Abstract
Citrus fruits are widely consumed for their nutritional and health benefits. They belong to the Rutaceae and have many varieties, such as sweet orange (Citrus sinensis), which is the most popular. Citrus fruits are rich in water (>80%), dietary fiber, and vitamins. They also contain bioactive components, which may modulate energy metabolism and lipid oxidation through various mechanisms. These mechanisms include stimulating β3-adrenergic receptors, increasing mitochondrial biogenesis and thermogenesis, activating AMP kinase and peroxisome proliferator-activated receptor-gamma coactivator-1α pathways, inhibiting lipogenesis and lipid accumulation, and inducing browning of white adipose tissue. This review summarizes the mechanisms and outcomes of citrus fruits and their metabolites on energy metabolism and body weight in different experimental models. The literature was searched for in vitro and in vivo animal and human studies that investigated the effects of citrus consumption on energy expenditure, thermogenesis, adipogenesis, and lipid accumulation. Citrus fruits and their metabolites have shown promising effects on energy metabolism and lipid oxidation in in vitro and in vivo animal studies. However, the evidence from human studies is limited and inconsistent. Possible reasons for the discrepancy are briefly discussed, and knowledge gaps and research needs are identified for future studies. Citrus fruits may have beneficial effects on energy metabolism and body weight, but more rigorous and well-designed human trials are needed to confirm their efficacy and safety.
Keywords: adipogenesis, citrus, energy metabolism, flavonoids, lipid metabolism, thermogenesis
INTRODUCTION
Citrus fruits, which are produced in almost all countries of the world, have an important place in the human diet in their fresh or processed forms. Although China, Brazil, India, Mexico, and the United States share the lead in the amount of citrus production in 2020, according to the report of the Food and Agriculture Organization, citrus is produced in >140 countries.1 Sweet orange (Citrus sinensis) constitutes more than half of citrus production. Citrus fruits are commonly consumed fresh, with about one-third used after processing.2 Orange juices constitute 85% of processed products.3 Classification of citrus fruits belonging to the Rutaceae is quite complex, and recent studies have adopted 4 basic classifications: C. maxima, C. medica, C. reticulata, and C. micrantha). However, there are still contradictions about the origin of certain varieties.4,5 The botanical names of edible citrus and their commonly used names are given in Table 1.5
Table 1.
Common and botanical names of citrus 5
| Botanical name | Common name |
|---|---|
| Citrus sinensis | Sweet orange |
| C. reticulata | Mandarin (tangerine) |
| C. grandis/maxima | Pummelo (pamplemousse) |
| C. medica | Citron |
| C. mitis | Calamondin |
| Fortunella margarita | Kumquat |
| C. aurantium | Sour or bitter orange |
| C. paradisi | Grapefruit |
| C. bergamia | Bergamot |
| C. aurantifolia | Lime |
| Poncirus trifoliata | Trifoliate orange |
Citrus fruits with >80% water content are rich in simple sugars and dietary fiber. The energy source of citrus, which has a very low protein and fat content, is carbohydrates.3,6 Citrus fruits are rich in micronutrients, including ascorbic acid, and are also a source of vitamins such as thiamine, pyridoxal phosphate, and folate. Additionally, they contain key minerals like potassium.7 In addition to all these primary metabolites, the secondary metabolites in citrus fruits have attracted attention in recent years.6 Among these are many bioactive components such as flavonoids (eg, naringenin, hesperidin), alkaloids (eg, synephrine), terpenes (eg, limonene), and carotenoids (eg, β-cryptoxanthin).8,9 These bioactive metabolites have antioxidant,10 antidiabetic,11 cardioprotective,12 anticancer, and anti-inflammatory properties.13 Citrus and its metabolites have been discussed in various aspects in terms of health in in vitro,14,15 in silico,16 and in vivo animal17 and human18 studies.
The effect of citrus fruits on body weight is 1 of the issues that has attracted attention in the past 20 years. Phytochemicals, especially, have attracted attention in the scientific world.15,19,20 Citrus flavonoids have been associated with reducing adiposity and regulating obesity-related enzymes such as carnitine palmitoyltransferase-I and stearoyl-coenzyme A desaturase, among others.21,22 In a meta-analysis, consumption of citrus and its extracts were reported to decrease body mass index (BMI), waist and hip circumference, and body weight, and these effects were more pronounced at higher doses.23 Wang et al24 found that orange juice consumption by adults was associated with lower BMI, waist circumference, and body fat percentage. It has been reported that bitter orange (C. aurantium) extract and its primary protoalkaloidal component, p-synephrine, have thermogenic and lipolytic activities, affect energy metabolism, and are effective in weight management.25 Additionally, there is scientific evidence that p-synephrine does not have any side effects in acute or long-term use.26 In this review, we investigated the effectiveness of bioactive components of citrus fruits on energy expenditure, weight management, and metabolic effects as reported in in vitro and in vivo studies.
LITERATURE REVIEW
In this study, PubMed (used to search MEDLINE), Web of Science, Cochrane Central Register of Controlled Trials, SPORTDiscus, and Scopus databases, and the Google Scholar website for gray literature, were searched without time limitation. During the screening, the botanical and common names of citrus given in Table 1,5 citrus metabolites (eg, vitamin C, dietary fiber, naringenin, synephrine) were used. Additionally, the keywords “resting metabolic rate,” “thermogenesis,” “energy metabolism,” “energy expenditure,” and “weight loss” were used to explore the role of citrus in energy metabolism and the outputs of the studies. The primary and secondary metabolites included in the review were determined assuming that citruses could be the main sources in the diet. Table 227–59 summarizes the English-language, full-text articles about the role of citrus and its metabolites in energy metabolism that were included in the study.
Table 2.
Summary of clinical studies with citrus fruits
| Reference | Type of study | Type of citrus | Doses and duration | Participants | Key results |
|---|---|---|---|---|---|
| Kegele et al (2019)27 | RCT | C. sinensis |
|
Male and female adults with abdominal fat >20% of body weight | Muscle mass increased by 0.58% in the 0.5 g group and 7.81% in the 1 g group. Fat mass decreased by 0.64% in the 0.5 g group and by 11.89% in the 1 g group. |
| Li et al (2020)28 | RCT | C. sinensis |
|
n = 16 healthy men and premenopausal women aged 20–45 y with BMI >25 kg/m2 | There was no significant change in HDL, LDL, TG, TC, and BP levels compared with baseline. |
| Cardile et al (2015)29 | RCT | C. sinensis |
|
n = 60 healthy people with a BMI between 25 and 35 kg/m2 | Body weight*, BMI*, WC*, and HC* values decreased in the supplement group. No change was found in the control group. |
| Niv et al (2012)30 | RCT | C. sinensis |
|
n = 48 healthy people (aged 18–60 y) | There was no significant change in body weight, HDL, LDL, TG, TC, and BP values in both groups. |
| Azzini et al (2017)31 | Cross-sectional Study | C. sinensis | Volunteers were given red orange juice 500 mL/d for 12 wk. | n = 20 women with a mean age of 36 ± (standard deviation) 7 y and a mean BMI of 34.4 ± 4.8 kg/m2. | There was no significant change in body weight, HDL, LDL, TG, TC, BP, WC, and HC values at the end of 12 wk. |
| Briskey et al (2022)32 | RCT | C. sinensis |
|
A total of 180 overweight (25 < BMI < 35 kg/m2) healthy men and women aged 20–65 y | There was a significant decrease in body weight*, BMI*, WC*, and HC* in both groups, and this decrease was higher in the extract group. There was no significant change in HDL, LDL, or TC levels. |
| Simpson et al (2012)33 | RCT | C. sinensis |
|
n = 32 overweight women aged 20–45 y with HOMA-IR >1.5 and a BMI of 27–35 kg/m2 | There was no significant change in LDL, HDL, TC, SBP, DBP, apo-A1, and apo-B levels; HOMA-IR; and body weight of the groups at the end of 12 wk. |
| Basile et al (2010)34 | Cross-Sectional Study | C. sinensis | For 8 wk, men were given 750 mL/d orange juice, and women were given 500 mL/d orange juice. | n = 21 healthy women aged 20–53 y and 20 healthy men aged 21–44 y | There was no significant change in orange juice consumption, body weight, and BMI in either sex. Although WC* decreased in women, no change was found in men. TC* and LDL* levels were decreased in both sexes. HDL* increased in women, whereas TG* and DBP* levels decreased significantly in men. |
| Cesar et al (2010)35 | Cohort Study | C. sinensis |
|
n = 53 male and female adults aged 36–44 y | No significant changes were found in the groups in BMI, WC, and HDL levels. TC** and LDL** levels were decreased in the hypercholesterolemic group. TG* level increased in the control group. |
| Asgary et al (2014)36 | RCT | C. sinensis |
|
n = 22 healthy volunteers (7 men and 15 women) aged 18–59 y | No significant change was found in the weight, BMI, WC, apo-B, HDL, TC, and TG levels of both groups. A significant decrease in LDL* level was found in the fresh orange juice group. |
| Pittaluga et al (2013)37 | Cohort Study | C. sinensis |
|
n = 22 healthy individuals who exercised regularly for 6 mo and were fed a Mediterranean diet | There was no significant change in BMI, body fat mass, and heart rate in both groups. |
| Büsing et al (2019)38 | Cohort study-cross-over | C. sinensis |
|
n = 26 healthy adults | There was no significant change in body weight between groups. |
| Hägele et al (2018)39 | Cohort Study-Cross-over | C. sinensis | Orange juice (43 kcal/100 mL and 8.9 g sugar/100 mL) in an amount to meet 20% of the daily energy needs 3 times/d for 2 wk was given to the first group with meals and the second group between meals. After 1 wk of washout, the application was repeated by crossing. | n = 26 healthy adults (13 women, 13 men) aged 20–45 y | There was no significant change in body weight, HOMA-IR, and TG groups and between groups. Consumption of orange juice with meals decreased body fat mass*, and consumption between meals increased body fat mass**. Fat mass** change was different between groups. |
