ABSTRACT
Background
Alectinib is a second‐generation anaplastic lymphoma kinase (ALK) inhibitor indicated for ALK‐mutated non‐small‐cell lung cancer. Recently, the association between alectinib and red cell morphological abnormalities has been reported in a few case series. This retrospective observational study aims to determine the frequency of occurrence of acanthocytosis in patients taking alectinib and to evaluate the red cell indices, biochemical markers of haemolysis and eosin‐5‐maleimide (EMA) binding assay results in patients receiving alectinib.
Methods
Patients who were on alectinib and had a complete blood count test performed in Queen Elizabeth Hospital Haematology Laboratory between 1 May 2021 and 31 August 2021 were included in the study. Haematological investigations that had been performed before and after the commencement of alectinib were reviewed.
Results
Fifty patients receiving alectinib were evaluated in this analysis. One hundred per cent of patients showed 3+ acanthocytes on the peripheral blood smears. Compared with the test results before starting alectinib, the post‐alectinib blood tests showed a significantly lower haemoglobin concentration, red blood cell count and haematocrit; and a significantly higher mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and red cell distribution width. All the tested patients showed a marked reduction in EMA mean channel fluorescence compared with normal control.
Conclusion
Our cohort revealed that alectinib caused significant acanthocytosis in all patients. Alectinib was also associated with changes in red cell indices and biochemical markers of haemolysis, compatible with a spherocytic and anisopoikilocytic morphology with haemolysis. Patients on alectinib had reduced EMA binding.
Keywords: acanthocytosis, alectinib, alectinib‐induced haemolysis, anaemia, drug‐induced haemolysis
This retrospective observational study aims to determine the frequency of occurrence of acanthocytosis in patients taking alectinib and to evaluate the red cell indices, biochemical markers of haemolysis and eosin‐5‐maleimide (EMA) binding assay results in patients receiving alectinib. Our cohort revealed that alectinib caused significant acanthocytosis in all patients. Alectinib was also associated with changes in red cell indicies and biochemical markers of haemolysis, compatible with a spherocytic and anisopoikilocytic morphology with haemolysis. Patients on alectinib had reduced EMA binding.
1. Introduction
Alectinib is a second‐generation anaplastic lymphoma kinase (ALK) inhibitor indicated for ALK‐mutated non‐small‐cell lung carcinoma. Alectinib is known to be associated with anaemia. In the ALEX study, 26% of the patients receiving alectinib showed anaemia, whereas 6% of them showed Grade 3 or worse anaemia [1].
Recently, the association between alectinib and red cell morphological abnormalities has been reported in a few case series or case reports [2, 3, 4, 5, 6, 7]. A recent study also demonstrated that alectinib induces marked red cell spheroacanthocytosis in almost all patients and is associated with reduced eosin‐5‐maleimide (EMA) binding [5].
Anaemia is common in patients with lung cancer, with an incidence of 50%–60% [8]. Morphological assessment of red cells by light microscopy and other haematological laboratory assays are frequently required in the workup in this situation. Therefore, knowledge of the red cell morphological changes and other laboratory parameters associated with alectinib may be beneficial in the diagnostic workup in this common clinical scenario.
This retrospective review aims to determine the frequency of occurrence of acanthocytosis in patients taking alectinib and to evaluate the red cell indices, biochemical markers of haemolysis and EMA binding assay results in patients receiving alectinib. We also aim to compare red cell morphological changes and changes in haematological laboratory parameters associated with alectinib and other ALK inhibitors indicated for the treatment of ALK‐mutated non‐small‐cell lung carcinoma, namely crizotinib and lorlatinib. To our best knowledge, this study consists of the largest cohort of patients on alectinib to date for the evaluation of alectinib‐induced red cell changes.
2. Materials and Methods
2.1. Study Population
Patients who were on alectinib, crizotinib or lorlatinib and had a complete blood count test performed in Queen Elizabeth Hospital Haematology Laboratory between 1 May 2021 and 31 August 2021 were included in the study.
