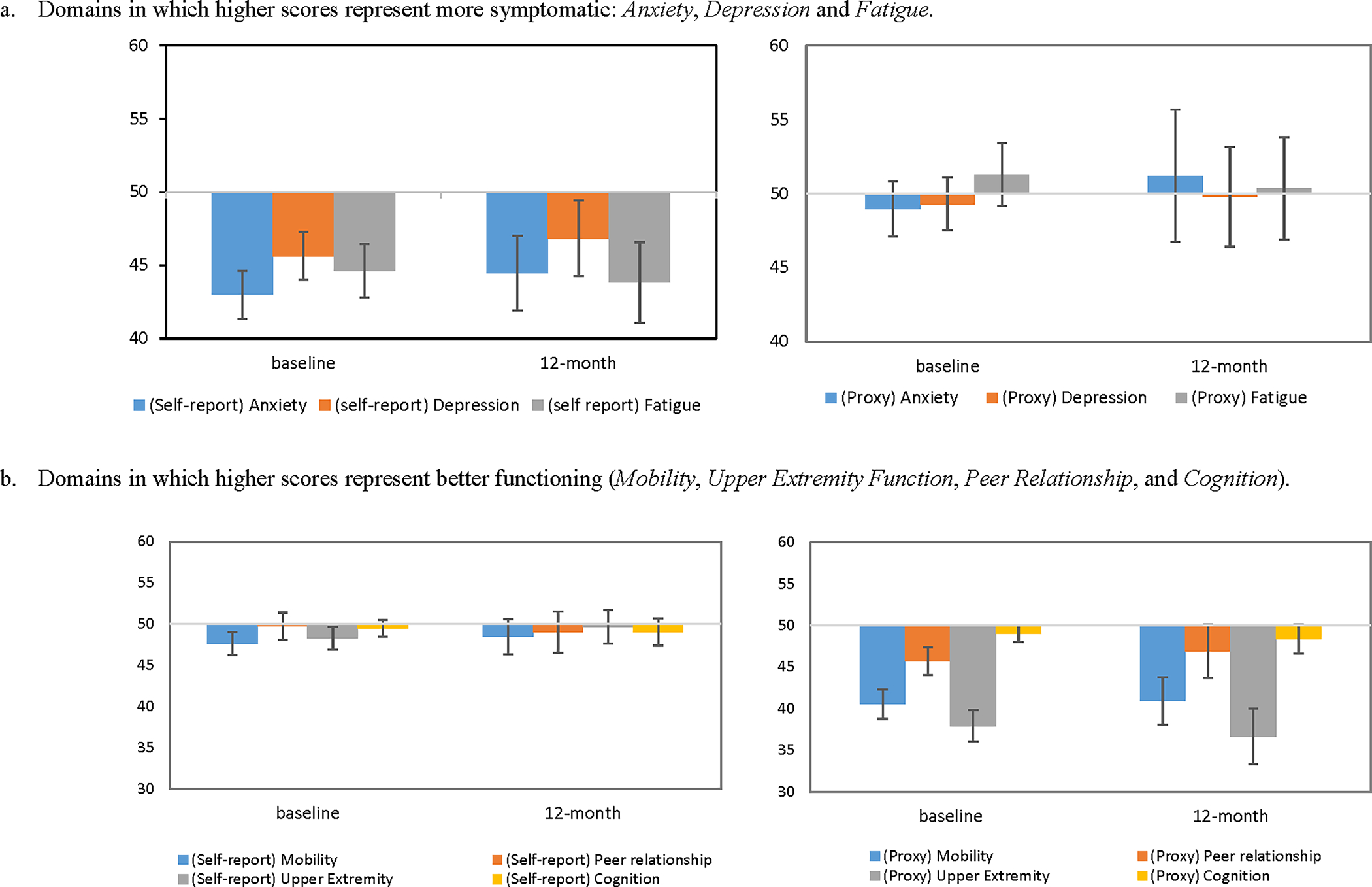
Patient- and parent-reported PROMIS measures at baseline and 12-month follow-up, compared to the norming sample mean=50 and standard deviation=10. Y-axis represents T-scores. Error bars represent 95% confidence intervals.
a. Domains in which higher scores represent more symptomatic: Anxiety, Depression and Fatigue.
b. Domains in which higher scores represent better functioning (Mobility, Upper Extremity Function, Peer Relationship, and Cognition).