| Stookey et al (2012)40 | RCT- crossover | C. sinensis | On 2 separate days, the groups were crossed and individuals consumed 500 mL of water or orange juice with breakfast. | Aged 11–17 y with a BMI <85th percentile. n = 7 adolescents and 10 adults (19–38 y) with a BMI 18.5–24.9 kg/m2 | At 180 min after breakfast, the plasma insulin to glucose ratio* increased in adolescents and adults compared with those drinking orange juice. 30 min after breakfast, fat oxidation* decreased compared with those drinking water at breakfast with an orange drink in both age groups. But NPREE and NPRER did not change. |
| Sakaki et al (2021)41 | Cohort Study | C. sinensis | The survey data were followed from 2004 to 2008 with an interval of 2 y. | n = 7301 children aged 9–16 y were included in the study. | Orange juice consumption was not associated with BMI and weight. |
| Aptekmann et al (2010)42 | Cohort Study | C. sinensis |
|
n = 30 physically active premenopausal women aged 30–48 y | Weight*, BMI*, SFT* (triceps, thigh), lactate, TC*, and LDL* levels decreased in the experimental group compared with the control group; HDL*, TG*, and SFT* values (biceps) increased. |
| Simpson et al (2016)43 | RCT | C. sinensis |
|
n = 36 healthy men aged 40–60 y with BMI 27–35 kg/m2 | BMI, weight, WC, HC, WHR, HOMA-IR, % body fat, TC, HDL, LDL, TG, apo-A1, and apo-B values did not change significantly between groups. |
| Ribeiro et al (2017)44 | RCT | C. sinensis |
|
n = 78 individuals aged 18–50 y with BMI: 30–40 kg/m2 | Weight, BMI, WC, HC, WHR, body fat, FG, HDL, TG, AST, and ALT values did not change. At wk 8 and 12, insulin* and HOMA-IR* values were decreased in the experimental group compared with the control group. At wk 12, LDL* level decreased. |
| O’Neil et al (2011)45 | Cross-sectional study | C. sinensis |
|
n = 7250 individuals between the ages of 2 and 18 y were included. | The group that consumed orange juice had lower levels of WC* and LDL* compared with the group that did not consume orange juice. Weight, BMI, SBP, DBP, TC, TG, HDL, apo-B, and insulin levels did not differ between groups. |
| Rumbold et al (2015)46 | RCT-crossover | C. sinensis | Participants were given 600 mL of skim milk or 600 mL (475 mL of orange juice and 125 mL of water) of orange juice after 30 min of cycling exercise. | Nine recreationally active women aged between 18 and 21 y and BMI of 19–25 kg/m2 were included. | Vo2peak was not different between groups. Relative energy intake* at the test meal decreased in milk consumption. |
| Fidélix et al (2020)47 | Cohort Study | C. sinensis | Participants followed a regular diet with no orange juice for 30 d, 300 mL of orange juice/d for 60 d, and no orange juice again for 30 d. | n = 10 healthy women aged between 20 and 35 y | BMI, weight, and body fat did not change over the 120-d period. During the drinking period of orange juice, FG*, insulin*, HOMA-IR*, TG*, TC*, and LDL* values decreased. |
| Rangel-Huerta et al (2015)48 | RCT | C. sinensis |
|
n = 100 individuals with a BMI 25–40 kg/m2 or a WC >94 cm in men and >80 cm women | Weight*, BMI,* WC*, and FPS* increased in both groups. SBP*, DBP*, insulin*, TG*, and apo-B* levels were decreased in the second group. Apo-A1* was decreased in the first group. HOMA-IR, TC, HDL, and LDL levels were unchanged. |
| Aptekmann et al (2013)49 | Cross-sectional Study | C. sinensis |
|
n = 129 individuals aged 18–66 y | There was no significant difference in TG, HDL, apo-A1, BMI, weight, body fat, and WC values. Normolipidemic or hypercholesterolemic individuals who consumed orange juice had less TC*, LDL*, and apo-B* compared with those who did not. |
| Dallas et al (2008)50 | RCT |
|
|
n = 20 healthy individuals aged 25–55 y with BMI 27–33 kg/m2 | At the end of wk 4 and 12, the experimental group's BMI*, weight*, and body fat* values had decreased compared with the placebo. |
| Silveira et al (2015)51 | Cross-sectional Study | C. sinensis |
|
n = 35 healthy and nonsmoking individuals aged 18–29 y | In the first group, after 8 wk, TC*, HDL*, LDL*, apo-A1*, FG*, HOMA-IR*, and SBP* decreased; weight, BMI, body fat, WC, apo-B, TG, and DBP values did not change. In the second group, TC, LDL, and DBP values decreased after 8 wk, whereas other values remained unchanged. |
| Penzak et al (2001)52 | Cross-sectional Cross-over | C. aurantium | Participants drank 8 oz (237 mL) of bitter orange juice (phase 1) or water (phase 2) 8 h before arriving at the study unit. After coming to the research unit, their measurements were taken, and 8 oz (237 mL) of bitter orange juice (phase 1) or water (phase 2) was given again. Measurements were repeated hourly for 5 h. | n = 12 healthy individuals aged 20–27 y | The bitter orange juice had no significant effects on SBP, DBP, or arterial pressure values, and heart rate compared with water. |
| Silver et al (2011)53 | RCT | C. paradisi |
|
n = 85 adults aged 21–50 y with BMI of 30–39.9 kg/m2 | BMI*, WC*, and body fat percentage* decreased in all groups at the end of 12 wk. There was no significant difference in REE, RQ, BMI, WC, HDL, TC, TG, SBP, or DBP values within and between groups. |
| Fujioka et al (2006)54 | RCT | C. paradisi |
|
n = 91 obese adult men and women with BMI = 30–40 kg/m2 | The fresh grapefruit group had greater weight loss* than the placebo group. HDL, TG, WC, and BP values did not change significantly. |
| Dow et al (2012)55 | RCT | C. paradisi |
|
n = 85 premenopausal healthy individuals with BMI ≥ 25–45 kg/m2 | WC***, WHR*, SBP*, TC*, and LDL*** values decreased in the experimental group after the intervention. BMI, WC, weight, HC, WHR, SBP, DBP, heart rate, TC, TG, HDL, and LDL did not change between the groups after the intervention. |
| Taghizadeh et al (2016)56 | RCT | C. aurantifolia |
|
n = 72 healthy aged 18–50 y with BMI ≥ 25 kg/m2 | In both experimental groups, weight***, BMI***, and FG*** values decreased compared with the placebo group. In addition, the high-dose group LDL* TC**, and TG* levels decreased compared with the low-dose and placebo groups. HOMA-IR, HOMA-β, and HDL values remained unchanged. |
| Hashemipour et al (2016)57 | RCT | C. aurantifolia |
|
n = 60 healthy individuals aged 10–18 y with BMI > 85th percentile | WC, NC, BMI, DBP, SBP, FG, TC, and LDL values did not change between groups after the intervention. HDL** increased in the placebo group. BMI***, SBP**, TC*, and LDL* levels decreased before the intervention in the experimental group after the intervention. BMI*** was also decreased in the placebo group. |
| Ferro et al (2020)58 | RCT | C. bergamia |
|
n = 102 individuals aged 30–75 y | BMI**, weight,** and controlled attenuation parameter score* decreased compared with the control group. WC, HC, TG, HDL, LDL, TC, FG, AST, and ALT values were unchanged. In subgroup analyses, men*, those older than 50 y**, those with android obesity*, and those with obese/overweight** had decreased weight in the experimental group compared with the control. |
| Lin et al (2022)59 | RCT | C. reticulata |
|
n = 20 healthy individuals (aged 18–70 y) with BMI ≥ 24 kg/m2 or body fat > 30% | At the end of 4, 6, and 8 wk, body weight* decreased in the experimental group. In addition, WC* decreased at the end of the 6th wk and AST* level decreased at the end of the 8th wk. Body fat, DBP, ALT, TC, TG, FG, and BMI levels did not change. |
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001.
Abbreviations: ALT, alanine transaminase; apo-A1, apolipoprotein A1; apo-B, apolipoprotein B; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; FG, fasting glucose; HC, hip circumference; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β-cell dysfunction; hs-CRP, high-sensitive C-reactive protein; LDL, low-density lipoprotein; NC, neck circumference; NHANES, National Health and Nutrition Examination Survey; NPREE, nonprotein resting energy expenditure; NPRER, nonprotein respiratory exchange ratio; RCT, randomized controlled trial; REE, resting energy expenditure; RQ, respiratory quotient; SBP, systolic blood pressure; SFT, skinfold thickness; TC, total cholesterol; TG, triglyceride; Vo2peak, peak oxygen uptake; WC, waist circumference; WHR, waist to hip ratio.
THE ROLE OF CITRUS METABOLITES IN ENERGY METABOLISM
Dietary fiber (citrus peel)
Citrus peel, which contains flavonoids, essential oils, and vitamins, is also a rich source of dietary fiber.60 According to their solubility, dietary fibers are divided into digestible dietary fiber (pectin) and nondigestible dietary fibers (cellulose, hemicellulose, and lignin).61 Although digestible dietary fibers have beneficial effects on blood sugar and cholesterol, nondigestible dietary fibers are essential for colon health.62 Citrus peels contain >60% dietary fiber, the majority of which is nondigestible dietary fiber.63 Although the history of the use of citrus peels dates back to the 10th century, the explanation of the activities of the components in the peel dates back to more recent times.64 It has been reported that boosting dietary fiber intake elevates the production of short-chain fatty acids in the intestine, supporting energy expenditure and aiding weight loss.65 In the study conducted by Chambers et al,66 the intake of propionate, the end product of dietary fiber fermentation, increased resting energy expenditure in 18 people aged 18–65 years. In another study carried out with rats fed a high-fat diet (HFD), extracts of orange, lemon, grapefruit, and tangerine peels were administered separately and in equal amounts in a combination to examine the effect on weight loss. Decreased appetite was reported in all groups, due to the satiety in the stomach caused by the pectin found in the citrus peels. Although the most beneficial effect in weight loss was observed in the group given the combination of all extracts, this effect was reported to be because of the stimulation of β-3 cell receptors and increased thermogenesis.67 C. sunki peel extract68 and C. ichangensis peel extract69 were slowed weight gain through β-oxidation and lipolysis in mice fed a HFD.