Sixty‐nine patients with ALK‐mutated non‐small‐cell lung cancer were recruited in the study, including 50 patients taking alectinib, 8 taking crizotinib and 11 taking lorlatinib. We labelled the patients taking alectinib as A1–A50, patients taking crizotinib as C1–C8, and patients taking lorlatinib as L1–L11. Among the lorlatinib‐treated patients, six of them had been treated with alectinib previously. None of the crizotinib‐treated patients had been treated with alectinib previously. Basic characteristics of the patients including sex, age and the duration of taking the drug are shown in Table 1.
TABLE 1.
Basic characteristics of the study population.
| Alectinib | Crizotinib | Lorlatinib | |
|---|---|---|---|
| Number of patients | 50 | 8 | 11 |
| Age (years) | 61 (40–85) | 63.5 (50–77) | 51 (38–82) |
| Female sex | 34 (68%) | 6 (75%) | 6 (55%) |
| Duration of taking the drug (months) | 14.0 (0.4–51.6) | 39.0 (9.0–74.5) | 19.0 (2.1–33.4) |
Note: The data are expressed as n, n (%) or median (range) where appropriate.
2.2. Sample Preparation and Analysis
Clinical information was collected via the electronic patient record system of the Hospital Authority. The information included basic demographics (age and sex), diagnosis, time of commencement of the ALK inhibitor.
Results of all diagnostic haematological laboratory investigations, including red cell indices [haemoglobin concertation (Hb), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW), red blood cell count (RBC), haematocrit (HCT) and reticulocyte count] [by Abbott Alinity hq analyzer (Abbott Diagnostics Division, Mountain View, CA, USA)], biochemical markers of haemoalysis [bilirubin, lactate dehydrogenase (LDH), haptoglobin levels], liver function test [alanine transaminase (ALT), alkaline phosphatase (ALP)], EMA binding assay (EMA dye: Thermo Fisher Scientific, Waltham, MA, USA), haemoglobin analysis [cation exchange high performance liquid chromatography on the VARIANT‐II Hemoglobin Testing System (Bio‐Rad Laboratories, Hercules, CA, USA) and i+Lab α‐thal immunochromatographic strip (i+Med Laboratories, Bangkok, Thailand)] and iron profile study that had been performed as a part of routine laboratory workup before and after commencement of ALK inhibitors were retrieved from the laboratory information system of Queen Elizabeth Hospital for review.
Peripheral blood smears that were retrievable from the archive of the Haematology Laboratory of Queen Elizabeth Hospital were analysed by experienced haematopathologists using light microscopy. All the peripheral blood smears were prepared from blood specimens collected in vacuum blood tubes containing Ethylenediamine Tetraacetic Acid Tripotassium Salt (K3EDTA) (Vacuette, Greiner Bio‐One GmbH, Kremsmünster, Austria) and stained with May‐Grunwald‐Giemsa (MGG) staining technique (MGG stain: Hematek Stain Pak, Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Classification and grading of abnormal red cells were reported according to the International Council for Standardization in Haematology (ICSH) recommendations [9]. More specifically, acanthocytes were defined as round, hyperchromic red cells with 2–20 irregularly spaced projections or spicules of variable length, thickness and shape, while some spicules have club‐shaped rather than pointed ends. The grading of 1+, 2+ and 3+ referred to <5%, 5–20% and >20% of acanthocytes respectively.
2.3. Statistical Analysis
Mean, median and range were used as descriptive statistics for continuous variables, while frequencies and percentages were used for categorical variables. Two‐tailed paired sample t‐test was used to compare the pre‐treatment and post‐treatment red cell indices and biochemical markers for the patients receiving alectinib. Two‐tailed independent sample t‐test was used to compare the red cell indices, biochemical markers and EMA mean channel fluorescence for the alectinib‐treated patient with the crizotinib‐ and lorlatinib‐treated patients respectively. All statistical analyses were performed using SPSS version 26.0 (SPSS, Inc., Chicago, IL). The results were considered significant if the p < 0.05.