Linear mixed models showed patient-reported Cognition (coefficient=−0.86, t=−2.11, p=0.037) and parent-reported Anxiety (coefficient=2.42, t=2.18, p=0.033) worsened over time. There were no significant differences between baseline and last assessment on other domains. No significant changes were on the SDS reported by both patients and parents.
Correlation coefficients between patient and parent change scores ranged in magnitude. The largest correlation was for Depressive Symptoms (r = 0.70, p<0.001; ICC = 0.82). As shown in Figure 2, patient- and parent-reported Depressive Symptoms shared the same patterns. Moderate (r) and good (ICC) correlations were found on physical health: Fatigue (r = 0.49, p=0.02; ICC = 0.66), Upper Extremity Function (r = 0.46, p=0.04; ICC = 0.62) and Mobility (r = 0.44, p=0.04; ICC = 0.59). Additionally, the correlation for Anxiety was also moderately large, though not statistically significant (r = 0.42, p=0.08; ICC = 0.59). Finally, the correlations for Peer Relationships (r = 0.19, p=0.46; ICC = 0.30) and Cognition (r = 0.12, p=0.36; ICC = 0.21) had small or marginal magnitudes.
Figure 2.
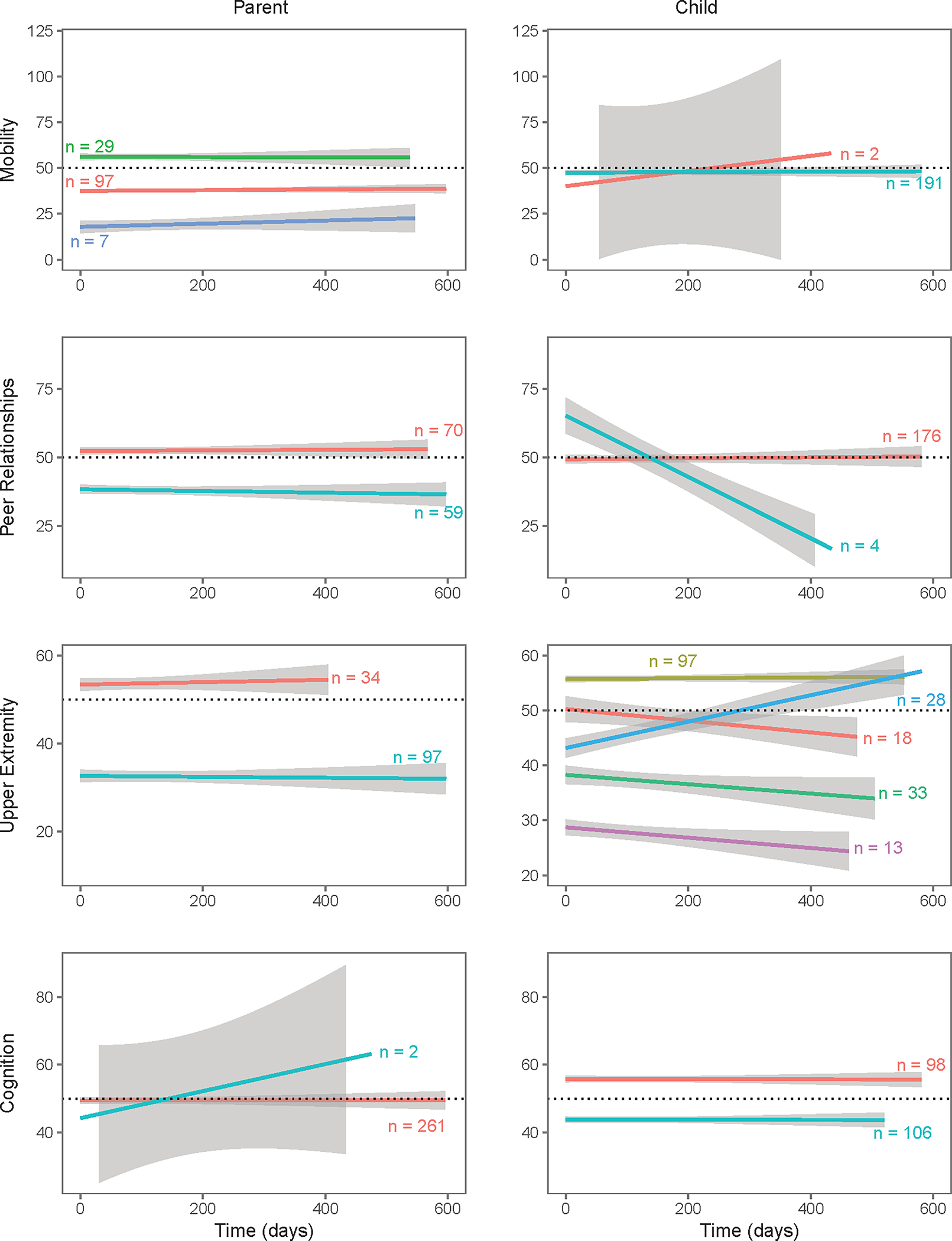
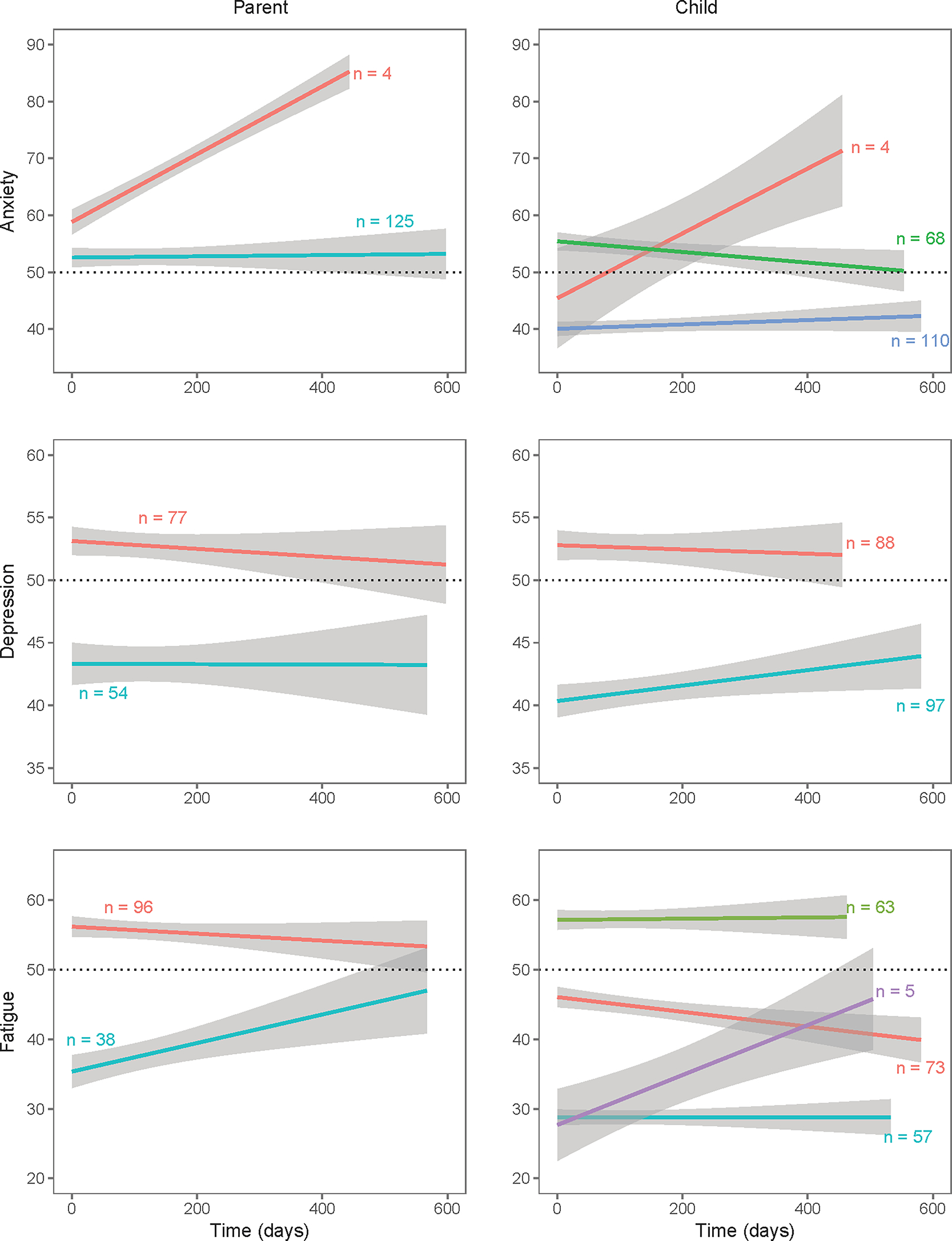
Class membership from the latent class growth analysis of all available follow-up data.
a. Domains in which higher scores representing more symptomatic: Anxiety, Depressive Symptoms and Fatigue.
b. Domains in which higher scores representing better functioning (Mobility, Upper Extremity Function, Peer Relationship, and Cognition).