Citrus flavonoids
Among the citrus flavonoids,70 some of which are responsible for the bitter taste of citrus, the most important group is flavanones (namely, hesperidin, hesperetin, naringenin, naringin, narirutin, eriocitrin, neohesperidin, dydimin, poncirin, and neoeriocitrin), flavones (tangeretin and nobiletin), and flavonols (quercetin, kaempferol).71,72 Additionally, citrus peel polymethoxyflavones (PMFs) are considered an important source of flavanone.73 However, the amount and subgroups of these flavonoids vary according to both citrus type and citrus tissue.72,74 Moreover, the type of fruit juice–processing technology and processing processes can also significantly reduce the flavonoid content.75,76 For this reason, the results of in vivo studies using citrus should be evaluated considering this difference in terms of citrus fruit,77 fruit juice,78 fruit peel,79 vitamins, and flavonoids.
Hesperidin
Citrus flavonoids can mediate energy expenditure in a variety of ways (Figure 1). Nishikawa et al80 showed that α-monoglucosyl hesperidin, the synthetic form of hesperidin with higher bioavailability and solubility, induced brown-fat adipocyte formation in mice and increased thermogenesis via uncoupled protein 1 (ucp-1) in white adipose tissue (WAT). Similarly, Shen et al81 reported that oral administration of 4G-α-glucopyranosyl hesperidin, a form of hesperidin with a greater absorption and solubility level, strengthened interscapular brown adipose tissue (BAT) sympathetic neuronal activity in rats, and brown fat caused by increased body temperature suggested increased thermogenesis in the tissue. Ohara et al,82 on the other hand, found that the treatment of glycosyl hesperidin and caffeine together in mice increased the expression of adipose tissue mass and liver lipogenic gene messenger RNAs (mRNAs), but the treatment alone did not show a significant effect. There is a limited number of human studies on this subject. According to the results of a randomized, placebo-controlled study of 40 amateur cyclists who were given 2S-hesperidin supplements for 8 weeks, a decrease in total fat mass and an increase in muscle mass were reported in the hesperidin group.19 In another randomized, placebo-controlled study, conducted by Yoshitomi et al83 with 60 healthy Japanese individuals aged 30–75 years who were given combined green tea and α-glucosyl hesperidin, body weight and visceral fat area were decreased in the entire intervention group. Individuals younger than 50 years in subgroup analyses had significant reductions in body weight, body fat percentage, visceral fat, and total abdominal fat areas.83
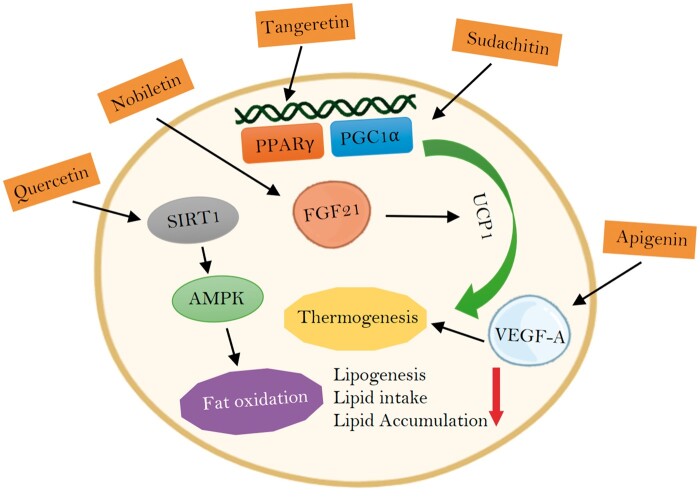
Figure 1.
Metabolic effects exhibited by various pathways of citrus fruit flavonoids. AMPK, AMP-activated protein kinase; FGF-21, fibroblast growth factor 21; PGC, peroxisome proliferator-activated receptor-gamma coactivator-1α; PPARγ, peroxisome proliferator-activated receptor-γ; SIRT1, sirtuin 1; UCP1, uncoupling protein 1; VEGF-A, vascular endothelial growth factor A.
Naringenin
Naringenin, another citrus flavanone, increased thermogenesis through ucp1, and pgc-1α upregulation, and thus lipid accumulation in BAT, in a murine study conducted by Bae et al.84 Zhang et al,85 on the other hand, showed that treatment of naringenin in mice fed an HFD was effective in preventing HFD-induced obesity and in increasing energy expenditure by activating thermogenesis and beiging through gut microbe–short-chain fatty acid–host interactions. In other animal studies, it has been shown that naringenin supplementation reduces intra-abdominal and subcutaneous adiposity,86 increases the expression of fat oxidation genes and β-hydroxybutyrate concentration in the liver,87 and contributes to energy expenditure by increasing mitochondrial biogenesis.88 Rebello et al89 showed that naringenin treatment of human white adipocyte cultures and abdominal subcutaneous adipose tissue promoted beiging by increasing the expression of key enzymes related to thermogenesis and fat oxidation. In vivo human studies involving naringenin supplementation appear promising in reducing weight, BMI, waist circumference, and visceral fat levels; however, more studies are needed.90–92
Eriocitrin
Eriocitrin flavanone, which is abundant in lemons, increased the mRNA expression of genes associated with thermogenesis and energy expenditure in mice to which it was administrated for 16 weeks and decreased lipogenesis and lipid uptake–related gene expression in WAT.93 The results of a randomized controlled trial using nutraceutical supplementation of this flavanone, which has been the subject of limited studies, show that eriocitrin is not effective in terms of weight, waist circumference, and BMI change.94
Tangeretin and nobiletin
Supplementation of tangeretin, a citrus flavone, to the diet of HFD-fed mice improved obesity by increasing thermogenic gene expression and reducing intestinal dysbiosis.95 In the in vitro study of Kihara-Negishi et al,96 another flavone, nobiletin, increased UCP-1 expression in HB2 brown adipocyte cell lines and mRNA expression of brown adipokines neuregulin-4 and fibroblast growth factor 21, and also has been suggested to activate BAT through β-adrenergic stimulation by increasing the secretion of fibroblast growth factor-21. Another in vitro study demonstrated the antiadipogenic effect of nobiletin in 3T3-L1 cells (a preadipocyte cell line) by modulating the peroxisome proliferator–activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathway.97 In contrast, an animal study suggested that the metabolic effects of nobiletin are independent of AMPK activation.98 Kou et al99 showed that nobiletin supplementation activates thermogenesis in BAT and WAT through its positive effects on gut microbiota in mice fed an HFD.
Apigenin
Another flavone found in citrus is apigenin.72 Apigenin can protect against inflammation and support beiging by activating the COX2/PGE2 axis in human adipocytes.100 In 3T3-L1 cells, C/EBPβ (a liver-enriched activating protein), a transcription factor involved in adipocyte differentiation) upregulates the expression of C/EBPβ inhibitors.101,102 Another study showed that WAT browning occurs by triggering the expression of vascular endothelial growth factor A.103 Sun and Qu,104 on the other hand, suggested that 12-week apigenin treatment in mice can reduce body weight by increasing energy expenditure without a change in energy intake. The number of studies on the effect of luteolin on energy expenditure is limited; it is thought to contribute by increasing browning and thermogenesis via AMPK/PGC1α.105
Quercetin, rutin, and kaempferol
There are in vivo human and animal studies in which the effect of quercetin, a citrus flavonol, on energy metabolism was examined. Some studies show that quercetin contributes to browning by increasing the expression of thermogenesis genes without increasing energy expenditure,106–108 whereas others show that it promotes lipophagy and prevents adiposity through the activation of AMPK signaling.109,110 Clinical studies have shown no significant effect of quercetin supplementation on REE,78 basal metabolic rate,79 total energy expenditure,79 body weight,111–113 body fat mass,111,112 lean body mass,111,112 and WHR.111,113 The glycosyl derivatives of quercetin, rutin, and isoquercetin are also found in citrus. Rutin can increase energy expenditure by increasing the expression of ucp-1 and other thermogenic genes20,114 and upregulating the AMPK pathway.115 In addition, in a clinical study using whey protein powder with added isoquercitrin, no significant effect was observed on total body mass, lean mass, and fat mass.116 Kaempferol, another flavonol, upregulated the cAMP-responsive gene for type 2 iodothyronine deiodinase by increasing the intracellular energy expenditure.117
Other flavonoids
Another component that contains more than 1 methoxy group on the flavone skeleton, which is abundant in citrus peels, are PMFs.118 Although there are more than 80 types of PMFs, which are specific to citrus fruits, the main ones found in citrus peels are tangeretin, nobiletin, sudachitin, and sinensetin.119 Administration of PMF-containing extracts obtained from citrus peels to mice fed an HFD reduced weight gain.79,120–124 Dietary supplements containing PMFs attenuate adipogenic differentiation by downregulating adipogenic factors such as PPARγ and upregulating AMPK in 3T3 L1 cells,125 whereas citrus peel extracts reduced mouse body weight and adipose tissue weight through microbiota or AMPK activation.120–122 Sudachitin extract, thought to result from sudachitin polyphenol found in the peel of C. sudachi, increases energy expenditure by increasing SIRT1, PGC1-α, and UCP-1 gene expression in skeletal muscle.126 Extracts containing the PMFs also reduced weight gain.127–130 To prevent obesity, PMFs play an important role in preventing hypertrophy by inhibiting lipid accumulation and apoptosis in 3T3-L1 cells.131,132 More in vivo and in vitro studies, especially clinical studies, are needed on the effect of citrus flavonoids on energy metabolism.