2.4. Ethics
The study has been reviewed and approved by the Research Ethics Committee (Kowloon Central/Kowloon East).
3. Results
3.1. Red Cell Morphology
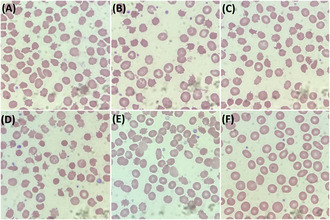
In the cohort of alectinib‐receiving patients, 100% (50/50) of patients showed acanthocytes with a grading of 3+ (Figure 1A–E).
FIGURE 1.
Peripheral blood smears (PBS) of patients taking alectinib. (A) PBS of patient A1, (B) PBS of patient A2, (C) PBS of patient A3, (D) PBS of patient A4, (E) PBS of patient A15 23 days after the start of alectinib use. Images of (A–E) shows 3+ acanthocytosis. (F) PBS of patient A15 before taking alectinib, which did not show significant acanthocytosis.
Peripheral blood smears before the commencement of alectinib were available for one of the patients (patient A15). The pre‐treatment peripheral blood smear did not show significant acanthocytosis (Figure 1F).
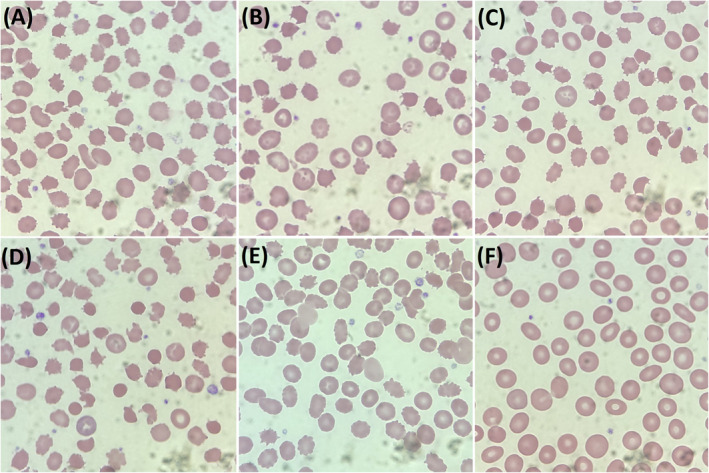
None of the patients taking crizotinib or lorlatinib showed significant acanthocytosis in the peripheral blood smears (Figure 2).
FIGURE 2.
Peripheral blood smear (PBS) of two patients receiving crizotinib and lorlatinib respectively. (A) PBS of patient C1, (B) PBS of patient C2, (C) PBS of patient L1, (D) PBS of patient L2. All of them did not show significant acanthocytosis.
3.2. Red Cell Indices
Among the patients taking alectinib, 16.0% (8/50) of them showed Grade 2 or above anaemia (Hb < 10 g/dL) and only 2% (1/50) of them showed Grade 3 or above anaemia (Hb < 8 g/dL). 65.3% (32/49) of the patients receiving alectinib showed reticulocytosis (>2%).
Complete blood count results before the commencement of alectinib were available in 47 patients of the alectinib‐treated group. Compared with the test results before starting alectinib, the post‐alectinib blood tests showed a significantly lower Hb, RBC and HCT; and a significantly higher MCH, MCHC and RDW. There were no significant differences in MCV between the two time points. A detailed overview of data is shown in Table 2.
TABLE 2.