Patterns of Individiual-Level Symptom Change over Time
Table 2 and Figure 2 show the LCGA results. Classes composed of sample sizes less than five were considered insignificant. Patient and parent reports diverged often, both in the number of classes identified, as well as in the trends of these classes. One exception was Depression, where trends reported by parents and patients were similar. With the exception of Mobility, parents tended to identify fewer classes than patients.
Table 2.
Class membership and predictors of each class across domains reported by patients and parents
| Domain | number of classes a | Sample n (by class) | Marital status | Gender (child) | IEPb | Parent rated QOL | Initial dx or recurrent | Number of tx receivedc | Length (chemo) d | Length (radiation) e | PSRf | Age (parent) | Age (child) | Years since dx | Years since last tx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety | child | 2a | 4 a; 68; 108 | * | *** | * | ||||||||||
| Depression | child | 2 | 88; 95 | *** | * | * | ** | |||||||||
| Fatigue | child | 4 | 73;63;54;5 | ** | *** | ** | ** | * | ||||||||
| Mobility | child | 1a | 2 a;189 | |||||||||||||
| UE | child | 5 | 17;96;35;26;13 | * | * | * | ** | * | ** | * | *** | *** | ** | |||
| Peer | child | 1a | 174; 4 a | * | ||||||||||||
| Cognition | child | 2 | 95;106 | * | *** | *** | ||||||||||
| Anxiety | parent | 1a | 4 a; 125 | |||||||||||||
| Depression | parent | 2 | 77; 54 | ** | ** | |||||||||||
| Fatigue | parent | 2 | 96;38 | *** | * | |||||||||||
| Mobility | parent | 3 | 97; 29; 7 | * | * | *** | *** | ** | ||||||||
| UE | parent | 2 | 34; 97 | ** | *** | ** | *** | *** | * | |||||||
| Peer | parent | 2 | 70; 59 | * | ||||||||||||
| Cognition | parent | 1a | 259; 2 a |
p<0.05;
p<0.01;
p<0.001
UE=Upper Extremity Function; Peer= Peer Relationships; Dx=diagnosis; Tx=treatment
Classes with sample size less than 5 were considered trivial.
IEP: “attending regular classroom without any individualized educational program” vs. “receiving any types of special education programs, including individualized educational program”
No treatment, one of three possible treatments, two of three possible treatments; all three possible treatments (surgery, chemotherapy and radiation).
Length (chemo): “never received chemotherapy” vs. “received the most recent chemotherapy within one year” vs. “received the most recent chemotherapy more than one year”
Length (radiation): “never received radiation” vs. “received the most recent radiation within one year” vs. “received the most recent radiation more than one year”
PSR: Functional performance rating; 40–80 vs. 90 vs. 100
Significant predictors of class membership are shown in Table 2, and these varied across domains and between parents and patients. Variables not significantly associated with class membership (e.g., parent gender and race) were not included in this table. Parent-rated child’s health-related quality of life was a significant predictor of all classes. Patient-reported Upper Extremity Function, Fatigue and Depressive Symptoms class membership was associated with some or all clinical characteristics of new diagnosis (vs. recurrent), number of treatments received (min=0, max=3), time since last chemotherapy (no chemotherapy; <=1 year; > 1 year), time since last radiation (no radiation; <= 1 year; > 1 year), years since diagnosis, and years since last treatment. However, these clinical characteristics were not associated with parent-reported domains except Depressive Symptoms vs. years since last treatment. Clinical variables were associated with class membership based on patient-reported measures but not on parent-reported measures.
Association of Symptoms with Patient Survival
Median follow-up time for survival was approximately one year. Results of Cox proportional hazards models indicated that better patient-reported Mobility (hazard ratio [HR]=0.725, 95% CI=[0.565, 0.929], p=0.011) and Upper Extremity Function (HR=0.703, 95% CI=[0.549, 0.902], p=0.006) were associated with longer survival. Additionally, longer time since diagnosis (HR per 60 days=0.972, p=0.01) and better performance rating (HR for 100 vs <100=0.37, p=0.021) were significantly associated with survival.