Citrus carotenoids and terpenes
Carotenoids are fat-soluble pigments responsible for the red, orange, and yellow coloration of citrus. Carotenoids, which are highly sensitive to enzymatic, chemical, and oxidative reactions, may cause different responses even if the dietary intake of individuals is the same. Therefore, when investigating their therapeutic effects, the biology, activity, and metabolism of carotenoids should be well investigated.133,134 As with flavonoids, carotenoids can be found in citrus in varying amounts depending on environmental conditions or species. β,β-Xanthophylls (primarily β-cryptoxanthin, zeaxanthin/lutein, and violaxanthin) constitute 90% of the carotenoid content of citrus, which contains approximately 115 different carotenoids.135 Terpenes, on the other hand, are the main components of essential oils, among the main ones in citrus are limonene, α-terpineol, β-pinene, carene, myrcene, and linalool, that give unique taste and odor to plants.136,137
β-Cryptoxanthin, occurs mainly in tangerine and sweet orange, and has been reported to have a higher effect on fat reduction and protection against oxidative stress at low doses compared with lycopene and β-carotene.138 Additionally, studies have reported that dietary consumption of tangerine increases serum β-cryptoxanthin levels in humans.139,140 Treatment of HFD-fed mice with β-cryptoxanthin, which is stored in adipose tissue after absorption, increased ucp-1 expression via the retinoic acid receptor pathway,141 increasing energy expenditure and reducing lipid accumulation. Xie et al142 also showed that zeaxanthin, another carotenoid, activates the β3-adrenergic receptor in HFD-fed mice, increasing the expression of prdm16, pgc-1α, and ucp-1; and WAT thermogenesis. The results of the meta-analysis conducted by Yao et al,143 including studies evaluating serum levels of β-cryptoxanthin and lutein/zeaxanthin,144–146 found a significant relationship between carotenoids and reduction of body weight, BMI, and waist circumference in overweight and obese individuals.
Limonene, 1 of the citrus terpenes, increased the expression of PRDM16 and UCP-1, and also modulated C/EBPβ in 3 T-L1 cells in the in vitro study of Lone and Yun.147 It was thought that it could induce beiging, as well.147
There are not enough in vitro and in vivo studies investigating the mechanism of action of citrus carotenoids and terpenes on energy expenditure. More studies are needed to elucidate this area.
Citrus alkaloids
Alkaloids of citrus metabolites are octopamine, synephrine (p-synephrine, m-synephrine, o-synephrine, and methylsynephrine), tyramine, N-methyltyramine, and hordenine.148 p-Synephrine, which is found as a primary alkaloid in many citrus fruits, has a key role in increasing energy expenditure and controlling body weight.6 p-Synephrine, the most common source of which is C. aurantium (bitter orange),149 is a phenylethylamine derivative with a hydroxyl group on the benzene ring.26 C. aurantium extract is standardized to contain 6%–10% p-synephrine.150 Ephedrine, popular for body weight loss because of its effect causing increased thermogenesis and decreased appetite, was banned for causing hypertension, heart disease, and stroke.151 Since then, the use of synephrine, which is like ephedrine in its structure and mechanism of action, has attracted attention.152
The amount of synephrine is higher in unripe citrus fruits and decreases as they mature. The amount of synephrine in C. deliciosa was found to be higher than in other citrus fruits.6,24 The French Agency for Food, Environmental and Occupational Health and Safety reported that the level of synephrine intake from food supplements should be <20 mg/d and should not be taken in combination with caffeine.119
p-Synephrine affects the resting metabolic rate by binding and stimulating β-3 adrenoceptors.153 It has been reported that activation of β-3 adrenoreceptors increases lipolysis in adipose tissue and decreases weight gain.154 p-Synephrine also reduces aP2 expression and lipid accumulation in 3T3-L1 cells.155 It has been reported that β-synephrine causes significant increases in resting metabolic rate in humans, and its use for up to 12 weeks can result in moderate weight loss.156 Stohs et al25 examined the effect of the consumption of p-synephrine alone or in combination with some flavonoids on the resting metabolic rate, and they reported that 50 mg of p-synephrine supplementation alone increased the resting metabolic rate by 6.9% compared with placebo. Jung et al157 reported that 20 mg of p-synephrine supplementation before training by 25 participants increased the respiratory quotient significantly compared with the placebo.
Gutiérrez‐Hellín and Del Coso158 showed that 3 mg/kg p-synephrine did not affect resting energy expenditure and fat oxidation compared with placebo. However, it can increase fat oxidation without affecting energy expenditure during exercise.159–161 Ratamess et al162 examined the effects of β-synephrine supplementation alone and in combination with caffeine on resistance exercise performance in 12 healthy men. The use of 100 mg of p-synephrine in the study provided significant increases in the number of repetitions compared with the placebo and control groups. Gougeon et al163 investigated whether alkaloids increase the thermic effect of food. They reported that the thermic effect was 20% lower in women than men after eating only, and the intake of capsule alkaloids (namely, synephrine, hordenine, octopamine, tyramine, and N-methyltyramine) with a meal increased the thermic effect by 29% only in women. Capsule use significantly increased the respiratory quotient in both sexes, whereas no change was observed in blood pressure. Hoffman et al164 found that a capsule containing 20 mg of methylsynephrine and methylhordenine together with caffeine tetradecylthioacetic acid, yerba mate extract, and methylphenylethylamine significantly increased the average 3-hour energy expenditure. Kaats et al165 tested the safety of synephrine with 67 individuals who consumed 50 mg of p-synephrine alone or together with citrus flavonoids (naringin and hesperidin) for 60 days. At the end of the study, there was no significant change in the systolic or diastolic blood pressures and blood findings of the groups.
EFFECTS OF CITRUS FRUIT CONSUMPTION ON CLINICAL OUTCOMES
In vitro and in vivo animal studies mentioned in the previous section discussed in detail that citrus bioactive components have an effect on energy expenditure through various pathways such as thermogenesis, beiging, or fat oxidation (Figure 1). Although these studies generally reported positive effects, direct consumption of a citrus component and consumption as a fruit juice or citrus fruit extract may not have the same effects on the organism, especially considering the variation of flavonoid amounts in the citrus type and processing duration.74–76 Therefore, it would not be correct to generalize the positive effects of a citrus component consumed as citrus fruit or fruit juice, in terms of preclinical studies to clinical studies. On the other hand, it is reasonable to base positive effects seen in clinical studies on theories from preclinical studies. In this section, we discuss the effects of consuming citrus fruits as fresh, juice, or extract in clinical studies on anthropometric and biochemical outcomes in humans.
It is widely accepted that if environmental factors such as dietary energy intake and physical activity change, the composition of the human body will change. Negative energy balance is associated with a significant reduction in body weight and visceral adipose tissue.166,167 The studies included in this review reported the effects of citrus consumption on anthropometric measures such as body weight, BMI, body fat, and waist, hip, and neck circumference. Of the 28 studies evaluating body weight and BMI, only 8 reported that citrus was effective in reducing body weight and BMI. Body fat mass was decreased in 4 of 12 studies, and body fat mass and body weight changes were correlated. Circumference measurements (waist, hip, neck) did not change significantly in most studies. Studies with positive results did not show a weighted distribution to any citrus species. However, the remarkable point is that the extract form is reported to be more effective than consuming citrus as fresh or fruit juice. Studies conducted using any of these consumption forms by humans and the observed effects are summarized in Table 2.27–59
Citruses can inhibit lipid accumulation by activating AMPK signaling and the PPARγ/AMPK pathway97 or increasing thermogenesis through ucp-1 expression.141 BAT has an active role in energy expenditure through thermogenesis.168 Increasing the activity of BAT can boost energy expenditure169 and may also lower blood lipid levels.170 A dysfunction in the AMPK pathway is linked to fat accumulation and high blood lipid levels.171 Based on these findings, the effect of citrus fruits on thermogenesis and their impact on triglycerides (TGs), total cholesterol (TC), and low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels, which are biochemical markers of lipid metabolism, is examined.
Consumption of 750 mL/d orange juice for 8 weeks significantly reduced TC,35 LDL,35 and HDL51 levels in adults. Consumption of 500 mL/d orange juice for the same duration in women also significantly reduced TC and LDL levels.34 Additionally, it significantly increased the HDL level in women and significantly decreased the TG levels in men.34 Consumption of 500 mL of orange juice for 12 weeks significantly decreased TG levels.48 Combining a low-energy diet with the same amount of orange juice consumption for the same duration significantly decreased the LDL44 level, whereas combining exercise decreased TC and LDL levels, and increased HDL and TG42 levels. Reducing the amount of orange juice to 300 mL/d for 60 days significantly decreased47 TG, TC, and LDL levels. As a result of a dose of 250 mL/d orange juice, LDL, HDL, and TC levels did not change.33,43 As seen in the studies, although orange juice consumption decreases TG, TC, and LDL levels and increases HDL levels in 2–3 months, these results are not consistent. This inconsistency may be due to the amount of orange juice consumption, duration, flavonoid content, and health status of the participants. When looking at the effects of different types of citruses, citrus lime consumption for 8 weeks significantly reduced LDL, TC, and TG levels.56 Consumption of half a grapefruit55 and lemon peel powder57 significantly reduced TC and LDL levels. Citruses also show activity in different biochemical findings. Orange juice consumption significantly decreased fasting glucose levels,51,56 homeostatic model assessment of insulin resistance values,47,51 and insulin47 and high-sensitivity C-reactive protein172 levels.