Comparison of red cell indices of the pre‐alectinib and post‐alectinib samples.
| Post‐alectinib (mean) | Pre‐alectinib (mean) | p value | |
|---|---|---|---|
| Hb (g/dL) | 11.4 | 12.5 | <0.001 |
| MCV (fL) | 85.9 | 85.2 | 0.619 |
| MCH (pg) | 29.3 | 28.2 | <0.001 |
| MCHC (g/dL) | 34.2 | 32.5 | <0.001 |
| RBC (×1012/L) | 3.92 | 4.50 | <0.001 |
| HCT | 0.333 | 0.384 | <0.001 |
| RDW | 14.7 | 13.7 | 0.001 |
Note: p values which are <0.05 are bolded.
Abbreviations: Hb, haemoglobin concertation; HCT, haematocrit; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell count; RDW, red cell distribution width.
Grade 2 or above anaemia were found in none of the patients receiving crizotinib or lorlatinib. 37.5% (3/8) and 9.1% (1/11) of patients on crizotinib and lorlatinib, respectively, showed reticulocytosis.
Alectinib‐treated patients had a significantly lower Hb, RBC, HCT, MCV and MCH; and a higher MCHC, RDW and reticulocyte count than crizotinib‐treated patients. They had a significantly lower Hb, RBC and HCT and a higher MCHC and reticulocyte count than lorlatinib‐treated patients.
Table 3 provides the detailed statistical comparisons.
TABLE 3.
Comparison of red cell indices of the alectinib‐treated patients versus crizotinib‐treated patients or lorlatinib‐treated patients.
| Alectinib (mean) | Crizotinib (mean) | p value | Lorlatinib (mean) | p value | |
|---|---|---|---|---|---|
| Hb (g/dL) | 11.4 | 13.2 | <0.001 | 13.5 | <0.001 |
| MCV (fL) | 86.0 | 92.3 | 0.002 | 87.2 | 0.538 |
| MCH (pg) | 29.4 | 31.3 | 0.005 | 28.7 | 0.339 |
| MCHC (g/dL) | 34.2 | 33.4 | 0.008 | 32.9 | <0.001 |
| RBC (×1012/L) | 3.90 | 4.30 | 0.031 | 4.74 | 0.001 |
| HCT | 0.332 | 0.396 | <0.001 | 0.412 | <0.001 |
| RDW | 14.7 | 13.0 | 0.002 | 13.7 | 0.089 |
| Reticulocyte count | 2.34% | 1.90% | 0.039 | 1.59% | <0.001 |
Note: p values which are <0.05 are bolded.
Abbreviations: Hb, haemoglobin concertation; HCT, haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; RBC, red blood cell count; RDW, red cell distribution width.
3.3. Biochemical Markers of Haemolysis
For patients receiving alectinib, the post‐treatment sample was associated with a significantly higher bilirubin level (mean: 18.6 vs. 7.0 μmol/L, p < 0.001). Fourteen per cent (7/50) of the patients receiving alectinib showed a bilirubin level greater than the reference interval (reference interval: ≤27 μmol/L). Furthermore, alectinib‐treated patients had a significantly higher bilirubin level than patients receiving crizotinib (mean: 18.6 vs. 5.3 μmol/L, p < 0.001) and lorlatinib (mean: 18.6 vs. 5.3 μmol/L, p < 0.001) respectively. Unconjugated bilirubin levels were evaluated in five of the patients (patient A3, A4, A15, A27 and A36). The median unconjugated bilirubin level was 17 μmol/L, and the range was 7–36 μmol/L (reference interval: 3–12 μmol/L). Among the five patients who were tested for unconjugated bilirubin levels, 60% (3/5) of them had elevated unconjugated bilirubin levels.
LDH and haptoglobin results were only available in four (patients A8, A11, A15 and A36) and two (patients A15 and A35) of the alectinib‐treated patients respectively. All samples showed increase in LDH (246–395 IU/L, median = 305 IU/L, reference interval: 110–210 IU/L) and low haptoglobin (both sample were <0.1 g/L) levels. Among these four patients, one of them had Grade 1 anaemia (patient A36, Hb 9.1 g/dL), the other three patients (patients A8, A11 and A15) did not have clinically significant anaemia (Hb 10.1–12.1 g/dL, median = 10.4 g/dL).