DISCUSSION
Studies have shown that childhood brain tumor survivors are at high risk of experiencing treatment late effects throughout their life span.18,32,33 Most current studies focused on survivors’ symptom burden at the group level. In this study, we examined symptom burden reported by patients and parents at both the group and the individual levels by evaluating symptom burden trajectories and identifying factors associated with these patterns. These results can assist investigators in designing targeted strategies to provide timely interventions and in educational efforts to help families prepare for managing symptoms.
At the group level, mixed effects analyses revealed significant small changes on patient-reported Cognition and parent-reported Anxiety. However, at the individual level, LCGA results indicated different individual trajectory patterns over time on almost all domains within as well as between patients and parents. Factors associated with trajectory patterns varied across domains and between patients and parents. In brief, clinical factors were associated with patient-reported trajectory patterns of Depressive Symptoms, Fatigue, Upper Extremity Function and Peer Relationship but none of the parent-reported domains. Literature has indicated low to moderate concordance between parent and child-reports.34,35 Perceived symptom burden is self-referenced phenmona and thus, patient-reports from children should be considered the primary source in this matter.36,37 Because patients tend to report more classes, and that these classes are more dynamic and were associated with clinical factors, we suggest patient-reports might be more sensitive to differences and changes in health. We speculate that child patients might not want, or are unwilling, to communicate with their parents for various reasons such as not wanting to increase their parents’ worries and wanting to be good patients. This speculation is echoed in Su and colleagues’ finding that parents seemed unaware of the specific difficulties that their children faced.38 The association between patient-, not parent, reported outcomes and survival provides further evidence to support this hypothesis that patient-report should be the primary sources in determining patients’ symptom burden. Additionally, the LCGA findings highlight the importance of understanding symptom burden at the individual level and further reiterate the need for individualized assessments uisng appropriate measurement tools in survivors’ follow-up care.9,18
Gotay and colleagues39 conducted a systematic review and concluded that PROs provide distinct prognostic information beyond standard clinical measures in adult cancer clinical trials. However, such research in children is in its infancy. In this study, we found Mobility and Upper Extremity Function, along with baseline functional performance status and days since diagnosis, significantly predicted survival. The current study was one of the first studies to explore PROs as predictors of survival in children with brain tumors. Yet given a moderate sample size and a small number of deaths (n=24), we considered results were preliminary and its generalizability is limited and should be interpreted with caution. Regardless, this finidng reiteritated the importance of listening to patients’ reports of their own health. Future studies across multiple sites with a larger sample size should be conducted to replicate these results.
The results should be considered in the context of study limitations. We used data from a heterogeneous sample with mixed types of brain tumors at any stage of disease continuum. Numerous factors could impact patients’ symptom experiences such as tumor characteristics (e.g., histologies, locations, and sizes) and treatments received. Though brain tumors are the most common pediatric solid tumor, low numbers of available patients (incident rate: 5.14 cases per 100,000 persons in the US40) with more than 100 different histological subtypes makes it challenging to investigate patterns within tumor type without long-term national and international collaborations. We thus focused on common symptoms and functioning concerns of general brain tumor patients rather than specific type of tumor to advance generalizability of the study results. In addition, we included patients receiving any types of treatment at any stage of the treatment continuum to evaluate treatment-related factors to trajectory patterns. Future studies are warranted to evaluate reproducibility of the results with a larger sample size, and replicability of trajectory patterns within brain tumor types. Additionally, this study followed up with patients for up to one year with considerable number of participants missing a 12-month follow-up visit, a common issue for longitudinal studies. We did not find significant differences on most variables between participants who completed versus not completed the 12-month follow-up assessments. We found patients who were sicker at the baseline likey opted out of the follow-up assessments. Impacts from these variables were taken into account in the LCGA (Table 2). We did not include histology in our model because 1) there was a small sample size in each histology and 2) it is confounded with treatment received. To ensure the replicability of the study findings, future studies are needed to evaluate the stability of these patterns for longer period of time.
In conclusion, childhood brain tumor survivors demonstrated different trajectory patterns of symptom burden, and factors influencing these patterns differed among domains as well as by reporter (patients vs. parents). Patient-reported Mobility and Upper Extremity Function were associated with survival. Though results were considered preliminary, it suggested a possible role of PROs in clinical care, especially individualized, tailored assessments such as validated PROMIS-based CATs.16,18,41,42
Acknowledgements:
The authors would like to thank all of the patients and parents who participated in the study. The authors would also like to thank Dr. Peter Manely, Dr. John Han-Chih Chang, Dr. William F. Hartsell, and Dr. Young Kwok for their assistance in recruitment.