CONCLUSIONS
Studies including citruses have explained the effect on energy metabolism and lipid oxidation of citrus components: dietary fiber, flavonoids, alkaloids, terpenes, and carotenoids. Citruses increase energy expenditure by increasing thermogenesis through stimulation of β-3 cell receptors and increasing mitochondrial biogenesis. In addition, BAT browning and increased thermogenesis are observed through AMPK/PGC1α or triggering vascular endothelial growth factor A expression. Similarly, energy expenditure is increased by increasing pgc1-α, sırt1, and ucp-1 gene expression and activating the β3-adrenergic receptor. In in vitro studies, citrus fruits had antiadipogenic effects by activating the AMPK signal and the PPARγ/AMPK pathway and inhibiting lipid accumulation in 3T3-L1 cells. Additionally, lipid accumulation is inhibited by the expression of UCP-1. Citrus increases mRNA expression of genes associated with energy expenditure and decreasing lipogenesis-related gene expression in WAT. It also upregulates the cAMP-responsive gene for type 2 iodothyronine deiodinase, increasing intracellular energy expenditure.
Even though in vivo animal studies and in vitro studies have reported the positive effects of citrus components, it is not possible to clearly say that citrus consumption has the same effect in humans when looking at clinical studies. Studies reporting the significant effects of citrus fruits on anthropometric and biochemical findings are limited. In addition, although the mechanisms by which citrus fruits increase energy expenditure and result in weight loss have been shown, there are limited clinical studies evaluating energy expenditure. This indicates the dose–time interaction that was reported to have efficacy in vivo animal studies would not have the same effect as adding citrus fruits to the human diet. This does not mean that citruses are not beneficial for human health and metabolism, but it does indicate that it is premature to recommend adding them to the diet in terms of promising weight loss.
This comprehensive review summarizes how citrus metabolites affect energy metabolism in different in vitro and in vivo animal studies and the results of different citrus varieties in human studies. More studies are needed on the effect of citrus on energy expenditure.
Acknowledgments
The authors thank the Gazi University proof team for checking the academic writing of the article.
Author contributions. M.N.A. and B.S.-K. contributed equally to this study and contributed to the conception, design, investigation, data curation, visualization, writing, preparation of tables and figures, review, and editing. N.A.T. contributed to the conception, design, critical revision, project administration, and supervision of the study. All authors have read and approved the final version of the manuscript.
Funding. No external funding was received to support this work.
Declaration of interest. The authors have no relevant interests to declare.
Contributor Information
Merve Nur Aslan, Faculty of Health Sciences, Department of Nutrition and Dietetics, Bolu Abant Izzet Baysal University, Bolu, Turkey; Department of Nutrition and Dietetics, Institute of Health Sciences, Gazi University, Ankara, Turkey.
Betül Sukan-Karaçağıl, Department of Nutrition and Dietetics, Institute of Health Sciences, Gazi University, Ankara, Turkey.
Nilüfer Acar-Tek, Faculty of Health Sciences, Department of Nutrition and Dietetics, Gazi University, Ankara, Turkey.
References
- 1. Food and Agriculture Organization of the United Nations. FAOSTAT. Available at: https://www.fao.org/faostat/en/#data/QCL. Accessed November 17, 2022.
- 2. Food and Agriculture Organization of the United Nations. Markets and Trade. Available at: https://www.fao.org/markets-and-trade/commodities/citrus/en/. Accessed November 17, 2022.
- 3. Inglese P, Sortino G, Citrus history, taxonomy, breeding, and fruit quality. In: Hazlett RW, ed. Oxford Research Encyclopedia of Environmental Science. Pennsylvania: Oxford University Press; 2019:1-22.
- 4. Curk F, Ollitrault F, Garcia-Lor A, et al. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann Bot. 2016;117:565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luro F, Curk F, Froelicher Y, et al. Recent insights on Citrus diversity and phylogeny. In: Zech-Matterne V, Fiorentino G, eds. AGRUMED: Archaeology and History of Citrus Fruit in the Mediterranean: Acclimatization, Diversifications, Uses. Publications du Centre Jean Bérard; 2017:16–28. [Google Scholar]
- 6. Lu X, Zhao C, Shi H, et al. Nutrients and bioactives in citrus fruits: different citrus varieties, fruit parts, and growth stages. Crit Rev Food Sci Nutr. 2023;63:2018–2041. [DOI] [PubMed] [Google Scholar]
- 7. US Department of Agriculture. FoodData Central [database]. Available at: https://fdc.nal.usda.gov/fdc-app.html#/?component=1162. Accessed November 20, 2022.
- 8. Chhikara N, Kour R, Jaglan S, et al. Citrus medica: nutritional, phytochemical composition and health benefits–a review. Food Funct. 2018;9:1978–1992. [DOI] [PubMed] [Google Scholar]
- 9. Shakour ZTA, Fayek NM, Farag MA. How do biocatalysis and biotransformation affect Citrus dietary flavonoids chemistry and bioactivity? A review. Crit Rev Biotechnol. 2020;40:689–714. [DOI] [PubMed] [Google Scholar]
- 10. Zou Z, Xi W, Hu Y, et al. Antioxidant activity of Citrus fruits. Food Chem. 2016;196:885–896. [DOI] [PubMed] [Google Scholar]
- 11. Gandhi GR, Vasconcelos ABS, Wu D-T, et al. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: a systematic review of in vitro and in vivo studies. Nutrients. 2020;12:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahmoud AM, Hernandez Bautista RJ, Sandhu MA, et al. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:5484138–5484119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manthey JA, Guthrie N, Grohmann K. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001;8:135–153. [DOI] [PubMed] [Google Scholar]
- 14. Jagetia GC, Reddy TK. Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin in vitro. Chem Biol Interact. 2011;190:121–128. [DOI] [PubMed] [Google Scholar]
- 15. Kim G-S, Park HJ, Woo J-H, et al. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complement Altern Med. 2012;12:31–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jahan S, Redhu NS, Siddiqui AJ, et al. Nobiletin as a neuroprotectant against NMDA receptors: an in silico approach. Pharmaceutics. 2022;14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muhtadi M, Haryoto H, Azizah T, et al. Antidiabetic and antihypercholesterolemic activities of Citrus sinensis peel: in vivo study. Natl J Physiol Pharm Pharmacol. 2015;5:382–385. [Google Scholar]
- 18. Nielsen ILF, Chee WS, Poulsen L, et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr. 2006;136:404–408. [DOI] [PubMed] [Google Scholar]
- 19. Noguera FJM, Alcaraz PE, Vivas JC, et al. 8 Weeks of 2 S-hesperidin supplementation improves muscle mass and reduces fat in amateur competitive cyclists: randomized controlled trial. Food Funct. 2021;12:3872–3882. [DOI] [PubMed] [Google Scholar]
- 20. Yuan X, Wei G, You Y, et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017;31:333–345. [DOI] [PubMed] [Google Scholar]
- 21. Mulvihill EE, Allister EM, Sutherland BG, et al. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor–null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nichols LA, Jackson DE, Manthey JA, et al. Citrus flavonoids repress the mRNA for stearoyl-CoA desaturase, a key enzyme in lipid synthesis and obesity control, in rat primary hepatocytes. Lipids Health Dis. 2011;10:36–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Li D, Liu F, et al. Dietary citrus and/or its extracts intake contributed to weight control: evidence from a systematic review and meta‐analysis of 13 randomized clinical trials. Phytother Res. 2020;34:2006–2022. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Lloyd B, Yang M, et al. Impact of orange juice consumption on macronutrient and energy intakes and body composition in the US population. Public Health Nutr. 2012;15:2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stohs SJ, Preuss HG, Keith SC, et al. Effects of p-synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int J Med Sci. 2011;8:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stohs SJ. Safety, efficacy, and mechanistic studies regarding Citrus aurantium (bitter orange) extract and p‐synephrine. Phytother Res. 2017;31:1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kegele CS, Oliveira J, Magrani T, et al. A randomized trial on the effects of CitrusiM®(Citrus sinensis (L.) Osbeck dried extract) on body composition. Clin Nutr Exp. 2019;27:29–36. [Google Scholar]
- 28. Li L, Lyall GK, Martinez-Blazquez JA, et al. Blood orange juice consumption increases flow-mediated dilation in adults with overweight and obesity: a randomized controlled trial. J Nutr. 2020;150:2287–2294. [DOI] [PubMed] [Google Scholar]
- 29. Cardile V, Graziano ACE, Venditti A. Clinical evaluation of Moro (Citrus sinensis (L.) Osbeck) orange juice supplementation for the weight management. Nat Prod Res. 2015;29:2256–2260. [DOI] [PubMed] [Google Scholar]
- 30. Niv E, Shapira Y, Akiva I, et al. Effect of levan supplement in orange juice on weight, gastrointestinal symptoms and metabolic profile of healthy subjects: results of an 8-week clinical trial. Nutrients. 2012;4:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azzini E, Venneria E, Ciarapica D, et al. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: a pilot study. Oxid Med Cell Longev. 2017;2017:1672567–1672569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Briskey D, Malfa GA, Rao A. Effectiveness of “Moro” blood orange Citrus sinensis Osbeck (Rutaceae) standardized extract on weight loss in overweight but otherwise healthy men and women—a randomized double-blind placebo-controlled study. Nutrients. 2022;14:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson E, Brown S, Mendis B, et al. The effect of daily orange juice consumption on insulin sensitivity and indices of the metabolic syndrome. Proc Nutr Soc. 2012;71:e182. [Google Scholar]