3.4. EMA Binding Assay
EMA binding assay was performed in 18, 5 and 6 of the alectinib‐, crizotinib‐ and lorlatinib‐treated patients respectively. All of the patients receiving alectinib had a marked reduction in MCF ratio (0.41–0.68, median = 0.57) (Figure 3A–F). EMA MCF ratio were within reference intervals for all the patients receiving crizotinib (1.03–1.08, median = 1.07) (Figure 3G) and lorlatinib (0.97–1.06, median = 1.00) (Figure 3H). The alectinib group had a significantly lower EMA MCF compared with crizotinib (p < 0.001) and lorlatinib (p < 0.001) group respectively.
FIGURE 3.
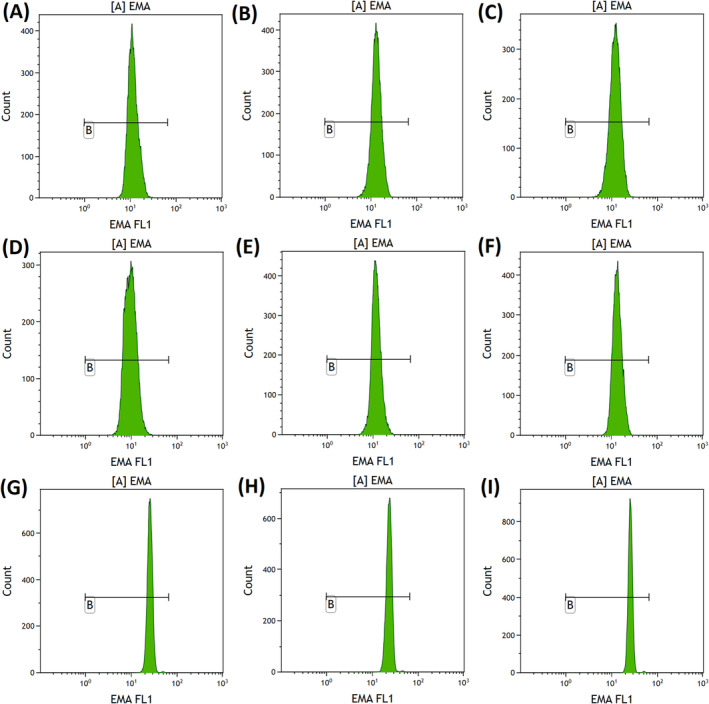
Eosin‐5‐maleimide (EMA) binding study results of the patient receiving alectinib, crizotinib and lorlatinib respectively. (A) EMA binding study results of patient A1, EMA mean channel fluorescence (MCF) = 0.55. (B) EMA binding study results of patient A1, EMA MCF = 0.56. (C) EMA binding study results of patient A3, EMA MCF = 0.51. (D) EMA binding study results of patient A4, EMA MCF = 0.41. (E) EMA binding study results of patient A5, EMA MCF = 0.50. (F) EMA binding study results of patient A6, EMA MCF = 0.58. (G) EMA binding study results of patient C1, EMA MCF = 1.07. (H) EMA binding study results of patient L1, EMA MCF = 0.98. (I) EMA binding study results of a normal control, EMA MCF = 1.05.
3.5. Haemoglobin Analysis and Iron Profile
Haemoglobin analysis and iron profile study were performed in seven (patients A11, A13, A22, A25, A27, A36 and A41) and five (patients A11, A13, A25, A27 and A36) alectinib‐treated patients respectively. All of these patients had a low MCV (<82 fL). Among these patients, four of them were found to have α‐thalassaemia trait and three of them were found to have β‐thalassaemia trait. None of them had low serum iron levels.
3.6. Liver Function Test
ALT and ALP levels were evaluated in all the alectinib‐treated patients.