Funding Source:
This study was funded by National Institutes of Health/National Cancer Institute (R01CA174452; PI: Jin-Shei Lai)
Footnotes
Conflict of Interests Disclosures:
All authors have no conflict of interests to be disclosed.
Contributor Information
Jin-Shei Lai, Medical Social Sciences and Pediatrics, Northwestern University Feinberg School of Medicine.
Jennifer L. Beaumont, Medical Social Sciences, Northwestern University Feinberg School of Medicine, Terasaki Research Institute, Los Angeles, California, USA.
Mary Jo Kupst, Pediatrics, Medical College of Wisconsin.
John Devin Peipert, Medical Social Sciences, Northwestern University Feinberg School of Medicine.
David Cella, Medical Social Sciences, Northwestern University Feinberg School of Medicine.
Allison Piazza Fisher, Medical Social Sciences, Northwestern University Feinberg School of Medicine.
Stewart Goldman, Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA; Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois, USA.
References
- 1.Lavigne JV, Faier-Routman J. Psychological adjustment to pediatric physical disorders: A meta-analytic review. J Pediatr Psychol. 1992;17(2):133–157. [DOI] [PubMed] [Google Scholar]
- 2.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol. 2005;30(1):9–27. [DOI] [PubMed] [Google Scholar]
- 3.Hays DM, Dolgin M, Steele LL, et al. Educational achievement, employment and workplace experience of adult survivors of childhood cancer. International Journal of Pediatric Hematology/Oncology. 1997;4:327–337. [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. The Journal of the American Medical Association. 2007;297(24):2762–2764. [DOI] [PubMed] [Google Scholar]
- 6.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. The Journal of the American Medical Association. 2007;297(24):2705–2715. [DOI] [PubMed] [Google Scholar]
- 7.Ellenberg L, Liu Q, Yasui Y, et al. Neurocognitive Status in Long-Term Survivors of Childhood CNS Malignancies: A Report From the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovén E, Lannering B, Gustafsson G, Boman KK. The met and unmet health care needs of adult survivors of childhood central nervous system tumors. Cancer. 2011;117(18):4294–4303. [DOI] [PubMed] [Google Scholar]
- 9.Kazak AE, Abrams AN, Banks J, et al. Psychosocial Assessment as a Standard of Care in Pediatric Cancer. Pediatr Blood Cancer. 2015;62(S5):S426–S459. [DOI] [PubMed] [Google Scholar]
- 10.Annett RD, Patel SK, Phipps S. Monitoring and Assessment of Neuropsychological Outcomes as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer. 2015;62(S5):S460–S513. [DOI] [PubMed] [Google Scholar]
- 11.Macartney G, Harrison MB, VanDenKerkhof E, Stacey D, McCarthy P. Quality of Life and Symptoms in Pediatric Brain Tumor Survivors A Systematic Review. J Pediatr Oncol Nurs. 2014;31(2):65–77. [DOI] [PubMed] [Google Scholar]
- 12.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella D, Yount S, Gershon R, Rothrock N. The Patient-Reported Outcomes Measurement Information System (PROMIS): Four years in and four to go. Paper presented at: International Society for Quality of Life Research (ISOQOL); October 22–25, 2008; Montevideo, Uruguay. [Google Scholar]
- 14.Cella D, Gershon RC, Lai JS, Bass M. The future of outcomes measurement: Item banking, tailored short forms, and computerized-adaptive assessment. Paper presented at: NIH and Robert Wood Johnson Foundation Critical Issues in eHealth Research Conference; June, 2005; Bethesda, MD. [DOI] [PubMed] [Google Scholar]
- 15.Lai J-S, Zelko F, Krull K, et al. Parent-reported cognition of children with cancer and its potential clinical usefulness. Qual Life Res. 2014;23(4):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. [DOI] [PubMed] [Google Scholar]