- 34. Basile LG, Lima CG, Cesar TB. Daily intake of pasteurized orange juice decreases serum cholesterol, fasting glucose and diastolic blood pressure in adults. Proc Fla State Hort Soc. 2010;123:228-233. [Google Scholar]
- 35. Cesar TB, Aptekmann NP, Araujo MP, et al. Orange juice decreases low-density lipoprotein cholesterol in hypercholesterolemic subjects and improves lipid transfer to high-density lipoprotein in normal and hypercholesterolemic subjects. Nutr Res. 2010;30:689–694. [DOI] [PubMed] [Google Scholar]
- 36. Asgary S, Keshvari M, Afshani MR, et al. Effect of fresh orange juice intake on physiological characteristics in healthy volunteers. Int Sch Res Notices. 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pittaluga M, Sgadari A, Tavazzi B, et al. Exercise-induced oxidative stress in elderly subjects: the effect of red orange supplementation on the biochemical and cellular response to a single bout of intense physical activity. Free Radic Res. 2013;47:202–211. [DOI] [PubMed] [Google Scholar]
- 38. Büsing F, Hägele FA, Nas A, et al. High intake of orange juice and cola differently affects metabolic risk in healthy subjects. Clin Nutr. 2019;38:812–819. [DOI] [PubMed] [Google Scholar]
- 39. Hägele FA, Büsing F, Nas A, et al. High orange juice consumption with or in-between three meals a day differently affects energy balance in healthy subjects. Nutr Diabetes. 2018;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stookey JD, Hamer J, Espinoza G, et al. Orange juice limits postprandial fat oxidation after breakfast in normal-weight adolescents and adults. Adv Nutr. 2012;3:629S–635S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakaki JR, Li J, Melough MM, et al. Orange juice intake and anthropometric changes in children and adolescents. Public Health Nutr. 2021;24:4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aptekmann NP, Cesar TB. Orange juice improved lipid profile and blood lactate of overweight middle-aged women subjected to aerobic training. Maturitas. 2010;67:343–347. [DOI] [PubMed] [Google Scholar]
- 43. Simpson E, Mendis B, MacDonald IA. Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; a randomised, controlled trial in an at-risk population. Food Funct. 2016;7:1884–1891. [DOI] [PubMed] [Google Scholar]
- 44. Ribeiro C, Dourado G, Cesar T. Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: a randomized controlled trial. Nutrition. 2017;38:13–19. [DOI] [PubMed] [Google Scholar]
- 45. O'Neil CE, Nicklas TA, Rampersaud GC, et al. One hundred percent orange juice consumption is associated with better diet quality, improved nutrient adequacy, and no increased risk for overweight/obesity in children. Nutr Res. 2011;31:673–682. [DOI] [PubMed] [Google Scholar]
- 46. Rumbold P, Shaw E, James L, et al. Milk consumption following exercise reduces subsequent energy intake in female recreational exercisers. Nutrients. 2015;7:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fidélix M, Milenkovic D, Sivieri K, et al. Microbiota modulation and effects on metabolic biomarkers by orange juice: a controlled clinical trial. Food Funct. 2020;11:1599–1610. [DOI] [PubMed] [Google Scholar]
- 48. Rangel-Huerta OD, Aguilera CM, Martin MV, et al. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr. 2015;145:1808–1816. [DOI] [PubMed] [Google Scholar]
- 49. Aptekmann NP, Cesar TB. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013;12:119–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dallas C, Gerbi A, Tenca G, et al. Lipolytic effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (SINETROL) in human body fat adipocytes. Mechanism of action by inhibition of cAMP-phosphodiesterase (PDE). Phytomedicine. 2008;15:783–792. [DOI] [PubMed] [Google Scholar]
- 51. Silveira JQ, Dourado GK, Cesar TB. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int J Food Sci Nutr. 2015;66:830–836. [DOI] [PubMed] [Google Scholar]
- 52. Penzak SR, Jann MW, Cold JA, et al. Seville (sour) orange juice: synephrine content and cardiovascular effects in normotensive adults. J Clin Pharmacol. 2001;41:1059–1063. [DOI] [PubMed] [Google Scholar]
- 53. Silver HJ, Dietrich MS, Niswender KD. Effects of grapefruit, grapefruit juice and water preloads on energy balance, weight loss, body composition, and cardiometabolic risk in free-living obese adults. Nutr Metab (Lond). 2011;8:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fujioka K, Greenway F, Sheard J, et al. The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J Med Food. 2006;9:49–54. [DOI] [PubMed] [Google Scholar]
- 55. Dow CA, Going SB, Chow H-HS, et al. The effects of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metabolism. 2012;61:1026–1035. [DOI] [PubMed] [Google Scholar]
- 56. Taghizadeh M, Memarzadeh MR, Abedi F, et al. The effect of Cumin cyminum L. plus lime administration on weight loss and metabolic status in overweight subjects: a randomized double-blind placebo-controlled clinical trial. Iran Red Crescent Med J. 2016;18:e34212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hashemipour M, Kargar M, Ghannadi A, et al. The effect of Citrus aurantifolia (lemon) peels on cardiometabolic risk factors and markers of endothelial function in adolescents with excess weight: a triple-masked randomized controlled trial. Med J Islam Repub Iran. 2016;30:429. [PMC free article] [PubMed] [Google Scholar]
- 58. Ferro Y, Montalcini T, Mazza E, et al. Randomized clinical trial: bergamot citrus and wild cardoon reduce liver steatosis and body weight in non-diabetic individuals aged over 50 years. Front Endocrinol (Lausanne). 2020;11:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin Y-K, Chung Y-M, Yang H-T, et al. The potential of immature poken (Citrus reticulata) extract in the weight management, lipid and glucose metabolism. J Complement Integr Med. 2022;19:279–285. [DOI] [PubMed] [Google Scholar]
- 60. Zhang Y, Qi J, Zeng W, et al. Properties of dietary fiber from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem. 2020;311:125873. [DOI] [PubMed] [Google Scholar]
- 61. Karn A, Zhao C, Yang F, et al. In-vivo biotransformation of citrus functional components and their effects on health. Crit Rev Food Sci Nutr. 2021;61:756–776. [DOI] [PubMed] [Google Scholar]
- 62. Stephen AM, Champ MM-J, Cloran SJ, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30:149–190. [DOI] [PubMed] [Google Scholar]
- 63. Cui J, Lian Y, Zhao C, et al. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr Rev Food Sci Food Saf. 2019;18:1514–1532. [DOI] [PubMed] [Google Scholar]
- 64. Koolaji N, Shammugasamy B, Schindeler A, et al. Citrus peel flavonoids as potential cancer prevention agents. Curr Dev Nutr. 2020;4:nzaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sukkar AH, Lett AM, Frost G, et al. Regulation of energy expenditure and substrate oxidation by short-chain fatty acids. J Endocrinol. 2019;242:R1–R8. [DOI] [PubMed] [Google Scholar]
- 66. Chambers ES, Byrne CS, Aspey K, et al. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes Metab. 2018;20:1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ezekwesili-Ofili JO, Gwacham NC. Comparative effects of peel extract from Nigerian grown Citrus on body weight, liver weight and serum lipids in rats fed a high-fat diet. Afr J Biochem Res. 2015;9:110–116. [Google Scholar]
- 68. Kang S-I, Shin H-S, Kim H-M, et al. Immature Citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet-induced obese mice. Biol Pharm Bull. 2012;35:223–230. [DOI] [PubMed] [Google Scholar]
- 69. Ding X, Fan S, Lu Y, et al. Citrus ichangensis peel extract exhibits anti-metabolic disorder effects by the inhibition of PPAR and LXR signaling in high-fat diet-induced C57BL/6 mouse. Evid-Based Complement Alternat Med. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li S, Wang Z, Ding F, et al. Content changes of bitter compounds in ‘Guoqing No. 1’ Satsuma mandarin (Citrus unshiu Marc.) during fruit development of consecutive 3 seasons. Food Chem. 2014;145:963–969. [DOI] [PubMed] [Google Scholar]
- 71. Addi M, Elbouzidi A, Abid M, et al. An overview of bioactive flavonoids from Citrus fruits. Appl Sci. 2021;12:29. [Google Scholar]
- 72. Peterson JJ, Dwyer JT, Beecher GR, et al. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J Food Compos Anal. 2006;19:S66–S73. [Google Scholar]
- 73. Xi W, Zhang Y, Sun Y, et al. Phenolic composition of Chinese wild mandarin (Citrus reticulata Balnco.) pulps and their antioxidant properties. Ind Crops Prod. 2014;52:466–474. [Google Scholar]
- 74. Wang S, Yang C, Tu H, et al. Characterization and metabolic diversity of flavonoids in citrus species. Sci Rep. 2017;7:10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Czech A, Malik A, Sosnowska B, et al. Bioactive substances, heavy metals, and antioxidant activity in whole fruit, peel, and pulp of citrus fruits. Int J Food Sci. 2021;2021:6662259–6662214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sánchez-Moreno C, Plaza L, Elez-Martínez P, et al. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J Agric Food Chem. 2005;53:4403–4409. [DOI] [PubMed] [Google Scholar]
- 77. Papandreou D, Magriplis E, Abboud M, et al. Consumption of raw orange, 100% fresh orange juice, and nectar-sweetened orange juice—effects on blood glucose and insulin levels on healthy subjects. Nutrients. 2019;11:2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ani PN, Aginam PC. Effect of Citrus maxima juice on fasting blood glucose, lipid profile, liver enzyme and body weight. NFS. 2018;48:755–763. [Google Scholar]