The median ALT level was 22 IU/L and the range was 7–66 IU/L (reference interval: <47 IU/L). Only 10% (5/50) of the cases had raised ALT levels, and the degrees of elevation of ALT levels were mild in these cases (range: 49–66 IU/L).
The median ALP level of male patients was 124 IU/L and the range was 46–266 IU/L (reference interval: 56–119 IU/L). The median ALP level of female patients was 120 IU/L and the range was 80–332 IU/L (reference interval: 34–97 IU/L). Seventy per cent (35/50) of the cases had elevated ALP, but most of the cases showed only Grade 1 ALP elevation according to the Common Terminology Criteria for Adverse Events (CTCAE) classification [14]. Only one patient showed Grade 2 ALP elevation and none of them showed Grade 3 or above ALP elevation.
4. Discussion
In this study, we examined the frequency of occurrence of acanthocytosis and other red cell morphological changes in peripheral blood smears of patients with ALK‐mutated non‐small‐cell lung carcinoma, treated with alectinib, crizotinib and lorlatinib. We also examined other changes in laboratory tests associated with these ALK inhibitors. To our best knowledge, this study comprises the largest cohort of patients on alectinib to date regarding the evaluation of alectinib‐induced red cell changes.
We found that all the patients taking alectinib had significant acanthocytosis (3+) on peripheral blood smears. This finding was compatible with the results of a previous study by Kuzich et al. [5]. Acanthocytosis is also associated with other clinical conditions, most notably severe liver disease [10]. In order to rule out the effects of liver dysfunction, we evaluated the ALT and ALP levels of the patients receiving alectinib and found that only 10% (5/50) of the cases had raised ALT levels, and the degrees of elevation of ALT levels were mild in these cases. ALP levels were elevated in 70% of the patients but the degrees of elevation were also mild in most of the cases, and only one patient had Grade 2 ALP elevation. These results showed that the patients in the alectinib‐treated group did not have severe liver disease. Other rarer causes of acanthocytosis include post‐splenectomy, abetalipoproteinaemia, McLeod syndrome, chorea‐acanthocytosis syndrome and anorexia nervosa [10]. Clinical histories of the patients have been evaluated by the authors and we conclude that the patients were unlikely to have the aforementioned conditions, although it was not possible to completely rule them out.
Interestingly, despite the high rate of red cell morphological changes, only a small percentage of alectinib‐treated patients showed significant anaemia. Only one of the alectinib‐treated patients developed anaemia that was severe enough to warrant red cell transfusion support. This case was reported by the authors in [6]. This patient had concomitant α‐thalassaemia trait and we hypothesise that concurrent haemoglobinopathy may increase the susceptible to alectinib‐induced haemolysis of the patient due to the pre‐existing impaired red cell survival.
The red cell indices after the commencement of alectinib indicated the development of a more anaemic (lower Hb, RBC and HCT) pattern, with a more spherocytic (higher MCHC) and anisopoikilocytic (higher RDW) morphology compared with pre‐alectinib results.
It is well known that increases in RDW values are associated with various clinical conditions, such as iron deficiency [11], thalassaemia [12], autoimmune diseases [13] and inflammation [13]. In order to evaluate the effects of iron deficiency and thalassaemia on RDW values in our patients, we retrieved the iron profile and haemoglobin analysis results of patients. In the alectinib group, seven patients had low MCV. Among them, four of them were found to have α‐thalassaemia trait and three of them were found to have β‐thalassaemia trait. None of them were found to have iron deficiency. All the remaining patients had a normal or raised MCV, hence, they were unlikely to have iron deficiency or thalassaemia. Other potential causes of elevated RDW values were evaluated by the authors by reviewing the clinical histories of patients. We conclude that they were unlikely to have those conditions based on our clinical judgement, although those conditions could not be completely ruled out.