- 17.Lai J-S, Beaumont JL, Nowinski CJ, et al. Computerized Adaptive Testing In Pediatric Brain Tumor Clinics. J Pain Symptom Manage. 2017;54(3):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai JS, Kupst MJ, Beaumont JL, et al. Using the Patient-Reported Outcomes Measurement Information System (PROMIS) to measure symptom burden reported by patients with brain tumors. Pediatr Blood Cancer. 2019;66(3):e27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai J-S, Butt Z, Zelko F, et al. Development of a Parent-Report Cognitive Function Item Bank Using Item Response Theory and Exploration of its Clinical Utility in Computerized Adaptive Testing. J Pediatr Psychol. 2011;36(7):766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai JS, Cella D, Choi SW, et al. How Item Banks and Their Application Can Influence Measurement Practice in Rehabilitation Medicine: A PROMIS Fatigue Item Bank Example. Arch Phys Med Rehabil. 2011;92(10 Supplement):S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS):depression, anxiety, and anger. Assessment. 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg SL, Paramanathan D, Khoury R, et al. A Patient-Reported Outcome Instrument to Assess Symptom Burden and Predict Survival in Patients with Advanced Cancer: Flipping the Paradigm to Improve Timing of Palliative and End-of-Life Discussions and Reduce Unwanted Health Care Costs. Oncologist. 2019;24(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XS, Shi Q, Lu C, et al. Prognostic value of symptom burden for overall survival in patients receiving chemotherapy for advanced nonsmall cell lung cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2010;116(1):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacourt TE, Kavelaars A, Ohanian M, et al. Patient-reported fatigue prior to treatment is prognostic of survival in patients with acute myeloid leukemia. Oncotarget. 2018;9(58):31244–31252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 27.Hedeker DR, Gibbons RD. Longitudinal data analysis Hoboken, N.J.: Wiley-Interscience; 2006. [Google Scholar]
- 28.Troxel AB, Fairclough DL, Curran D, Hahn EA. Statistical analysis of quality of life with missing data in cancer clinical trials. Stat Med. 1998;17(5–7):653–666. [DOI] [PubMed] [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 30.Cohen J Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 31.Joseph L Fleiss BL, Paik Myunghee Cho. The Measurement of Interrater Agreement. In: Joseph L. Fleiss BL, Myunghee Cho Paik, ed. Statistical Methods for Rates and Proportions. 3rd ed.: John Wiley & Sons, Inc.; 2003:598–626. [Google Scholar]
- 32.Duckworth J, Nayiager T, Pullenayegum E, et al. Health-related quality of life in long-term survivors of brain tumors in childhood and adolescence: a serial study spanning a decade. J Pediatr Hematol Oncol. 2015;37(5):362–367. [DOI] [PubMed] [Google Scholar]
- 33.Schulte F, Barrera M. Social competence in childhood brain tumor survivors: a comprehensive review. Support Care Cancer. 2010;18(12):1499–1513. [DOI] [PubMed] [Google Scholar]
- 34.Wiener L, Baird K, Crum C, et al. Child and parent perspectives of the chronic graft-versus-host disease (cGVHD) symptom experience: a concept elicitation study. Support Care Cancer. 2013:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theunissen NC, Vogels TG, Koopman HM, et al. The proxy problem: child report versus parent report in health-related quality of life research. Qual Life Res. 1998;7(5):387–397. [DOI] [PubMed] [Google Scholar]
- 36.Lai JS, Cella D, Kupst MJ, et al. Measuring fatigue for children with cancer: development and validation of the pediatric Functional Assessment of Chronic Illness Therapy-Fatigue (pedsFACIT-F). J Pediatr Hematol Oncol. 2007;29(7):471–479. [DOI] [PubMed] [Google Scholar]
- 37.Sheffler LC, Hanley C, Bagley A, Molitor F, James MA. Comparison of self-reports and parent proxy-reports of function and quality of life of children with below-the-elbow deficiency. J Bone Joint Surg Am. 2009;91(12):2852–2859. [DOI] [PubMed] [Google Scholar]
- 38.Su CT, Wang JD, Lin CY. Child-rated versus parent-rated quality of life of community-based obese children across gender and grade. Health Qual Life Outcomes. 2013;11:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2716–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai J-S, Bregman C, Zelko F, et al. Parent-reported cognitive function is associated with leukoencephalopathy in children with brain tumors. Qual Life Res. 2017;26(9):2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinds PS, Wang J, Cheng YI, et al. PROMIS pediatric measures validated in a longitudinal study design in pediatric oncology. Pediatr Blood Cancer. 2019. [DOI] [PubMed] [Google Scholar]