- 79. Kobayashi H, Mitani M, Minatogawa Y, et al. Extracts of Citrus sudachi peel attenuate body weight gain in C57BL/6 mice fed a high-fat diet. J Med Invest. 2017;64:20–23. [DOI] [PubMed] [Google Scholar]
- 80. Nishikawa S, Hyodo T, Nagao T, et al. α-Monoglucosyl hesperidin but not hesperidin induces brown-like adipocyte formation and suppresses white adipose tissue accumulation in mice. J Agric Food Chem. 2019;67:1948–1954. [DOI] [PubMed] [Google Scholar]
- 81. Shen J, Nakamura H, Fujisaki Y, et al. Effect of 4G-α-glucopyranosyl hesperidin on brown fat adipose tissue-and cutaneous-sympathetic nerve activity and peripheral body temperature. Neurosci Lett. 2009;461:30–35. [DOI] [PubMed] [Google Scholar]
- 82. Ohara T, Muroyama K, Yamamoto Y, et al. A combination of glucosyl hesperidin and caffeine exhibits an anti‐obesity effect by inhibition of hepatic lipogenesis in mice. Phytother Res. 2015;29:310–316. [DOI] [PubMed] [Google Scholar]
- 83. Yoshitomi R, Yamamoto M, Kumazoe M, et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: a randomized placebo-controlled clinical trial. Sci Rep. 2021;11:19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bae J, Yang Y, Xu X, et al. Naringenin, a citrus flavanone, enhances browning and brown adipogenesis: role of peroxisome proliferator-activated receptor gamma. Front Nutr. 2022;9:1036655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang S, Li J, Shi X, et al. Naringenin activates beige adipocyte browning in high fat diet-fed C57BL/6 mice by shaping the gut microbiota. Food Funct. 2022;13:9918–9930. [DOI] [PubMed] [Google Scholar]
- 86. Ke J-Y, Kliewer KL, Hamad EM, et al. The flavonoid, naringenin, decreases adipose tissue mass and attenuates ovariectomy-associated metabolic disturbances in mice. Nutr Metab (Lond). 2015;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Burke AC, Telford DE, Edwards JY, et al. Naringenin supplementation to a chow diet enhances energy expenditure and fatty acid oxidation, and reduces adiposity in lean, pair‐fed Ldlr−/− mice. Mol Nutr Food Res. 2019;63:e1800833. [DOI] [PubMed] [Google Scholar]
- 88. Yang Y, Wu Y, Zou J, et al. Naringenin attenuates non-alcoholic fatty liver disease by enhancing energy expenditure and regulating autophagy via AMPK. Front Pharmacol. 2021;12:687095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rebello CJ, Greenway FL, Lau FH, et al. Naringenin promotes thermogenic gene expression in human white adipose tissue. Obesity (Silver Spring). 2019;27:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Murugesan N, Woodard K, Ramaraju R, et al. Naringenin increases insulin sensitivity and metabolic rate: a case study. J Med Food. 2020;23:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Naeini F, Namkhah Z, Tutunchi H, et al. Effects of naringenin supplementation on cardiovascular risk factors in overweight/obese patients with nonalcoholic fatty liver disease: a pilot double-blind, placebo-controlled, randomized clinical trial. Eur J Gastroenterol Hepatol. 2022;34:345–353. [DOI] [PubMed] [Google Scholar]
- 92. Namkhah Z, Naeini F, Mahdi Rezayat S, et al. Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double‐blind, placebo‐controlled, clinical trial. Int J Clin Pract. 2021;75:e14852. [DOI] [PubMed] [Google Scholar]
- 93. Kwon E-Y, Choi M-S. Eriocitrin improves adiposity and related metabolic disorders in high-fat diet-induced obese mice. J Med Food. 2020;23:233–241. [DOI] [PubMed] [Google Scholar]
- 94. Cesar TB, Ramos FMM, Ribeiro CB. Nutraceutical eriocitrin (Eriomin) reduces hyperglycemia by increasing glucagon-like peptide 1 and downregulates systemic inflammation: a crossover-randomized clinical trial. J Med Food. 2022;25:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen Q, Wang D, Gu Y, et al. Tangeretin prevents obesity by modulating systemic inflammation, fat browning, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. J Nutr Biochem. 2022;101:108943. [DOI] [PubMed] [Google Scholar]
- 96. Kihara-Negishi F, Ohkura N, Takahashi Y, et al. Nobiletin and 3′-demethyl nobiletin activate brown adipocytes upon β-adrenergic stimulation. Biol Pharm Bull. 2022;45:528–533. [DOI] [PubMed] [Google Scholar]
- 97. Choi Y, Kim Y, Ham H, et al. Nobiletin suppresses adipogenesis by regulating the expression of adipogenic transcription factors and the activation of AMP-activated protein kinase (AMPK). J Agric Food Chem. 2011;59:12843–12849. [DOI] [PubMed] [Google Scholar]
- 98. Morrow NM, Burke AC, Samsoondar JP, et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J Lipid Res. 2020;61:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kou G, Li P, Hu Y, et al. Nobiletin activates thermogenesis of brown and white adipose tissue in high‐fat diet‐fed C57BL/6 mice by shaping the gut microbiota. Faseb J. 2021;35:e21267. [DOI] [PubMed] [Google Scholar]
- 100. Okla M, Al Madani JO, Chung S, et al. Apigenin reverses interleukin‐1β‐induced suppression of adipocyte browning via COX2/PGE2 signaling pathway in human adipocytes. Mol Nutr Food Res. 2020;64:e1900925. [DOI] [PubMed] [Google Scholar]
- 101. Guo L, Li X, Tang Q-Q. Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem. 2015;290:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kim M-A, Kang K, Lee H-J, et al. Apigenin isolated from Daphne genkwa Siebold et Zucc. inhibits 3T3-L1 preadipocyte differentiation through a modulation of mitotic clonal expansion. Life Sci. 2014;101:64–72. [DOI] [PubMed] [Google Scholar]
- 103. Sreekumar S, Vijayan V, Singh F, et al. White to brown adipocyte transition mediated by apigenin via VEGF‐PRDM16 signaling. J Cell Biochem. 2022;123:1793–1807. [DOI] [PubMed] [Google Scholar]
- 104. Sun Y-S, Qu W. Dietary apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-fed mice. Food Chem Toxicol. 2019;133:110780. [DOI] [PubMed] [Google Scholar]
- 105. Zhang X, Zhang Q, Wang X, et al. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1α pathway-mediated mechanism. Int J Obes (Lond). 2016;40:1841–1849. [DOI] [PubMed] [Google Scholar]
- 106. Choi H, Kim C-S, Yu R. Quercetin upregulates uncoupling protein 1 in white/brown adipose tissues through sympathetic stimulation. J Obes Metab Syndr. 2018;27:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kuipers EN, Dam A, Held NM, et al. Quercetin lowers plasma triglycerides accompanied by white adipose tissue browning in diet-induced obese mice. Int J Mol Sci. 2018;19:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pei Y, Otieno D, Gu I, et al. Effect of quercetin on nonshivering thermogenesis of brown adipose tissue in high-fat diet-induced obese mice. J Nutr Biochem. 2021;88:108532. [DOI] [PubMed] [Google Scholar]
- 109. Fukaya M, Sato Y, Kondo S, et al. Quercetin enhances fatty acid β-oxidation by inducing lipophagy in AML12 hepatocytes. Heliyon 2021;7:e07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jiang H, Horiuchi Y, Hironao K-y, et al. Prevention effect of quercetin and its glycosides on obesity and hyperglycemia through activating AMPKα in high-fat diet-fed ICR mice. J Clin Biochem Nutr. 2020;67:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Askari G, Ghiasvand R, Paknahad Z, et al. The effects of quercetin supplementation on body composition, exercise performance and muscle damage indices in athletes. International Journal of Preventive Medicine. 2013;4:21–26. [PMC free article] [PubMed] [Google Scholar]
- 112. Egert S, Rimbach G, Müller MJ. No evidence for a thermic effect of the dietary flavonol quercetin: a pilot study in healthy normal-weight women. Eur J Appl Physiol. 2011;111:869–873. [DOI] [PubMed] [Google Scholar]
- 113. Rezvan N, Moini A, Janani L, et al. Effects of quercetin on adiponectin-mediated insulin sensitivity in polycystic ovary syndrome: a randomized placebo-controlled double-blind clinical trial. Hormone Metabol Res. 2017;49:115–121. [DOI] [PubMed] [Google Scholar]
- 114. Cheng L, Shi L, He C, et al. Rutin‐activated adipose tissue thermogenesis is correlated with increased intestinal short‐chain fatty acid levels. Phytother Res. 2022;36:2495–2510. [DOI] [PubMed] [Google Scholar]
- 115. Ma B, Hao J, Xu H, et al. Rutin promotes white adipose tissue “browning” and brown adipose tissue activation partially through the calmodulin-dependent protein kinase kinase β/AMP-activated protein kinase pathway. Endocr J. 2022;69:385–397. [DOI] [PubMed] [Google Scholar]
- 116. Omi N, Shiba H, Nishimura E, et al. Effects of enzymatically modified isoquercitrin in supplementary protein powder on athlete body composition: a randomized, placebo-controlled, double-blind trial. J Int Soc Sports Nutr. 2019;16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Da-Silva WS, Harney JW, Kim BW, et al. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes 2007;56:767–776. [DOI] [PubMed] [Google Scholar]
- 118. Gao Z, Gao W, Zeng S-L, et al. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J Funct Foods. 2018;40:498–509. [Google Scholar]
- 119. Zhang M, Zhu S, Ho C-T, et al. Citrus polymethoxyflavones as regulators of metabolic homoeostasis: recent advances for possible mechanisms. Trends Food Sci Technol. 2021;110:743–753. [Google Scholar]
- 120. Guo J, Tao H, Cao Y, et al. Prevention of obesity and type 2 diabetes with aged citrus peel (chenpi) extract. J Agric Food Chem. 2016;64:2053–2061. [DOI] [PubMed] [Google Scholar]
- 121. Kou G, Hu Y, Jiang Z, et al. Citrus aurantium L. polymethoxyflavones promote thermogenesis of brown and white adipose tissue in high-fat diet induced C57BL/6J mice. Journal of Functional Foods. 2020;67:103860. [Google Scholar]