In the alectinib group, the post‐treatment samples showed a significantly higher bilirubin level compared with pre‐treatment samples. Alectinib‐treated patients also had a significantly higher bilirubin than crizotinib‐ or lorlatinib‐treated patients. A raise in bilirubin level may signify an association between alectinib and chronic haemolysis. However, most of the patients on alectinib had not undergone extensive biochemical workups for haemolysis, this was probably because the majority of the alectinib‐treated patients showed mild anaemia, and only a minority of cases developed clinically significant anaemia with Hb <10 g/dL. As a result, the clinicians who treated the patients did not pursue further workup in most of the cases. Hence the results of LDH and haptoglobin were only available in a small number of the alectinib‐treated patients. These limited data of LDH and haptoglobin levels showed patterns compatible with chronic haemolysis, but further studies with larger sample sizes and more extensive workups of biochemical markers of haemolysis would provide a better insight into the effect of haemolysis by alectinib.
Kuzich et al. also showed that alectinib treatment was associated with reduced EMA binding. We confirmed the findings and demonstrated that all the tested patients had a marked reduction in EMA MCF ratio. The results were well below the diagnostic cut‐off for hereditary spherocytosis, indicating that the drug may affect erythrocyte cytoskeleton.
It should be noted that none of the patients on crizotinib or lorlatinib had significant acanthocytosis on peripheral blood smear or a reduced EMA binding. This implied that the red cell changes caused by alectinib are likely an off‐target effect unrelated to ALK inhibition. Moreover, among the lorlatinib‐treated patients, six of them had been treated with alectinib previously, but had already stopped when the tests for the study were performed. The median duration of cessation of alectinib was 21.4 months. The lack of significant red cell changes in these patients suggested that the reversibility of effects of alectinib on red cells.
This study had limitations that merit mention. First, due to the retrospective nature of the study, we could not acquire the pre‐treatment peripheral blood smears for most of the subjects. In addition, biochemical markers of haemolysis other than bilirubin were not available in most of the patients. Moreover, despite having the largest cohort of patients on alectinib to date for evaluating alectinib induced red cell changes, the sample size of the study was still relatively small. This may limit the statistical power when comparing some of the haematological parameters in the study. However, this problem should be minimal since we have obtained statistically significant results in many parameters when comparing pre‐alectinib and post‐alectinib samples and also comparing alectinib‐treated patients and patients treated with other ALK inhibitors. It should also be noted that the study is single centre in nature, and therefore, we could not compare the effects between sites nor explore the generalisability of effects of alectinib across institutions. Last but not least, the effects of other ALK inhibitors, for example, ceritinib and brigatinib had not been evaluated in this study.
In summary, our observations indicated that alectinib treatment was associated with the development of prominent acanthocytosis, and a marked reduction in EMA binding. This was a ubiquitous phenomenon that occurred in every tested patient. These changes were not found in crizotinib‐ or lorlatinib‐treated patients. Alectinib treatment was also associated with red cell indices and biochemical changes there were compatible with chronic haemolysis. The degrees of haemolysis were usually mild and only a small percentage of patients showed significant anaemia. The red cell changes seen in alectinib‐treated patients were very likely to be reversible.
Knowledge of these laboratory changes associated with alectinib is important for haematologists and oncologists to work up cases of anaemia in patients taking alectinib and helps avoiding misdiagnosis of other red cell disorders. Our findings also have implication in the treatment, the reversibility of red cell changes indicates that it is a reasonable approach to manage patients with significant haemolysis associated with alectinib by switching to alternative ALK inhibitors, such as lorlatinib.
Author Contributions
Ting Hon Stanford Li: analysed and interpreted the patient's data, performed morphological examination of the peripheral blood film, produced the images used in the manuscript and wrote the manuscript. Yin Kwan Jeannie Chik: treated the patient and wrote the manuscript. Ka Yan Ng: performed and interpreted the EMA binding assay. Wai Shan Wong: analysed and interpreted the patient's data, performed morphological examination of the peripheral blood film.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee (Kowloon Central/Kowloon East) (Ref.: KC/KE‐22‐0007/ER‐2) on 22 March 2022.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Mok T., Camidge D. R., Gadgeel S. M., et al., “Updated Overall Survival and Final Progression‐Free Survival Data for Patients With Treatment‐Naive Advanced ALK‐Positive Non‐Small‐Cell Lung Cancer in the ALEX Study,” Annals of Oncology 31 (2020): 1056–1064. [DOI] [PubMed] [Google Scholar]
- 2. Yuan Y., Mapp S., and Xu W., “Two Cases of Marked Red Cell Anisopoikilocytosis and Haemolysis With Alectinib, an Anaplastic Lymphoma Kinase Inhibitor,” British Journal of Haematology 190 (2020): 642. [DOI] [PubMed] [Google Scholar]
- 3. Ashby M. and Low M. S. Y., “Spheroacanthocytes Secondary to Novel Tyrosine Kinase Inhibitors,” eJHaem 1 (2020): 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gullapalli V., Xu W., Lewis C. R., Anazodo A., and Gerber G. K., “A Multi‐Centre Case Series of Alectinib‐Related Erythrocyte Membrane Changes and Associated Haemolysis,” Journal of Hematopathology 14 (2021): 131–136. [Google Scholar]
- 5. Kuzich J. A., Heynemann S., Geoghegan N., et al., “Alectinib Induces Marked Red Cell Spheroacanthocytosis in a Near‐Ubiquitous Fashion and Is Associated With Reduced Eosin‐5‐Maleimide Binding,” Pathology 53 (2021): 608–612. [DOI] [PubMed] [Google Scholar]
- 6. Li T. H. S., Chik Y. K. J., and Wong W. S., “Alectinib‐Induced Red Cell Morphological Changes in a Patient With Underlying α‐Thalassaemia Trait,” International Journal of Laboratory Hematology 44, no. 1 (2022): 65–66. [DOI] [PubMed] [Google Scholar]
- 7. Kunz J., Wiedemann C., Grosch H., et al., “Early Development of Ubiquitous Acanthocytosis and Extravascular Hemolysis in Lung Cancer Patients Receiving Alectinib,” Cancers (Basel) 14, no. 11 (2022): 2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langer C. J., Choy H., Glaspy J. A., and Colowick A., “Standards of Care for Anemia Management in Oncology: Focus on Lung Carcinoma,” Cancer 95 (2002): 613–623. [DOI] [PubMed] [Google Scholar]
- 9. Palmer L., Briggs C., McFadden S., et al., “ICSH Recommendations for the Standardization of Nomenclature and Grading of Peripheral Blood Cell Morphological Features,” International Journal of Laboratory Hematology 37 (2015): 287–303. [DOI] [PubMed] [Google Scholar]
- 10. Shah P. R., Grewal U. S., and Hamad H., Acanthocytosis (Treasure Island (FL): StatPearls, 2023), https://www.ncbi.nlm.nih.gov/books/NBK549788/. [Google Scholar]
- 11. Sultana G. S., Haque S. A., Sultana T., and Ahmed A. N., “Value of Red Cell Distribution Width (RDW) and RBC Indices in the Detection of Iron Deficiency Anemia,” Mymensingh Medical Journal 22, no. 2 (2013): 370–376. [PubMed] [Google Scholar]
- 12. Baqar M. S., Khurshid M., and Molla A., “Does Red Blood Cell Distribution Width (RDW) Improve Evaluation of Microcytic Anaemias?” Journal of the Pakistan Medical Association 43, no. 8 (1993): 149–151. [PubMed] [Google Scholar]
- 13. Mohamed O. S. D., Azmy G. J., and Elfadl E. M. A., “Clinical Significance of Red Blood Cell Distribution Width in Systemic Lupus Erythematosus Patients,” Egyptian Rheumatology and Rehabilitation 47 (2020): 38. [Google Scholar]
- 14. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES , Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 2017. National Institutes of Health, accessed 14 June 2024, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