- 122. Tung Y-C, Chang W-T, Li S, et al. Citrus peel extracts attenuated obesity and modulated gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2018;9:3363–3373. [DOI] [PubMed] [Google Scholar]
- 123. Zeng S-L, Li S-Z, Xiao P-T, et al. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci Adv. 2020;6:eaax6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang M, Zhu J, Zhang X, et al. Aged citrus peel (chenpi) extract causes dynamic alteration of colonic microbiota in high-fat diet induced obese mice. Food Funct. 2020;11:2667–2678. [DOI] [PubMed] [Google Scholar]
- 125. Pan M-H, Li M-Y, Tsai M-L, et al. A mixture of citrus polymethoxyflavones, green tea polyphenols and lychee extracts attenuates adipogenesis in 3T3-L1 adipocytes and obesity-induced adipose inflammation in mice. Food Funct. 2019;10:7667–7677. [DOI] [PubMed] [Google Scholar]
- 126. Tsutsumi R, Yoshida T, Nii Y, et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr Metab (Lond). 2014;11:32–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Feng K, Lan Y, Zhu X, et al. Hepatic lipidomics analysis reveals the antiobesity and cholesterol-lowering effects of tangeretin in high-fat diet-fed rats. J Agric Food Chem. 2020;68:6142–6153. [DOI] [PubMed] [Google Scholar]
- 128. Kim MS, Hur HJ, Kwon DY, et al. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol Cell Endocrinol. 2012;358:127–134. [DOI] [PubMed] [Google Scholar]
- 129. Lee Y-S, Cha B-Y, Choi S-S, et al. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J Nutr Biochem. 2013;24:156–162. [DOI] [PubMed] [Google Scholar]
- 130. Mulvihill EE, Assini JM, Lee JK, et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 2011;60:1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Burke AC, Sutherland BG, Telford DE, et al. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr−/− mice. J Lipid Res. 2018;59:1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu N, Li X, Zhao P, et al. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021;365:130585. [DOI] [PubMed] [Google Scholar]
- 133. Ngamwonglumlert L, Devahastin S. Carotenoids. In: Melton L, Shahidi F, Varelis P, eds. Encyclopedia of Food Chemistry. Pennsylvania: Elsevier; 2018:40–52. [Google Scholar]
- 134. Pradhan SP, Padhi S, Dash M, et al. Carotenoids. In: Kour J, Nayik GA, eds. Nutraceuticals and Health Care. Pennsylvania: Elsevier; 2021;135–157. [Google Scholar]
- 135. Ma G, Zhang L, Yungyuen W, et al. Accumulation of carotenoids in a novel citrus cultivar ‘Seinannohikari’ during the fruit maturation. Plant Physiol Biochem. 2018;129:349–356. [DOI] [PubMed] [Google Scholar]
- 136. Buckle J. Basic plant taxonomy, chemistry, extraction, biosynthesis, and analysis (chapter 3), clinical aromatherapy. In: Buckle J, ed. Essential Oils in Practice. Churchill Livingstone; 2003;38–75. [Google Scholar]
- 137. Busetta G, Ponte M, Barbera M, et al. Influence of citrus essential oils on the microbiological, physicochemical and antioxidant properties of Primosale cheese. Antioxidants 2022;11:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Llopis S, Rodrigo MJ, González N, et al. β-Cryptoxanthin reduces body fat and increases oxidative stress response in Caenorhabditis elegans model. Nutrients. 2019;11:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sugiura M, Kato M, Matsumoto H, et al. Serum concentration of β-cryptoxanthin in Japan reflects the frequency of Satsuma mandarin (Citrus unshiu Marc.) consumption. J Health Sci. 2002;48:350–353. [Google Scholar]
- 140. Turner T, Burri BJ, Jamil KM, et al. The effects of daily consumption of β-cryptoxanthin–rich tangerines and β-carotene–rich sweet potatoes on vitamin A and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low vitamin A status in a randomized controlled trial. Am J Clin Nutr. 2013;98:1200–1208. [DOI] [PubMed] [Google Scholar]
- 141. Hara H, Takahashi H, Mohri S, et al. β-Cryptoxanthin induces UCP-1 expression via a RAR pathway in adipose tissue. J Agric Food Chem. 2019;67:10595–10603. [DOI] [PubMed] [Google Scholar]
- 142. Xie J, Liu M, Liu H, et al. Zeaxanthin ameliorates obesity by activating the β3-adrenergic receptor to stimulate inguinal fat thermogenesis and modulating the gut microbiota. Food Funct. 2021;12:12734–12750. [DOI] [PubMed] [Google Scholar]
- 143. Yao N, Yan S, Guo Y, et al. The association between carotenoids and subjects with overweight or obesity: a systematic review and meta-analysis. Food Funct. 2021;12:4768–4782. [DOI] [PubMed] [Google Scholar]
- 144. Kimmons JE, Blanck HM, Tohill BC, et al. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Medscape Gen Med. 2006;8:59. [PMC free article] [PubMed] [Google Scholar]
- 145. Suzuki K, Inoue T, Hioki R, et al. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin Nutr. 2006;25:780–789. [DOI] [PubMed] [Google Scholar]
- 146. Suzuki K, Ito Y, Ochiai J, et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac J Cancer Prevent. 2003;4:259–266. [PubMed] [Google Scholar]
- 147. Lone J, Yun JW. Monoterpene limonene induces brown fat-like phenotype in 3T3-L1 white adipocytes. Life Sci. 2016;153:198–206. [DOI] [PubMed] [Google Scholar]
- 148. Pellati F, Benvenuti S. Fast high-performance liquid chromatography analysis of phenethylamine alkaloids in Citrus natural products on a pentafluorophenylpropyl stationary phase. J Chromatogr A. 2007;1165:58–66. [DOI] [PubMed] [Google Scholar]
- 149. Stohs SJ, Shara M, Ray SD. p‐Synephrine, ephedrine, p‐octopamine and m‐synephrine: comparative mechanistic, physiological and pharmacological properties. Phytother Res. 2020;34:1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on the safety of caffeine. EFSA J. 2015;13:4102. [Google Scholar]
- 151. Haaz S, Fontaine K, Cutter G, et al. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obes Rev. 2006;7:79–88. [DOI] [PubMed] [Google Scholar]
- 152. Koncz D, Tóth B, Bahar MA, et al. The safety and efficacy of Citrus aurantium (bitter orange) extracts and p-synephrine: a systematic review and meta-analysis. Nutrients. 2022;14:4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gutiérrez‐Hellín J, Del Coso J. Dose–response effects of p‐synephrine on fat oxidation rate during exercise of increasing intensity. Phytother Res. 2018;32:370–374. [DOI] [PubMed] [Google Scholar]
- 154. Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p‐synephrine. Phytother Res. 2011;25:1421–1428. [DOI] [PubMed] [Google Scholar]
- 155. Guo LX, Chen G, Yin ZY, et al. p‐Synephrine exhibits anti‐adipogenic activity by activating the Akt/GSK3β signaling pathway in 3T3‐L1 adipocytes. J Food Biochem. 2019;43:e13033. [DOI] [PubMed] [Google Scholar]
- 156. Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int J Med Sci. 2012;9:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Jung YP, Earnest CP, Koozehchian M, et al. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J Int Soc Sports Nutr. 2017;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gutiérrez‐Hellín J, Del Coso J. Acute p‐synephrine ingestion increases fat oxidation rate during exercise. Br J Clin Pharmacol. 2016;82:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gutiérrez-Hellín J, Baltazar-Martins G, Rodríguez I, et al. p-Synephrine, the main protoalkaloid of Citrus aurantium, raises fat oxidation during exercise in elite cyclists. Eur J Sport Sci. 2021;21:1273–1282. [DOI] [PubMed] [Google Scholar]
- 160. Gutiérrez-Hellín J, Del Coso J. Effects of p-synephrine and caffeine ingestion on substrate oxidation during exercise. Med Sci Sports Exerc. 2018;50:1899–1906. [DOI] [PubMed] [Google Scholar]
- 161. Gutiérrez-Hellín J, Ruiz-Moreno C, Del Coso J. Acute p-synephrine ingestion increases whole-body fat oxidation during 1-h of cycling at Fatmax. Eur J Nutr. 2020;59:3341–3345. [DOI] [PubMed] [Google Scholar]
- 162. Ratamess NA, Bush JA, Kang J, et al. The effects of supplementation with p-synephrine alone and in combination with caffeine on metabolic, lipolytic, and cardiovascular responses during resistance exercise. J Am Coll Nutr. 2016;35:657–669. [DOI] [PubMed] [Google Scholar]
- 163. Gougeon R, Harrigan K, Tremblay JF, et al. Increase in the thermic effect of food in women by adrenergic amines extracted from Citrus aurantium. Obes Res. 2005;13:1187–1194. [DOI] [PubMed] [Google Scholar]
- 164. Hoffman JR, Kang J, Ratamess NA, et al. Thermogenic effect of an acute ingestion of a weight loss supplement. J Int Soc Sports Nutr. 2009;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kaats GR, Miller H, Preuss HG, et al. A 60day double-blind, placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem Toxicol. 2013;55:358–362. [DOI] [PubMed] [Google Scholar]
- 166. Ross R, Soni S, Houle S. Negative energy balance induced by exercise or diet: effects on visceral adipose tissue and liver fat. Nutrients. 2020;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Verheggen R, Maessen M, Green DJ, et al. A systematic review and meta‐analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17:664–690. [DOI] [PubMed] [Google Scholar]
- 168. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. [DOI] [PubMed] [Google Scholar]
- 169. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. [DOI] [PubMed] [Google Scholar]
- 170. Wang L, Qiu Y, Gu H, et al. Regulation of adipose thermogenesis and its critical role in glucose and lipid metabolism. Int J Biol Sci. 2022;18:4950–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wu L, Zhang L, Li B, et al. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol. 2018;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kim MJ, Hwang JH, Ko HJ, et al. Lemon detox diet reduced body fat, insulin resistance, and serum hs-CRP level without hematological changes in overweight Korean women. Nutr Res. 2015;35:409–420. [DOI] [PubMed] [Google Scholar]